0% found this document useful (0 votes)
1K views28 pagesClassification of Organometallic Compounds
The document classifies organometallic compounds based on their bonding features and ligand types. It discusses several types of organometallic compounds including:
1. σ-bonded compounds containing metal-carbon sigma bonds such as alkyl and aryl derivatives.
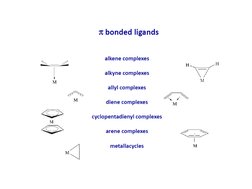
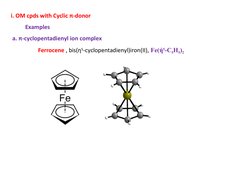
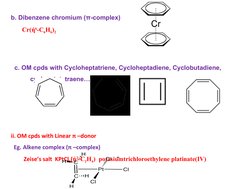
2. π-bonded compounds where the transition metal forms bonds to unsaturated hydrocarbons using d-orbitals, such as ferrocene.
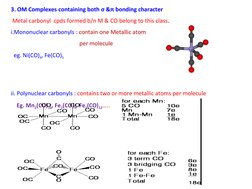
3. Compounds containing both σ and π bonding such as metal carbonyls.
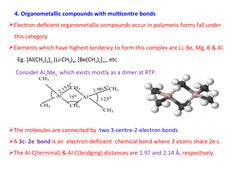
4. Compounds with multicenter bonds like dialkylaluminum compounds which form 3-center-2-electron bonds.
It also provides examples and details of metal-
Uploaded by
Dingetegna GodanaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPT, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
1K views28 pagesClassification of Organometallic Compounds
The document classifies organometallic compounds based on their bonding features and ligand types. It discusses several types of organometallic compounds including:
1. σ-bonded compounds containing metal-carbon sigma bonds such as alkyl and aryl derivatives.
2. π-bonded compounds where the transition metal forms bonds to unsaturated hydrocarbons using d-orbitals, such as ferrocene.
3. Compounds containing both σ and π bonding such as metal carbonyls.
4. Compounds with multicenter bonds like dialkylaluminum compounds which form 3-center-2-electron bonds.
It also provides examples and details of metal-
Uploaded by
Dingetegna GodanaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPT, PDF, TXT or read online on Scribd
- Classification of Organometallic Compounds: Introduces the features of organometallic compounds categorized by their bonding characteristics, focusing on π-bonded groups.
- OM Complexes with σ and π bonding characters: Discusses metal carbonyl compounds that exhibit both σ and π bonding attributes.
- Organometallic Compounds with Multicentre Bonds: Covers organometallic compounds with multicenter bonds, emphasizing electron-deficient complexes and structural features.
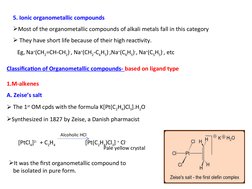
- Ionic Organometallic Compounds: Explores ionic organometallic compounds, with emphasis on alkali metal complexes.
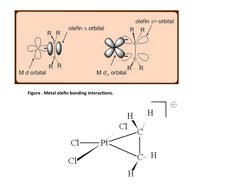
- Synthesis of Metal Alkenes: Covers methods for the synthesis of metal alkenes through different chemical reactions.
- Reactions of Metal Alkenes: Discusses the typical reactions involving metal alkenes including insertion and oxidative addition.
- η2-coordination to a Single Metal Center: Focuses on η2-coordination, explaining its significance in complex formation with single metal centers.
- Bridging Metal Alkyne Complexes: Details the bridging coordination methods using metal alkyne complexes and their properties.