Thermodynamics 2 (TRDMIA2)
Lecture 13
2024
�Thermodynamic air cycles
Chapter 6 (2) – Stirling and Ericsson cycles
� Stirling cycle
Stirling cycle is the ideal cycle for external combustion engines that incorporate a regenerator. The
regenerator is used to briefly store and release thermal energy.
Fig.1 p-V and T-S diagrams of Stirling
engine
3
�Stirling cycle
The processes are as follows:
1-2: heat is supplied from an external source as the gas expands
isothermally (i.e. isothermal expansion so, T1 =T2)
Q1-2 = W1-2 = p1V1ln(V2/V1) = mRT1 ln(V2/V1)
where, V2/V1= r (expansion ratio)
2-3: constant volume cooling ( from T2 to T3=T4),
the heat rejected, Q2-3=mcvΔT is briefly stored externally (in the
regenerator) and used to heat the gas during process 4-1;
4
�Stirling cycle
The processes are as follows:
3-4: isothermal compression, heat rejected to the atmosphere is
Q3-4 = W3-4 = -p3V3ln(V4/V3) =mRT3 ln(V3/V4),
where V3/V4=r (compression ratio)
4-1: constant volume heating, using the heat Q2-3 stored briefly in the
regenerator) such that,
Q4-1 = Q2-3
Then, Wnet = Qsupplied – Qrejected = mRT1 ln(r) – mRT3 ln(r) = mR ln(r)[T1 – T3]
Therefore,
ηSterling = mRln(r)[T1 – T3]/ mRT1 ln(r) = (T1 – T3)/T1 = 1- T3/T1
5
� Stirling cycle
𝑻3
𝜼𝑺𝒕𝒊𝒓𝒍𝒊𝒏𝒈 =1 −
𝑻1
Notes:
The assumption is that the heat rejected in 2-3 is stored in a
regenerator and used to heat the process 4-1, ideally and reversibly.
The regenerative process takes place at constant volume and is
internal to the cycle
The efficiency of Stirling cycle is the same as that of a Carnot cycle:
1 – (T3/T1)
6
�Stirling cycle
Notes:
• The Stirling engine can use any form of heat (from
conventional or indigenous fuel to solar and
nuclear sources) providing the temperature
created is high enough Fig.2 Model of a Stirling engine [1]
• Possible range of applications: marine use,
electricity generation for peak loads and as a
stand-by unit; in situations where a
nonconventional fuel is used.
• Most important application is the reversed Stirling
used as a refrigerator capable of reaching
cryogenic regions. Fig. 3 Soda can Stirling engine [2]
1. https://www.pasco.com/products/lab-apparatus/thermodynamics/heat-engine/se-8636
7
2. https://en.wikiversity.org/wiki/File:SODA_CAN_STIRLING_ENGINE.jpg
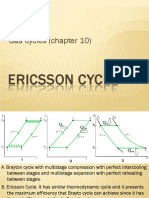
�Ericsson cycle
The Ericsson cycle is similar to Sterling cycle except that the two isothermals are connected by
constant pressure processes.
Fig.4 p-V and T-S diagrams of Ericsson engine
8
�Ericsson cycle
The processes are as follows:
1-2: isothermal expansion (T1 =T2), heat supplied from an
external is
Q1-2 = W1-2 = p1V1ln(V2/V1) = mRT1 ln(V2/V1)
2-3: constant pressure cooling ( from T2 to T3=T4),
the heat rejected, Q2-3=mcpΔT is briefly stored externally
(in the regenerator) and used to heat the gas during
process 4-1;
9
�Ericsson cycle
The processes are as follows:
3-4: isothermal compression, heat rejected to the
atmosphere is
Q3-4 = W3-4 = p3V3ln(V4/V3) =mRT3 ln(V4/V3)
4-1: constant pressure heating, using the heat Q2-3 (stored
briefly in the regenerator) such that,
Q4-1 = Q2-3= mcpΔT
10
�Ericsson cycle
Notes:
• The actual implementation of both Stirling and
Ericsson cycles is complex and impractical.
However, there is rising interest in these cycles
because of high thermal efficiency and potential
for better emission control
• The Ericsson cycle has the same efficiency as
Carnot cycle
Fig.5 Diagram of an Ericsson engine and cycle
11
�Comparison of Carnot, Stirling and Ericsson cycles
12