Professional Documents
Culture Documents
Protocol Summary HT
Protocol Summary HT
Uploaded by
api-6426923630 ratings0% found this document useful (0 votes)
19 views28 pagesOriginal Title
protocol summary ht
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
19 views28 pagesProtocol Summary HT
Protocol Summary HT
Uploaded by
api-642692363Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 28
Protocol A011202
Alliance A011202/NCT 01901094
A011202
• Alliance for Clinical Trials in Oncology (Sponsor)
• National Cancer Institute (NCI)
• Canadian Cancer Trials Group
• A Randomized Phase III Trial Comparing Axillary Node Dissection
(ALND) to Axillary Radiation in Breast Cancer Patients (cT1-3 N1) who
have Positive Sentinel Lymph Node Disease after Neoadjuvant
Chemotherapy.
Participants
• 2012 Participants.
• 1246 Study locations.
Primary Objective
• In term of recurrence-free interval in patients with positive sentinel lymph
node after completion of neoadjuvant chemotherapy.
• To evaluate whether radiation to the undissected axilla and regional lymph
nodes is not inferior to axillary lymph node dissection with radiation to
the regional lymph nodes but not to the dissected axilla.
Secondary Objective
• In term of loco-regional recurrences in patients with positive sentinel
lymph node after completion of neoadjuvant chemotherapy.
• To evaluate whether radiation to the undissected axilla and regional lymph
nodes is not inferior to axillary lymph node dissection with radiation to
the regional lymph nodes but not to the dissected axilla.
• To obtain an estimate of the distribution of residual disease burden scores.
• To estimate the distribution of overall survival.
Pre-Registration Eligibility Criteria
• Clinical stage T1-3 N1 M0 at diagnosis (prior to the start of neoadjuvant chemotherapy).
• No inflammatory breast cancer.
• No other malignancy within 5 years of registration, with the exception, of basal cell or squamous
cell carcinoma of the skin treated with local resection only, or carcinoma in situ of the cervix.
• All patients must have had an axillary ultrasound with fine needle aspiration (FNA) or core
needle biopsy (CNB) of axillary lymph nodes, documenting axillary metastasis at the time of
diagnosis, prior to or at most 14 days after starting neoadjuvant chemotherapy.
• Patients must have had estrogen receptor, progesterone receptor and HER2 status evaluated on
diagnostic core biopsy prior to start of neoadjuvant chemotherapy.
Pre-Registration Eligibility Criteria
• Patients must have completed all planned neoadjuvant chemotherapy prior to
surgery.
• Patients with HER-2 positive tumors must have received approved
neoadjuvant anti-HER-2 therapy.
• At the completion of neoadjuvant chemotherapy, patients must have a
clinically negative axilla on physical examination documented.
• No more than 8 weeks of neoadjuvant endocrine therapy prior to the start of
neoadjuvant chemotherapy.
Pre-Registration Eligibility Criteria
• No neoadjuvant radiation therapy.
• No sentinel lymph node (SLN) surgery/excisional biopsy for pathological
confirmation of axillary status prior to or during neoadjuvant chemotherapy.
• No prior history of ipsilateral malignant or benign breast cancer.
• No prior ipsilateral axillary surgery.
• No history of prior or concurrent contralateral invasive or benign breast cancer.
• Patients are not pregnant or nursing.
Intra-Operative Registration/Randomization
Criteria:
• Breast surgery (lumpectomy or mastectomy) and sentinel lymph node surgery must
be completed within 56 days of the completion of the last dose of neoadjuvant
chemotherapy.
• A minimum of 1 sentinel node and a maximum of 8 total nodes (sentinel + non-
sentinel) are identified and excised; more than 8 nodes identified by either surgeon
or pathologist is NOT allowed.
• At least one lymph node (sentinel or non-sentinel) excised during sentinel lymph
node surgery with a metastasis greater than 0.2 mm in greatest dimension
identified on intra-operative pathologic assessment.
Post-Operative Registration/Randomization
Criteria:
• For cases where ALND has not been performed and one of the following is true:
• 1) intra-operative evaluation of sentinel lymph node could not be/was not performed, and final pathology identified a positive lymph node
(sentinel or non-sentinel) with metastasis greater than 0.2 mm
• 2) lymph node (sentinel or non-sentinel) considered negative on intra-operative evaluation was found to be positive on final pathology (with
metastasis greater than 0.2 mm)
• Breast surgery (lumpectomy or mastectomy) and sentinel lymph node surgery must be completed within 56 days of the
completion of the last dose of neoadjuvant chemotherapy.
• At least one lymph node (sentinel or non-sentinel) with a metastasis greater than 0.2 mm in greatest dimension identified on
final pathology.
• At least one and no more than 8 lymph nodes (sentinel and non-sentinel) were found by the pathologists to have been excised
during sentinel lymph node procedure.
• For patients who also undergo contralateral breast surgery, if invasive disease is found in the contralateral breast, the patient is
not eligible for registration /randomization
Treatment Arms
• Arm I:
• Patients undergo Axillary Lymph Node Dissection (ALND). After 3-12 weeks
following surgery, patient undergo radiation treatment (3DCRT, IMRT or PRT) 5
days a week over 5-6 weeks.
• Arm II
• Patient undergo axillary and nodal radiation treatment (3DCRT, IMRT or PRT) 5
days a week over 5-6 weeks.
Simulation
• Supine
• Gating or breath hold (ABC) if required/allowed.
• Breast-board devices to improve positioning.
• Scars are marked and identified by radio-opaque wires.
• Expanders for reconstruction.
Radiation Treatment Planning
• Radiation treatment methods (3DCRT, IMRT, and PRT) and Dose Volume parameters are
followed by RTOG criteria.
• Radiation treatment should not begin earlier than 3 weeks (21days) and no later than 12 weeks
(84 days) following surgery.
• Arm I (ALND & Nodal RT)
• PTV - breast/chest wall, undissected axilla, supraclavicular nodes and internal mammary nodes in the first 3
intercostal spaces.
• Arm II (Axillary & Nodal RT)
• PTV - breast/chest wall, full axilla including Levels I, II, III, supraclavicular nodes and internal mammary
nodes in the first 3 intercostal spaces.
Target Delineated
• Lumpectomy
• Lumpectomy GTV.
• Lumpectomy CTV (GTV + 1cm 3D expansion).
• Lumpectomy PTV (CTV + .7cm 3D expansion).
• Lumpectomy PTV Eval (PTV with .5cm exclusion from skin).
• Breast CTV.
• Breast PTV (CTV + .7cm 3D expansion).
• Breast PTV Eval (PTV with .5cm exclusion from skin).
Target Delineated
• Mastectomy
• Mastectomy Scar GTV.
• Mastectomy Scar CTV ( GTV + 1cm 3D expansion).
• Mastectomy Scar PTV ( CTV + .7cm 3D expansion).
• Mastectomy Scar PTV Eval ( PTV with .3cm exclusion from skin).
• Chestwall CTV.
• Chestwall PTV (CTV + .7cm 3D expansion)
• Chestwall PTV Eval (PTV with .3cm exclusion from skin).
Target Delineated
• Regional Nodes
• Supraclavicular CTV.
• Supraclavicular PTV (CTV + .5cm 3D expansion *except medially)
• Axillary CTV
• Arm I: remain “undissected” (usually level III)
• Arm II: all level I, II, III.
• Axillary PTV (CTV + .5cm 3D expansion *except medially)***Arm II only***
• Internal Mammary Node CTV.
• Internal Mammary Node PTV ( CTV + .5cm expansion*medical and lateral only)
Dose Specifications
• Post Lumpectomy Breast + Boost:
• Total tumor bed dose: 60-64Gy
• Breast: 50 Gy, 25 fx, 2 Gy/fx (5 days/week).
• Lumpectomy Boost: 10-14 Gy,5-7 fx, 2 Gy/fx (5 days/week).
• Post Mastectomy:
• Total tumor bed dose: 60-64Gy
• Chestwall, undissected axilla, supraclavicular nodes and internal mammary nodes: 50Gy, 25 fx, 2 Gy/fx (5 days/week).
• Chestwall/scar boost: 10-14 Gy, 5-7 fx, 2 Gy/fx (5 days/week).
• Regional Nodal Irradiation:
• Total dose: 46-50Gy
• Undissected axilla, supraclavicular nodes and internal mammary nodes: 50Gy, 25 fx, 2 Gy/fx (5 days/week).
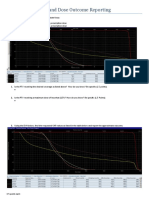
PTV Constraints
• Lumpectomy PTV Eval:
• Per protocol: V60-64 ≥ 95%
• Variation Acceptable: V60-64 ≥ 90%
• Per protocol: V66-70.4 ≤ 5%
• Variation Acceptable: V66-70.4 ≤ 10%
• Per protocol: Dmax ≤ 69-73.6Gy
• Variation Acceptable: Dmax ≤ 72-76.8 Gy
• Chestwall/Scar PTV Eval:
• Per protocol: V60-64 ≥ 95%
• Variation Acceptable: V60-64 ≥ 90%
PTV Constraints
• Chestwall or Breast:
• Per protocol: V50 ≥ 95%
• Variation Acceptable: V50 ≥ 90%
• Per protocol: Dmax ≤ 57.5 Gy
• Variation Acceptable: Dmax ≤ 65 Gy
• Supraclavicular (SCL):
• Per protocol: V46-50 ≥ 95%
• Variation Acceptable: V46-50 ≥ 90%
PTV Constraints
• Axillary Nodes:
• Per protocol: V46-50 ≥ 95%
• Variation Acceptable: V46-50 ≥ 90%
• Internal Mammary Nodes (IMN):
• Per protocol: V46-50 ≥ 90%
• Variation Acceptable: V46-50 ≥ 80%
OAR Constraints
• Contralateral Breast:
• Per protocol: V3 ≤ 5%
• Variation Acceptable: V3 ≤ 8%
• Ipsilateral Lung:
• Per protocol: V20 ≤ 34%
• Variation Acceptable: V20 ≤ 38%
• Contralateral Lung:
• Per protocol: V5 ≤ 10%
• Variation Acceptable: V5 ≤ 15%
OAR Constraints
• Heart:
• L Breast:
• Per protocol: V25 ≤ 5%
• Variation Acceptable: V25 ≤ 10%
• R Breast:
• Per protocol: V25 ≤ 2%
• Variation Acceptable: V25 ≤ 1%
• Or
• Per protocol: Mean dose ≤ 4 Gy
• Variation Acceptable: Mean dose ≤ 5 Gy
Protocol Deviations
• Per Protocol: All specified DVH requirements identified as Per Protocol
have been met.
• Variation Acceptable: specified DVH requirements within the Variation
Acceptable have been met.
• Deviation Unacceptable: specified DVH requirements within Variation
Acceptable have not met.
Volume Deviations
• Per Protocol: All specified contoured volumes are drawn as specified in the
protocol.
• Contoured Volumes Variation Acceptable: Delineation of specified contoured
volumes deviates from protocol guidelines, but the protocol intended volumes
are adequately covered by the prescribed doses.
• Contoured Volumes Variation Unacceptable: Delineation of specified
contoured volumes deviates significantly from protocol guidelines, but the
protocol intended volumes are not adequately covered by the prescribed doses.
Summary
• Arm I:
• Patients undergo Axillary Lymph Node Dissection (ALND). After 3-12 weeks
following surgery, patient undergo radiation treatment (3DCRT, IMRT or PRT) 5
days a week over 5-6 weeks.
• Arm II
• Patients undergo axillary and nodal radiation treatment (3DCRT, IMRT or PRT) 5
days a week over 5-6 weeks.
The Question?
• Is Radiation Therapy alone as effective as lymph node dissection with
breast cancer previously treated with chemotherapy and surgery?
Reference
• 1. NIH. NCT01901094 Comparison of Axillary Lymph Node Dissection With Axillary
Radiation for Patients With Node-Positive Breast Cancer Treated With Chemotherapy.
Accessed July 25, 2023. https://clinicaltrials.gov/study/NCT01901094?
cond=Breast&spons=CALGB%20OR%20NCCTG%20OR%20ACOSOG%20OR%20SWOG
%20OR%20ECOG%20OR%20%22Gynecologic%20Oncology%20Group%22%20OR
%20%22Alliance%20for%20Clinical%20Trials%20in%20Oncology%22%20OR%20RTOG
%20OR%20NSABP%20OR%20ACRIN%20....%20&page=9&rank=82
• 2. Wong S, Santos J, Basik M. Eliminating Surgery in Early-Stage Breast Cancer: Pipe-Dream
or Worthy Consideration in Selected Patients? Curr Breast Cancer Rep (2017) 9:148-155.
Published June 2017. Doi: 10.1007/s12609-017-0242-y
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5814)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Fall 3rd EvalDocument3 pagesFall 3rd Evalapi-642692363No ratings yet
- Fall 1st EvalDocument3 pagesFall 1st Evalapi-642692363No ratings yet
- Outline IIIIDocument5 pagesOutline IIIIapi-642692363No ratings yet
- Fall 2nd EvalDocument3 pagesFall 2nd Evalapi-642692363No ratings yet
- NolaDocument3 pagesNolaapi-642692363No ratings yet
- Caselogtotals 1Document2 pagesCaselogtotals 1api-642692363No ratings yet
- Employer ServiceDocument1 pageEmployer Serviceapi-642692363No ratings yet
- Summary A011202 - HTDocument2 pagesSummary A011202 - HTapi-642692363No ratings yet
- Certificate LarynxDocument1 pageCertificate Larynxapi-642692363No ratings yet
- Intact Breast TangentsDocument2 pagesIntact Breast Tangentsapi-642692363No ratings yet
- 3 FLD With WedgesDocument2 pages3 FLD With Wedgesapi-642692363No ratings yet
- Master Competency ListDocument1 pageMaster Competency Listapi-642692363No ratings yet
- 2nd Spring EvalDocument3 pages2nd Spring Evalapi-642692363No ratings yet
- Phase I RodDocument6 pagesPhase I Rodapi-642692363No ratings yet
- DVH and Dose Reporting Outcomes 1Document3 pagesDVH and Dose Reporting Outcomes 1api-642692363No ratings yet
- Certificate-Left ParotidDocument1 pageCertificate-Left Parotidapi-642692363No ratings yet
- Citicompletioncertificate SocialDocument1 pageCiticompletioncertificate Socialapi-642692363No ratings yet
- Hieu Tran Lung Treatment Planning LabDocument14 pagesHieu Tran Lung Treatment Planning Labapi-642692363No ratings yet
- Citicompletioncertificate BiomedicalDocument1 pageCiticompletioncertificate Biomedicalapi-642692363No ratings yet
- Prostate PaperDocument15 pagesProstate Paperapi-642692363No ratings yet
- Certificate Glottic Larynx HTDocument1 pageCertificate Glottic Larynx HTapi-642692363No ratings yet
- Certificate Brainstem HTDocument1 pageCertificate Brainstem HTapi-642692363No ratings yet