Professional Documents
Culture Documents
Management of Retinal Vascular Occlusions
Uploaded by
khetpal0 ratings0% found this document useful (0 votes)
5 views55 pagesCopyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views55 pagesManagement of Retinal Vascular Occlusions
Uploaded by
khetpalCopyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 55
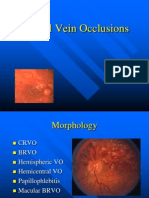
Management of Retinal
Vascular Occlusions
University of Florida - Jacksonville
CRAO
Central Retinal Artery Occlusion
– Painless vision loss ocurring over several
seconds
– May have preceding h/o amaurosis episodes
– Visual acuity CF’s to LP unless cilioretinal
sparing
– NLP suggests choroidal or optic nerve
involvement
Emboli visible in 20-40%
Most common variant is glistening, yellow
cholesterol embolus (Hollenhorst plaque).
– Most commonly arise from atherosclerotic deposits in
the carotid arteries
Calcific emboli less common
– Tend to be larger and cause more severe obstructions.
– Usually originate from the cardiac valves
Low interobserver agreement on type of
plaque, therefore fundus appearance should
not be only guidance for workup
Visible retinal arterial embolus is associated
with increased mortality
– Mortality rate(cardiac disease) of 56% over
nine years as compared to a rate of 27% during
the same time in an age-matched control group
without arterial emboli
20% of eyes develop rubeosis
– Develop at a mean of 4-5 weeks after
obstruction
– 65% regress with PRP
Ischemic necrosis of inner layers of retina
causes whitening of posterior pole except in
fovea, cherry red spot due to ability to see
through to RPE and choroid
Opacification requires hours to become
apparent
Opacification diminishes outside posterior
pole
Histopathologic findings in chronic branch retinal artery obstruction elucidate the
damage to the inner retina with arterial obstructive disease. The retina on the right is
unaffected, whereas on the left the inner layers have atrophied to the inner nuclear
layer. (Courtesy of Dr Jerry A. Shields, Philadelphia and the Armed Forces Institute
of Pathology, Washington D.C.)
Retinal opacification resolves over 4-6
weeks leaving optic nerve pallor, NFL
dropout, and arteriolar attenuation
With severe obstruction may see boxcarring
of arteries and veins
CRAO
IVFA
– May have delayed retinal arterial filling
– Most sensitive sign is delayed arterio-venous
transit time(Normal <= 11 seconds)
ERG
– Decreased B-Wave amplitude(Muller/bipolar
cells)
BRAO
Retinal whitening only in area perfused by
affected branch
Same systemic associations as CRAO
Combined retinal artery and vein occlusion
– H/o sudden vision loss
– Retinal opacification with cherry-red spot
combined with hemorrhagic component, dilated
and tortuous retinal veins, swollen optic disc,
and marked retinal edema in posterior pole
– Workup similar to CRAO, aminoglycoside
toxicity within differential
CRAO
Systemic Associations
– Most common include emboli, intraluminal
thrombosis, hemorrhage under an
atherosclerotic plaque, vasculitis, spasm,
circulatory collapse, dissecting aneurysm, and
hypertensive arterial necrosis
– HTN in 2/3 of patients, DM in ¼ of patients
Systemic Associations
– Structural cardiac pathology was seen in nearly
50% of patients with acute retinal arterial
occlusion though only 10% of these patients
had pathology severe enough to warrant
anticoagulation or cardiac surgery
CRAO
Systemic Associations
– Study by Inatomi – TTE found cardiac lesions
in 27% of patients, TEE found lesions in 59%
– 18% of patients with carotid artery stenosis
60% or greater
Etiology Retinal Arterial
Occlusion
Systemic arterial hypertension (via
atherosclerotic plaque formation)
Cardiac valvular disease
Left ventricular hypertrophy
Thrombus after myocardial infarction
Cardiac myxoma
Tumors
Intravenous drug abuse
Lipid emboli (pancreatitis)
Purtscher's retinopathy (trauma)
Loiasis
Radiologic studies (carotid angiography,
lymphangiography, head and neck corticosteroid
injection, retrobulbar injection)
Deep vein thrombosis (via paradoxical embolus
through cardiac wall defect)
Traumatic causes
– Retrobulbar injection
– Orbital fracture repair
– Anesthesia
– Penetrating injury
– Nasal surgery
– Eyelid capillary hemangioma injection
Coagulation abnormalities
– Sickle cell disease
– Homocystinuria
– Oral contraceptives
– Platelet abnormalities
– Pregnancy
– Lupus anticoagulants
Coagulation abnormalities
– Protein S deficiency
– Protein C deficiency
– Antithrombin III deficiency
– Activated protein C resistance
– Factor V Leiden abnormalities
Ocular Conditions
– Prepapillary arterial loops
– Optic disc drusen
– Increased intraocular pressure (with sickling
hemoglobinopathy)
– Toxoplasmosis
– Optic neuritis
Collagen Vascular Disease
– Systemic lupus erythematosus
– Polyarteritis nodosa
– Giant cell arteritis
– Wegener's granulomatosis
Other vasculitides
– Orbital mucormycosis
– Radiation retinopathy
– Behçet's disease
Miscellaneous
– Ventriculography
– Fabry's disease
– Sydenham's chorea
– Migraine
– Hypotension
– Fibromuscular hyperplasia
– Nasal oxymethazolone use
– Lyme disease
Workup/Treatment
Cardiac history, h/o IV drug abuse
TTE if cardiac history/risk or young patient
Carotid Dopplers in patients over 30 years
If age > 55 years without emboli on exam obtain
ESR (GCA accounted for 1-2% in series at Wills
Eye)
Homocysteine in young patients/no other etiology
identified
Antigoagulation studies (Protein C and S, Anti
thrombin III)
Treatment
Studies in rhesus monkeys show
irreversible damage at 90-100 minutes
In clinical situations rarely a complete
obstruction therefore timescale may differ
Recommended to treat if seen within 24
hours
Treatment
Ocular Massage
– Study by Russell showed 16% increase in
retinal arterial diameter when IOP increased by
60mmHg
– Study by Ffytche showed 86% increase in flow
when increase in IOP followed by sudden
decrease
Treatment
95% oxygen/5% carbon dioxide mixture
– May produce normal pO2 at retinal surface via
diffusion from choroid
AC paracentesis
– Attempt to increase arterial perfusion pressure
– Study by Atebara in 40 patients showed no
significant benefit over 47 patients without this
therapy
Treatment
Antifibrinolytic agents
– No randomized study, not currently
recommended
– With supraorbital artery injection 100 times
dose is delivered compared with IV, study by
Watson noted 50% improvement
Laser therapy to emboli vs. PPV with
manipulation of emboli
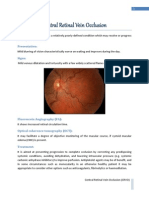
CRVO
Sudden painless loss of vision vs. gradual
vision loss with less severe occlusions
DBH/flame-shaped in all quadrants
Dilated/tortuous venules
Disc edema, macular edema, and CWS
present to varying degrees
CRVO
Retinal heme with gradually resolve, may
have subsequent RPE changes
Macular edema may persiste after
resolution of heme
Epiretinal membranes may form
Shunt vessels may form on disc
Neovascular proliferation
– Includes NVD, NVE, NVI/NVG
– NVA may occur without NVI in 6-12%
– NVI/NVA present in 16% of eyes with 10-29
disc areas of nonperfusion and 52% of eyes
with 75 or more disc areas of nonperfusion in
CVOS
NVI/NVA
– In CVOS occurred in 5% of eyes 20/40 or
better, 14.8% in eyes with 20/50 to 20/200, and
30.8% in eyes with worse than 20/200 acuity
CVOS criteria
– Perfused CVO (also termed nonischemic,
incomplete or partial) - less than 10 disc areas
of capillary nonperfusion
– Generally less hemorrhages
– Generally better visual acuity
CVOS criteria
– Nonperfused CRVO – 10 or more disc areas of
capillary nonperfusion
– Generally more hemorrhages, disc edema, and
macular edema
– Indeterminate when extent of hemorrhages
prevents full evaluation of extent of capillary
nonperfusion
CVOS
– 10% of perfused vs. 35% nonperfused
developed NVI/NVA
– 34% of initially perfused eyes converted to
nonperfused status after 3 years
– 38 eyes (83%) with an indeterminate CVO at
baseline were ultimately determined to be
nonperfused
ERG
– Reduction to 60% or less of normal mean b-
wave amplitude suggests ischemic CRVO
Pathogenesis
Histopathologic studies of eyes enucleated with
h/o CVO demonstrated a thrombus occluding the
lumen of the central retinal vein at or just
proximal to the lamina cribrosa
In retrolaminar portion of the optic nerve central
retinal artery and vein are aligned parallel to each
other with common tissue sheath
Central retinal artery and vein are naturally
compressed as they cross through the rigid sieve-
like openings of lamina cribosa
Central retinal artery and vein are naturally
compressed as they cross through the rigid
sieve-like openings of lamina cribosa
Pathogenesis
May be subject to compression from
mechanical stretching of the lamina from
increases in intraocular pressure, causing a
posterior shift or bowing of the lamina with
subsequent impingement on the central
retinal vein
Pathogenesis
Hemodynamic alterations may produce
stagnant flow and subsequent thrombus
formation in the central retinal vein,
including diminished blood flow, increased
blood viscosity, and an altered lumen wall
(Virchow's triad)
Pathogenesis
Experimentally, occlusion of both the
retrolaminar CRA and CRV, posterior to
the lamina cribrosa and before collateral
channels branch from the main trunk, was
required to produce the clinical appearance
of ischemic CVO. This implies that
concurrent retinal artery insufficiency or
occlusion may play a role in an ischemic
CVO
Systemic Associations
Systemic vascular diseases: Diabetes
mellitus, hypertension, carotid insufficiency
Ocular diseases: Open angle glaucoma,
ischemic optic neuropathy, pseudotumor
cerebri, tilted optic nerve heads, optic nerve
head drusen
Hematologic alterations: Hyperviscosity
syndromes: dysproteinemias (multiple myeloma),
blood dyscrasias (polycythemia very, lymphoma,
leukemia, sickle cell disease or trait). Anemia,
elevated plasma homocysteine, factor XII
deficiency, anti-phospholipid antibody syndrome,
activated protein C resistance, protein C
deficiency, protein S deficiency
Inflammatory/autoimmune vasculitis: Systemic
lupus erythematosus
Medications: Oral contraceptives,
diuretics, hepatitis B vaccine
Infectious vasculitis: HIV, syphilis, herpes
zoster, sarcoidosis
Other: After retrobulbar block,
dehydration, pregnancy
Workup
Generally no workup indicated if > 60 y/o and
known systemic vascular risk factors
Limited systemic work up for those with a prior
occlusion in the fellow eye, prior systemic
thrombotic disease, family history of thrombosis,
or other symptoms suggestive of a hematologic or
rheumatologic condition
Initial laboratory investigation may include ESR,
ANA, antiphospholipid antibody, and fasting
plasma homocysteine levels
Treatment
Identify systemic vascular risk factors
Pentoxyfilline improved retinal vascular
flow velocity, found in small retrospective
series to improve ME, however no VA
improvement
Treatment
CVOS
– Grid laser for ME showed no improvement in
VA, 31% showed resolution of ME
– PRP recommended for patients with NVI/NVA,
no benefit from prophylactic therapy(NVI in
20% early treatment(n=90) vs. 34% no early
treatment(n=91))
– Prophylactic PRP may be considered for high
risk cases with poor anticipated follow up
Treatment
Chorioretinal venous anastomosis(CRA) have
been created surgically through a trans-retinal
venipuncture technique or laser energy delivered
through argon or Nd-YAG systems directed at a
branch retinal vein to rupture the posterior vein
wall and Bruch's membrane
Successful CRA creation is reported in 10-54% of
perfused CVO with subsequent conversion to
ischemic CVO in 0-8% of eyes
Treatment
Radial Optic Neurotomy
– Opremcak and colleagues have proposed combining pars
plana vitrectomy with transvitreal incision of the nasal
scleral ring to release pressure on the central retinal vein at
the level of the scleral outlet
– Retrospective report of 11 eyes by Opremcak and colleagues,
successful RON was performed with no complications.
There was clinical improvement in retinal hemorrhages and
venous congestion. With a mean follow up of 9 months, 73%
of patients had improved Snellen acuity; Two eyes with
preoperative INV had worsening of acuity associated with
the development of neovascular glaucoma
Treatment
RON
– Recent study total of 117 male and female
patients, age >40 years (range 44-93 years)
with CRVO and severe loss of vision (visual
acuity of ≤20/200), underwent pars plana
vitrectomy with radial optic neurotomy
Treatment
RON
– Anatomic improvement of CRVO was found in 95% (106
of 111) of patients. Disc edema improved in by 1 week
– Visual acuity improved in 71% (79 of 111) of patients, with
an average of 2.5 lines of vision gained (range 1-12 lines).
Two or more lines of improvement were found in 53% (59
of 111) of patients, three or more in 41% (45 of 111), four
or more in 25% (27 of 111), and six or more in 14% (14 of
111)
– Twenty-three percent (25 of 111) of patients had unchanged
visual acuity, and 6% (7 of 111) had worse visual acuity
Treatment
Intravitreal triamcinolone
– Study by Ramezani
– CMT before, 2 and 4 months after injection was 565
(50), 259 (15), and 290 (53) microns in the treatment
group and 629 (43), 470 (62) and 413 (59) microns in
the sham group, respectively. The 2- month difference
was significant (P=0.003). The difference between VA
changes (0.39 logMAR) was significant only at 1
month (P=0.013)
Treatment
Intravitreal triamcinolone
– Study by Goff et al, retrospective
– Twenty-nine eyes were treated with intravitreal
injection. The median initial Early Treatment Diabetic
Retinopathy Study visual acuity was 20/250 (median
logMAR, 1.1). The median visual acuity 3 months after
injection was 20/125 (median logMAR, 0.8). This
difference was statistically significant. The median
final visual acuity was 20/250 (median logMAR, 1.1).
This difference in visual acuity was not statistically
significant
Treatment
Intravitreal Avastin
– Study Iturralde et al, retrospective, no adverse events
– There were 16 eyes of 15 consecutive patients
Intravitreal triamcinolone had been previously
administered to 9 patients. The patients received a
mean of 2.8 injections of bevacizumab per eye. The
mean central macular thickness at baseline was 887
microm and decreased to a mean of 372 microm at
month 1 (P < 0.001). The mean baseline acuity was
20/600 (logMAR = 1.48) and the mean acuity at month
1 was 20/200 (logMAR = 1.05)
Treatment
Intravitreal Avastin
– At last follow-up, a mean of 3 months after the
first injection, the mean visual acuity was
20/138 (logMAR = 0.84), which was
significantly better than baseline (P < 0.001).
Visual acuity improvement, defined as a
halving of the visual angle, was seen in 14 of
the 16 eyes
You might also like
- Retinal Vascular Occlusions: Classification, Presentation and ManagementDocument62 pagesRetinal Vascular Occlusions: Classification, Presentation and ManagementUlquiorra SchifferNo ratings yet
- Understanding Branch Retinal Vein OcclusionDocument31 pagesUnderstanding Branch Retinal Vein OcclusionChinmay KharadeNo ratings yet
- RvoDocument43 pagesRvoOrchlon LkNo ratings yet
- Neurology Equations Made Simple: Differential Diagnosis and NeuroemergenciesFrom EverandNeurology Equations Made Simple: Differential Diagnosis and NeuroemergenciesNo ratings yet
- Retinal Artery Occlusion Case: 62yo F Sudden Vision Loss Right EyeDocument24 pagesRetinal Artery Occlusion Case: 62yo F Sudden Vision Loss Right EyeAravind MahendranNo ratings yet
- Central Retinal Artery Occlusion GuideDocument16 pagesCentral Retinal Artery Occlusion GuidermurtiaNo ratings yet
- Case 29.07.09Document51 pagesCase 29.07.09kanavgNo ratings yet
- 4 RetinaDocument59 pages4 RetinaLala Komala Sari HakimNo ratings yet
- Retinal Vascular DisordersDocument52 pagesRetinal Vascular Disorderssushma shresthaNo ratings yet
- 4 RetinaDocument59 pages4 RetinajanechaterineNo ratings yet
- Hemi Central Retinal Vein OcclusionDocument30 pagesHemi Central Retinal Vein Occlusiondrvamsi_kNo ratings yet
- Central Serous Chorioretinopathy: Causes, Imaging, TreatmentDocument7 pagesCentral Serous Chorioretinopathy: Causes, Imaging, TreatmentshintasissyNo ratings yet
- Veno Occlusive Disease of RetinaDocument81 pagesVeno Occlusive Disease of RetinaPreetiNo ratings yet
- Management of CRAODocument3 pagesManagement of CRAOvennieNo ratings yet
- Non-Ischemic Central Retinal Vein OcclusionDocument6 pagesNon-Ischemic Central Retinal Vein OcclusionMahmoud Ahmed MahmoudNo ratings yet
- Crvo CraoDocument80 pagesCrvo CraoRakshit AgrawalNo ratings yet
- Central Retinal Artery OcclusionDocument9 pagesCentral Retinal Artery OcclusionAzhar Dzulhadj B. ArafahNo ratings yet
- Intracranial Aneurysms and SAH: Philippe Younes MD Neurosurgeon Head of Neuroscience Department BMCDocument37 pagesIntracranial Aneurysms and SAH: Philippe Younes MD Neurosurgeon Head of Neuroscience Department BMCHussein TarhiniNo ratings yet
- Centralretinalarteryocclusion 150821150708 Lva1 App6891 PDFDocument50 pagesCentralretinalarteryocclusion 150821150708 Lva1 App6891 PDFDhivya SekarNo ratings yet
- Vitreous HemorrhageDocument7 pagesVitreous HemorrhageindahNo ratings yet
- Essential Ophthalmology NotesDocument31 pagesEssential Ophthalmology NotesHashim Ahmad50% (2)
- Central Serous Retinopathy: Michael Colucciello, MDDocument9 pagesCentral Serous Retinopathy: Michael Colucciello, MDRani Henty NovitaNo ratings yet
- Hypertensive Retinopathy DR KatariaDocument48 pagesHypertensive Retinopathy DR KatariaDr Sandeep KatariaNo ratings yet
- Peripheral Arterial OcclusionDocument50 pagesPeripheral Arterial Occlusionshefalika mandremNo ratings yet
- Ocular Ischemic Syndrome Survey OphthalmologyDocument33 pagesOcular Ischemic Syndrome Survey OphthalmologyamaiacNo ratings yet
- Visual Loss April 8, 2010: Disease Pathophysiology Etiology Epidemiology Clinical Presentation TreatmentDocument3 pagesVisual Loss April 8, 2010: Disease Pathophysiology Etiology Epidemiology Clinical Presentation Treatmentkep1313No ratings yet
- Ax & DX SAHDocument5 pagesAx & DX SAHMohammadAwitNo ratings yet
- Retinal disorders overviewDocument82 pagesRetinal disorders overviewfebienaNo ratings yet
- Vitreous Hemorrhage: Major ReviewDocument4 pagesVitreous Hemorrhage: Major Reviewmutya yulindaNo ratings yet
- Dr. Bhosale SystemicDocument87 pagesDr. Bhosale SystemicdrksuhasNo ratings yet
- Hyphema: Dr. Dedeh KurniasihDocument24 pagesHyphema: Dr. Dedeh KurniasihFaishal Bawor Banyumas CockfightNo ratings yet
- Causes and Presentation of Peripheral Anterior SynechiaeDocument11 pagesCauses and Presentation of Peripheral Anterior SynechiaeMarisa SukoNo ratings yet
- Hypertensive Retinopathy Presenting as Angle Closure Glaucoma CaseDocument5 pagesHypertensive Retinopathy Presenting as Angle Closure Glaucoma CaseyunjaealwaysNo ratings yet
- Penjelasan Dan Jawaban CRVODocument4 pagesPenjelasan Dan Jawaban CRVONuruel SazaNo ratings yet
- Retinal Vascular Disorders: Causes and ManagementDocument46 pagesRetinal Vascular Disorders: Causes and ManagementSubash BasnetNo ratings yet
- Treating Traumatic Hyphema: Understanding Causes, Grading, and Managing Elevated IOPDocument13 pagesTreating Traumatic Hyphema: Understanding Causes, Grading, and Managing Elevated IOPAfiazka LuthfitaNo ratings yet
- Anesthesia For Cerebral - AneurysmsDocument14 pagesAnesthesia For Cerebral - AneurysmsDaniel BellemareNo ratings yet
- Frosted Branch AngiitisDocument9 pagesFrosted Branch AngiitisDragos-Constantin LuncaNo ratings yet
- Diabetic Retinopathy Screening and Management GuideDocument6 pagesDiabetic Retinopathy Screening and Management Guidegdudex118811No ratings yet
- Hypertensive RetinopathyDocument27 pagesHypertensive RetinopathyChikita Artia SariNo ratings yet
- Hypertensive Retinopathy - Yanoff and DukerDocument13 pagesHypertensive Retinopathy - Yanoff and DukerriveliNo ratings yet
- Pa Pill EdemaDocument13 pagesPa Pill Edemarutuparna383No ratings yet
- Ischemic optic neuropathy causes and treatmentDocument7 pagesIschemic optic neuropathy causes and treatmentJhoni Akbar DalimuntheNo ratings yet
- Subarachnoid Hemorrhage: Hania El Jarkass, MD Neurology MGHDocument30 pagesSubarachnoid Hemorrhage: Hania El Jarkass, MD Neurology MGHamal najaNo ratings yet
- Secondary HypertensionDocument9 pagesSecondary HypertensionGautam JoshNo ratings yet
- Sudden Loss of Vision & Optic NeuropathyDocument30 pagesSudden Loss of Vision & Optic NeuropathyIshak IzharNo ratings yet
- CT Angiogram for Blue Toe SyndromeDocument5 pagesCT Angiogram for Blue Toe Syndromejhk0428No ratings yet
- Marfan Syndrome: Presenter: DR - Shasidhar ReddyDocument18 pagesMarfan Syndrome: Presenter: DR - Shasidhar ReddyshravaniNo ratings yet
- Posterior SegmentDocument102 pagesPosterior SegmentAlice ChirilaNo ratings yet
- NCP Rheum 0268Document9 pagesNCP Rheum 0268drheriNo ratings yet
- Hifema: Classification and CharacteristicsDocument17 pagesHifema: Classification and CharacteristicsMarisa SukoNo ratings yet
- Fact Sheet Central Retinal Vein OcclusionDocument2 pagesFact Sheet Central Retinal Vein OcclusionAini Nur Syafa'ahNo ratings yet
- Retina/Vitreous: Question 1 of 130Document51 pagesRetina/Vitreous: Question 1 of 130safasayedNo ratings yet
- Cryptogenic StrokeDocument52 pagesCryptogenic Strokemrabhilekh100% (1)
- By /khaled Wael Elsayed ID/397Document10 pagesBy /khaled Wael Elsayed ID/397Salmonella TyphiNo ratings yet
- Maternal Lec Semi Final.Document26 pagesMaternal Lec Semi Final.Kate Onniel RimandoNo ratings yet
- Nasal Oxygen Catheter TechniqueDocument8 pagesNasal Oxygen Catheter TechniqueMegha V Kurian100% (1)
- Manual de Fixação Interna Rigida Craniofacial PDFDocument14 pagesManual de Fixação Interna Rigida Craniofacial PDFFernandaJolyMacedoNo ratings yet
- Acfrogbpct50utxi Ygf7nagcrvxsznh4jb9pxe5obiap5r9akhldg Tsatlrg6rqvztj 6to3p1cshsnpir568ly7efdd4 Eqryh8b7xshanq4pkq2vkbx-Svf0muql5xenzr4dcjfqxrowi2omDocument58 pagesAcfrogbpct50utxi Ygf7nagcrvxsznh4jb9pxe5obiap5r9akhldg Tsatlrg6rqvztj 6to3p1cshsnpir568ly7efdd4 Eqryh8b7xshanq4pkq2vkbx-Svf0muql5xenzr4dcjfqxrowi2omyebadem228No ratings yet
- Management of Advanced Peritonitis PP TDocument106 pagesManagement of Advanced Peritonitis PP Tvedant kanadeNo ratings yet
- Carpal Tunnel Syndrome Power Point PresentationDocument6 pagesCarpal Tunnel Syndrome Power Point Presentationapi-237061134No ratings yet
- MRI BasicsDocument7 pagesMRI Basicsmustafa mohammedNo ratings yet
- Scapular Region: Figure 17.1 Scapular Muscles (Posterior View)Document12 pagesScapular Region: Figure 17.1 Scapular Muscles (Posterior View)Anjali TanwarNo ratings yet
- Acute urinary retention: causes, symptoms, and emergency treatmentDocument4 pagesAcute urinary retention: causes, symptoms, and emergency treatmentRobert DanielNo ratings yet
- Max. MAJOR CONNECTORSDocument51 pagesMax. MAJOR CONNECTORSأمال داودNo ratings yet
- To Evaluate The Efficacy of Ultrasonography Guided Pectoral Nerve Block For Postoperative Analgesia in Breast SurgeriesDocument4 pagesTo Evaluate The Efficacy of Ultrasonography Guided Pectoral Nerve Block For Postoperative Analgesia in Breast SurgeriesInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Day Case Surgery SajeebDocument34 pagesDay Case Surgery SajeebMahbubur Rahman0% (1)
- Left Ventricle Size, Mass, Volume and Function Reference RangesDocument33 pagesLeft Ventricle Size, Mass, Volume and Function Reference RangesEka Rahayu UtamiNo ratings yet
- Coronary CT Angiography ExamDocument4 pagesCoronary CT Angiography ExamCarlos F Muñoz NúñezNo ratings yet
- 1601-004.16 Declaration of ConformityDocument3 pages1601-004.16 Declaration of ConformityPhạm Đỗ Thanh Tùng0% (1)
- Management of Post Burn ContracturesDocument65 pagesManagement of Post Burn ContracturesJetto TubeNo ratings yet
- A Clinical Study On The Inguinal Hernia and Its Management in The General Surgical Practice at Tertiary Care HospitalDocument4 pagesA Clinical Study On The Inguinal Hernia and Its Management in The General Surgical Practice at Tertiary Care Hospitalmelon segerNo ratings yet
- Procedural Report Breast Augmentation Drug Study Propofol AnecDocument15 pagesProcedural Report Breast Augmentation Drug Study Propofol AnecSamer SumalinogNo ratings yet
- Um en DG5000 0-48-0060 Ne DG5000 Ang 05.12.05 PDFDocument90 pagesUm en DG5000 0-48-0060 Ne DG5000 Ang 05.12.05 PDFpaninaro2011No ratings yet
- Lemone/Burke/Bauldoff, Medical-Surgical Nursing 6Th Edition Test BankDocument61 pagesLemone/Burke/Bauldoff, Medical-Surgical Nursing 6Th Edition Test Banknurse homeNo ratings yet
- The Right Choice: Smith&nephewDocument4 pagesThe Right Choice: Smith&nephewapi-19808945No ratings yet
- My First Tooth in An Hour CaseDocument35 pagesMy First Tooth in An Hour CaseRacovitanCNo ratings yet
- An Interesting Case of Gluteal Swelling - Intramuscular Myxoma (IMM) by DR Zothansanga ZadengDocument3 pagesAn Interesting Case of Gluteal Swelling - Intramuscular Myxoma (IMM) by DR Zothansanga ZadengCJ MazualaNo ratings yet
- Jadwal Operasi Sabtu 17 Juli 2021Document2 pagesJadwal Operasi Sabtu 17 Juli 2021Sri lovianaNo ratings yet
- SituationDocument32 pagesSituationGo IdeasNo ratings yet
- ER تجميعة 2Document8 pagesER تجميعة 2lclkNo ratings yet
- Suprahyoid Muscles and Prevertebral MusclesDocument13 pagesSuprahyoid Muscles and Prevertebral MusclesHemalatha D kNo ratings yet
- Runway Magazine ClassicDocument64 pagesRunway Magazine ClassicRunway Magazine50% (2)
- Amputation-Dr N K BeheraDocument8 pagesAmputation-Dr N K BeheraSheel GuptaNo ratings yet
- Fisher & Paykel MR 850Document20 pagesFisher & Paykel MR 850NithinBesentNNo ratings yet