0% found this document useful (0 votes)
1K views23 pagesCDM Process Overview
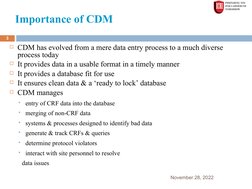
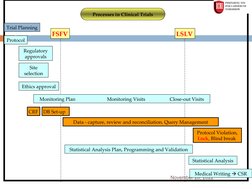
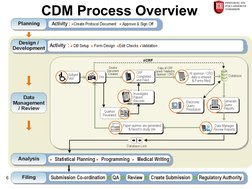
The document provides an overview of clinical data management (CDM) processes. It discusses how CDM has evolved from data entry to a diverse process that ensures clean, usable data in a timely manner. The objectives of CDM are to ensure data integrity and accuracy so that clinical trial results are correct. Key parts of the CDM process include study start-up activities like designing case report forms and databases, study conduct activities such as data entry and query resolution, and study close-out activities to finalize the database for analysis and reporting.
Uploaded by
Seema MishraCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPT, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
1K views23 pagesCDM Process Overview
The document provides an overview of clinical data management (CDM) processes. It discusses how CDM has evolved from data entry to a diverse process that ensures clean, usable data in a timely manner. The objectives of CDM are to ensure data integrity and accuracy so that clinical trial results are correct. Key parts of the CDM process include study start-up activities like designing case report forms and databases, study conduct activities such as data entry and query resolution, and study close-out activities to finalize the database for analysis and reporting.
Uploaded by
Seema MishraCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPT, PDF, TXT or read online on Scribd