Professional Documents
Culture Documents
Drugs Related To Clotting
Uploaded by
Big PigOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Drugs Related To Clotting
Uploaded by
Big PigCopyright:
Available Formats
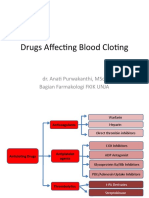
DRUGS RELATED TO
COAGULATION
Dong Ngoc Quang
OMFS Postgrad Student
HEPARIN
MECHANISM
Combine with
antithrombin-III
Inactivate many clotting
factors (including
thrombin (II) and VIII
XII)
Encourage the release of
tissue factor pathway
inhibitor (TFPI)
UNFRACTIONATED HEPARIN
Half life 30 mins, unpredictable
Mainly metabolised by endothelial cells,
liver, kidney
Subcutaneous:
Low dose
Bioavailability: 30%
Takes effect after up to 1 hour
NOT for emergency
UNFRACTIONATED HEPARIN
IV route:
Rapid (immediate)
Higher bioavailability
Can be used in emergency
LMWH
Low Molecular Weight Heparin
More predictable dose response
Greater subcutaneous bioavailability
(90%) more effective
Greater effect on Xa than IIa
Same anticoagulant effect as UFH
Reduce the risk of bleeding
Heparin Side Effects
Haemorrhage, increase by alcohol
Osteoporosis (less w/ LMWH)
Thrombocytopenia (life threatening):
caused by Heparin induced antiplatelet
antibodies
Hyperkalemia: inhibition of aldosteron
secretion
Hypersensitivity
aPTT
Activated Partial Thromboplastin Time
Heparin works on intrinsic pathway and is
measured by aPTT
Normal: 25 39 seconds
Prolonged when: using Heparin,
Haemophilia
WARFARIN
Mechanism: inhibits enzymatic reduction
of Vitamin K to its active form
(hydroquinone).Vitamin K is required for
factor II, VII, IX, X, protein C and S
Half life average 40 hours
Crosses the placenta, tiatrogenic
Early stages: defect, Later stages: foetal
haemorrhage
DO NOT use in pregnancy
WARFARIN
Metabolised by cytochrome P450 system,
interacts w/ many drugs
Monitored by Prothrombin time, expressed
in INR.
Dose of warfarin is adjusted to give INR of 2-4
WARFARIN
Adverse effects:
Haemorrhage: bowel and brain.
Treated by Vitamin K or fresh plasma
containing clotting factors
Teratogenicity
Necrosis of soft tissue (buttock, breast): rare
but serious
WARFARIN
Things increase warfarin effects:
Liver disease
High metabolis states
Drugs:
Inhibit synthesis of Vitamin K
Inhibit hepatic metabolism
Inhibit platetlet
Displace warfarin from its binding site on albumin
WARFARIN
Things that decrease warfarin effects:
State: pregnancy, hyperthyrodism
Drugs
Vitamin K, food rich of Vitamin K (broccoli)
Induce hepatic cytochrome P450 enzymes
ASPIRIN
Antiplatelet
Mechanism: permanently inactivate COX
activity of prostaglandin H synthetase
1 and 2 (COX-1, COX-2)
These catalyze the process to convert
Arachidonic acid to PGH
2
PGH
2
is the cursor of PGD
2
, PGE
2
, PGF
2
,
PGI
2
, TA
2
ASPIRIN
Platelet function and mechanisms of antiplatelet therapy. ADP=adenosine diphosphate;
Ecs=endothelial cells; Gi=inhibitory G protein; GP=glycoprotein; PG=prostaglandin; P2=type 2
platelet purinergic receptor; TX=thromboxane; HETE=hydroxyeicosatetraenoic acid;
HPETE=hydroperoxyeicosatetraenoic acid.
Source: Peter, J.M. et al (2005); Aspirin Resistance and
Atherothrombotic Disease, Journal of the American College of
Cardiology, 46(6), p. 987
ASPIRIN
Rapidly absorbed in the stomach and
upper instestine
Inhibition of TXA
2
dependent platelet
function by 1 hour.
Half-life 15 20 mins, inhibitory effect last
for the life span of platelet
ASPIRIN
Doses: use lowest effective dose is the
best strategy
AF:
Effective but < warfarin
DVT:
Effective but better methods of
thrombophylaxis available (LMWH,
rivaroxaban, dabigatran) not
recommended
ASPIRIN
Does not cause a generalized bleeding
abnormality unless used in patients w/
underlying hemostatic defect
Balance between preventing &
haemorrhage depends on absolute
thrombotic vs haemorrhagic risk
GI toxicity: even low dose
NOVEL ANTICOAGULANTS
Focus on the selective inhibition of clotting
factors
Two main factors: Xa and IIa (thrombin)
block both pathways
DABIGATRAN
Rapid absorbtion (onset within 2 hours)
Not metabolized by cytochrome P450
low risk of drug interaction
Bioavailability 6.5%, required high doses
80% excreted via kidney, contraindicated
in patient w/ renal failure
Indication: VTE treatment/prevention,
Stroke prevention in AF
RIVAROXABAN
Factor Xa inhibitor
High bioavailability
Rapid onset
Metabolized by liver, kidney
Can be administered in fixed dose w/o
coagulation monitoring
Minimal drug interactions
Indications: VTE treatment/prevention,
Stroke prevention in AF, ACS
APIXABAN
Factor Xa inhibitor
Bioavailabillity 50%
Excreted mainly in feces, 25% via kidney
Indications: VTE treatment/prevention,
Stroke prevention in AF, ACS
NOVEL ANTICOAGULANTS
Dabigatran extexilate Rivaroxaban Apixaban
Target Thrombin Factor Xa Factor Xa
Prodrug Yes No No
Bioavailability 6.5 >80 >50
Time to peak level
(hours)
2-3 2-4 3
Half-life (hours) 14-17 9 9-14
Renal excretion (%) 80 33 (67% by liver) 25(~70% in feces)
Dosing Once or twice daily Once or twice daily Twice daily
Drug interactions Potent CYP3A4 and P-
glycoprotein inhibitors
Potent CYP3A4 and
P-glycoprotein
inhibitors
Potent CYP3A4 and
P-glycoprotein
inhibitors
Antidode No No No
Properties of dabigatran etexilate, rivaroxaban, and apixaban
Source: Lip. Y. G. et al (2013) Handbook of Oral Anticoagulation. 2
nd
end. Springer
Healthcare. [Online]
Available at: http://www.springerhealthcare.com
THANK YOU
You might also like
- Anticoagulation PharmacologyDocument36 pagesAnticoagulation PharmacologyaymenNo ratings yet
- Apixaban Eliquis MonographDocument14 pagesApixaban Eliquis MonographTran Minh NgocNo ratings yet
- GI DrugsDocument19 pagesGI DrugsHarshwardhan SinhNo ratings yet
- Tishk International University: ApixabanDocument4 pagesTishk International University: ApixabanDyar MzafarNo ratings yet
- Anticoagulants by DR TariqDocument46 pagesAnticoagulants by DR Tariqsinan kNo ratings yet
- Drugs Study of Omeprazole, Metoclopramide EtcDocument12 pagesDrugs Study of Omeprazole, Metoclopramide EtcMargaret Cortinas75% (4)
- GerdDocument38 pagesGerdZANo ratings yet
- Envenomation and IntoxicationDocument37 pagesEnvenomation and IntoxicationYoelBagusGiartoNo ratings yet
- Receiving Concurrent Moderate CYP3A4 Inhibitors (Erythromycin, Saquinavir, Verapamil, Fluconazole) - 25 MG Once Daily InitiallyDocument272 pagesReceiving Concurrent Moderate CYP3A4 Inhibitors (Erythromycin, Saquinavir, Verapamil, Fluconazole) - 25 MG Once Daily InitiallyFatima Doran PandaogNo ratings yet
- 07 Miscellaneous Drugs Group ADocument64 pages07 Miscellaneous Drugs Group AKamal GhimireNo ratings yet
- Paracetamol Poisoning: G. Rajapandi 521625111 Final MbbsDocument18 pagesParacetamol Poisoning: G. Rajapandi 521625111 Final MbbsAntony PrakashNo ratings yet
- Acetaminophen ToxicityDocument16 pagesAcetaminophen ToxicityMohil PratapNo ratings yet
- Antiprotozoal Drugs: Halia Wanadiatri, DR., M.SiDocument34 pagesAntiprotozoal Drugs: Halia Wanadiatri, DR., M.SiBaiqLinaAnggrianNo ratings yet
- Farmakologi Obat AntikoagulanDocument20 pagesFarmakologi Obat AntikoagulanHenderi SaputraNo ratings yet
- PCM PoisonDocument23 pagesPCM PoisonPrabhat KcNo ratings yet
- Antiprotozoal Antimalarial DrugsDocument49 pagesAntiprotozoal Antimalarial DrugsFredericka QuayeNo ratings yet
- Anticoagulant, Fibrinolytic, and Antiplatelet DrugsDocument22 pagesAnticoagulant, Fibrinolytic, and Antiplatelet DrugsVera Waty100% (1)
- Exam 4 NOTESDocument20 pagesExam 4 NOTESDoctorDrapionNo ratings yet
- Pharmacology of GITDocument29 pagesPharmacology of GITMohammed Bahnasy100% (1)
- MalariaDocument45 pagesMalariaSiya PatelNo ratings yet
- Point of View Heparin ResistanceDocument20 pagesPoint of View Heparin ResistanceSameer Goyal100% (1)
- MIMS Drug StudyDocument6 pagesMIMS Drug StudyKaloy KamaoNo ratings yet
- 34-Paracetamol PoisoningDocument26 pages34-Paracetamol PoisoningSumaiyyaNo ratings yet
- Anticoagulation Monitoring Hand-OutDocument69 pagesAnticoagulation Monitoring Hand-OutApril OcampoNo ratings yet
- Tylenol OverdoseDocument9 pagesTylenol OverdoseTyler HempelNo ratings yet
- Study Guide - Pharmacology: Agonist vs. AntagonistDocument4 pagesStudy Guide - Pharmacology: Agonist vs. Antagonistb2ktookie9455No ratings yet
- Drug Name Dose, Route, Frequency Mechanism of Drug Indications Adverse Effects Contraindications Nursing ResponsibilitiesDocument15 pagesDrug Name Dose, Route, Frequency Mechanism of Drug Indications Adverse Effects Contraindications Nursing ResponsibilitiesitsmechachaNo ratings yet
- Preoperative PremedicationsDocument90 pagesPreoperative PremedicationsMorad SatariNo ratings yet
- Antimalarial DrugsDocument56 pagesAntimalarial DrugsKasturiRangan SrivatsaNo ratings yet
- Drugs Used in GIT DisordersDocument46 pagesDrugs Used in GIT Disordersسلطان القلحNo ratings yet
- Xi - Drug Study: Drugs Action Indication Contraindication Adverse Effect Nursing ConsiderationDocument18 pagesXi - Drug Study: Drugs Action Indication Contraindication Adverse Effect Nursing ConsiderationlicservernoidaNo ratings yet
- Anticoagulants and AntiplateletsDocument25 pagesAnticoagulants and AntiplateletsIdrissa John Sebeh ContehNo ratings yet
- Farmakologi Sistem Gastrointestinal 1 - FiksDocument77 pagesFarmakologi Sistem Gastrointestinal 1 - FiksINDRINo ratings yet
- KU - Lesson 5 - Drugs For Hyperlipidaemia PDFDocument51 pagesKU - Lesson 5 - Drugs For Hyperlipidaemia PDFchristine gisembaNo ratings yet
- Anaesthesia For Liver DieseaseDocument43 pagesAnaesthesia For Liver DieseaseShehan WijayasiriwardanaNo ratings yet
- XIV. Antimicrobial Drugs (D, E, F, G), ZI-WA, AY18-19Document64 pagesXIV. Antimicrobial Drugs (D, E, F, G), ZI-WA, AY18-19Hala Al-siyabiNo ratings yet
- AbemaciclibDocument2 pagesAbemaciclibIna SimacheNo ratings yet
- Drugs Affecting Blood Cloting 2019Document39 pagesDrugs Affecting Blood Cloting 2019Mutiara RizkiNo ratings yet
- Propofol 2Document22 pagesPropofol 2Bhaskar PendyalaNo ratings yet
- Anesthesia For Emergency AppendectomyDocument44 pagesAnesthesia For Emergency AppendectomyPrincess Lorenzo MiguelNo ratings yet
- Endocrine DrugsDocument6 pagesEndocrine DrugsdiriniumNo ratings yet
- Olanzapine For Nausea and VomitingDocument42 pagesOlanzapine For Nausea and Vomitingsitiradziah subariNo ratings yet
- Drugs Used in Cardiovascular SystemDocument57 pagesDrugs Used in Cardiovascular SystemSandeep ChaudharyNo ratings yet
- 2020 Drugs On Pain (Analgesics) - DENTISTRYDocument28 pages2020 Drugs On Pain (Analgesics) - DENTISTRYVisayan Alliah GailNo ratings yet
- Organophosphate PoisoningDocument40 pagesOrganophosphate PoisoningMadhu Sudhan PandeyaNo ratings yet
- 3 and 4 Management of Medically CompromisedDocument63 pages3 and 4 Management of Medically Compromisedranareda499No ratings yet
- 21 - Gastrointestinal PharmacologyDocument53 pages21 - Gastrointestinal PharmacologySuhayb CumarNo ratings yet
- Drugs in PregnancyDocument72 pagesDrugs in PregnancyNu Joe100% (1)
- Camuso OtcDocument15 pagesCamuso Otcapi-548307464No ratings yet
- Management of Tuberculosis in Special Situations: Prof. Dr. Zafar Hussain IqbalDocument27 pagesManagement of Tuberculosis in Special Situations: Prof. Dr. Zafar Hussain IqbalFahadKamalNo ratings yet
- Nichols GI 09Document59 pagesNichols GI 09marviecute22No ratings yet
- 12 MalariaDocument61 pages12 MalariaMewael TesfamichaelNo ratings yet
- The Biochemical Pathway of Paraquat ToxicityDocument16 pagesThe Biochemical Pathway of Paraquat ToxicitysuderiNo ratings yet
- Therapeutic Drug Monitoring Applied Pharmacokinetics: Concordance (Compliance)Document9 pagesTherapeutic Drug Monitoring Applied Pharmacokinetics: Concordance (Compliance)anon_954125025No ratings yet
- 02 - Common PoisonsDocument33 pages02 - Common PoisonsTrishenth FonsekaNo ratings yet
- Anti ArrhythmicsDocument46 pagesAnti Arrhythmicsnk999999No ratings yet
- Nonsteroidal Anti-Inflammatory Drugs (Nsaids)Document46 pagesNonsteroidal Anti-Inflammatory Drugs (Nsaids)b3djo_76No ratings yet
- Acetaminophen Toxicity Final 1 0Document23 pagesAcetaminophen Toxicity Final 1 0عزالدين الطيارNo ratings yet
- Deficiencies Bariatric SurgeryDocument10 pagesDeficiencies Bariatric SurgeryYazen JoudehNo ratings yet
- Pharmacology of The GIT System: CIC Ragasa, RN-MDDocument70 pagesPharmacology of The GIT System: CIC Ragasa, RN-MDCarmencita Ileen Ragasa - AhmedNo ratings yet
- FibromaDocument3 pagesFibromaAsiyath HNo ratings yet
- The Prevention and Cure Effects of Aspirin Eugenol Ester On Hyperlipidemia and Its MetabonomicsDocument108 pagesThe Prevention and Cure Effects of Aspirin Eugenol Ester On Hyperlipidemia and Its MetabonomicsKavisa GhoshNo ratings yet
- Vasofix SafetyDocument6 pagesVasofix Safetydex99No ratings yet
- Abnormal Psychology Movie ReviewDocument3 pagesAbnormal Psychology Movie ReviewAthirah NarawiNo ratings yet
- Hospital Infections PDFDocument794 pagesHospital Infections PDFJOSEPH APPIAHNo ratings yet
- PrescriptionDocument2 pagesPrescriptionELvin LozandeNo ratings yet
- College of Nursing: Name: Krizia Mae A. Mendoza Section: BSN 2-1Document9 pagesCollege of Nursing: Name: Krizia Mae A. Mendoza Section: BSN 2-1Nikki Coleen SantinNo ratings yet
- A Leading Surgical Gastroenterologist in Hyderabad Dr. Dinesh ReddyDocument4 pagesA Leading Surgical Gastroenterologist in Hyderabad Dr. Dinesh Reddydrdineshreddy02No ratings yet
- 1-29-20 Diabetes Protocol Draft With Pandya and Alvarez EditsDocument12 pages1-29-20 Diabetes Protocol Draft With Pandya and Alvarez Editsapi-552486649No ratings yet
- WHO - HQ - Reports G2 PROD EXT TBCountryProfileDocument1 pageWHO - HQ - Reports G2 PROD EXT TBCountryProfileAngelo Santos EstrellaNo ratings yet
- Hirschsprung's Disease, PDFDocument1 pageHirschsprung's Disease, PDFMr. LNo ratings yet
- Makalah Leigh Disease by Boys KDocument6 pagesMakalah Leigh Disease by Boys KAzizul HakimNo ratings yet
- Maternal and Child HealthDocument60 pagesMaternal and Child HealthStar AcademyNo ratings yet
- Carcinocine in PaediatricsDocument5 pagesCarcinocine in Paediatricssimiliadoc100% (1)
- Needle CricothyroidotomyDocument9 pagesNeedle Cricothyroidotomyhatem alsrour100% (2)
- Review Material Exam TypeDocument9 pagesReview Material Exam TypeFelimon BugtongNo ratings yet
- Jaw Fractures Managing The Whole PatientDocument2 pagesJaw Fractures Managing The Whole PatientElisa BaronNo ratings yet
- Blocked Goat Urolithiasis HandoutDocument22 pagesBlocked Goat Urolithiasis Handoutapi-262327869100% (1)
- What Is A Physiatrist PowerpointDocument17 pagesWhat Is A Physiatrist PowerpointsirniravNo ratings yet
- Endocrine Glands - 1st - ChapterDocument12 pagesEndocrine Glands - 1st - Chaptervarun kumarNo ratings yet
- Thrombocytopenia: Decreased Production Increased Destruction Sequestration PseudothrombocytopeniaDocument43 pagesThrombocytopenia: Decreased Production Increased Destruction Sequestration PseudothrombocytopeniaDea Tasha MeicitaNo ratings yet
- Disentri AmubaDocument8 pagesDisentri AmubaVivi DeviyanaNo ratings yet
- How We Can Spread AwerenessDocument47 pagesHow We Can Spread AwerenessFaurel AzmiNo ratings yet
- 社區要學pptDocument41 pages社區要學pptMK Camera100% (1)
- Epidemiology, Risk Factors, Pathogenesis, and Natural History of Thoracic Aortic AneurysmDocument5 pagesEpidemiology, Risk Factors, Pathogenesis, and Natural History of Thoracic Aortic AneurysmNathaliazuosNo ratings yet
- TALLY 79 Respondents FinalDocument7 pagesTALLY 79 Respondents FinalMarissa AsimNo ratings yet
- DM 2021-0114 Guidelines On The MGMT and Admin of The Initial 600,000 Donated SARS-CoV-2 Vaccine (Vero Cell) Inactivated Coronavac (Sinovac) DosesDocument17 pagesDM 2021-0114 Guidelines On The MGMT and Admin of The Initial 600,000 Donated SARS-CoV-2 Vaccine (Vero Cell) Inactivated Coronavac (Sinovac) DosesRalph Julius MendozaNo ratings yet
- Exercise Tolerance TestDocument15 pagesExercise Tolerance TestahmedNo ratings yet