Professional Documents
Culture Documents
Chapter (1) Temperature and Thermometry
Uploaded by
Bǿ DYOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter (1) Temperature and Thermometry
Uploaded by
Bǿ DYCopyright:
Available Formats
Temperature and first law of thermodynamics
Chapter (1)
Temperature and Thermometry
Two Basic Concept must be defined before we start this section, namely,
Thermal contact and thermal equilibrium. Two objects are in thermal contact with
each other if energy can be exchanged between them by these processes due to a
temperature difference while thermal equilibrium is a situation in which two objects
would not exchange energy by heat or electromagnetic radiation if they were placed
in thermal contact.
1.1. Zeroth law of thermodynamics
If objects A and B are separately in thermal equilibrium with a third object C,
then A and B are in thermal equilibrium with each other.
1.2. Temperature and Temperature Scales
The temperature of a body is its degree of hotness (or coldness).
There are many types of thermometers, but each makes use of a particular
thermometric physical property (i.e. a property whose value changes with
temperature T). For example: a mercury in glass thermometer makes use of the
change in length (l) of a column of mercury confined in the capillary
tube of uniform bore (l o T), a platinum resistance thermometer makes use of the
increase in electrical resistance with increasing temperature (R o T),
In order to establish a temperature scale it is necessary to make use of fixed
points: a fixed point is a single temperature at which certain physical property
always occurs. Three such point are defined below.
The ice point
(Upper fixed point)
is the temperature at which pure ice can exist in equilibrium
with pure water at standard atmospheric pressure.
The Steam point
(Lower fixed point)
is the temperature at which pure water can exist in
equilibrium with pure water vapor at standard atmospheric
pressure.
4
Temperature and first law of thermodynamics
Fundamental
intervals
is the interval between the lower and upper fixed points on
the temperature scale
Triple point is unique temperature at which ice, pure water and pure
water vapor can exist together in equilibrium.
The SI unit of temperature is the Kelvin (K). An interval of one Kelvin is
defined 1/273.15 of the temperature of the triple point of water as measured on
thermodynamic scale of temperature.
Another unit, the degree Celsius (C), is often used and defined by
15 . 273 =
K C
T T (1-1)
Where T
C
=temperature in C, and T
K
=temperature in K.
A mercury in glass thermometer could be calibrated by marking the position of
mercury both at ice and steam points and then dividing the interval (fundamental
interval) between them into 100 equal divisions.
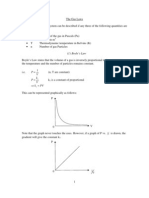
Figure (1.1) Temperature scales
The Celsius temperature corresponding to a length l
u
of the mercury column
would be given by
100
0 100
0
=
T
C
T
...(1-2)
Where l
0
and l
100
are the length of mercury column at 0 C and at 100 C
4
Temperature and first law of thermodynamics
The relationship between the three temperature scales discussed graphically in
fig. (1-1). The Kelvin scale is called the absolute temperature scale, and its zero
point is called the absolute zero.
So, we have
32
5
9
) 32 (
9
5
+ = =
C F F C
T T or T T
and
3 27 273 + = =
C K K C
T T or T T
We can use all this equation to conclude that
AT
C
=AT
K
=
9
5
AT
F
Example 1-1
On a day when the temperature reaches 50 C, what is the temperature in degrees
Celsius and in Kelvin?
Solution
Using equation 1-2 to convert to Celsius scale.
C T
T
T T
o
C
C
F C
10 ) 32 50 (
9
5
32 212
32 50
100
32 212
32
0 100
0
= =
To convert to Kelvin scale
K T
T
T T
K
K
F K
283 273 ) 32 50 (
9
5
180
32 50
100
273
32 212
32
273 373
273
= + =
4
Temperature and first law of thermodynamics
4
Example 1-2
A Pan of water is heated from 25 C to 80 C. What is the change in its temperature
on the Kelvin and on a Fahrenheit scale?
Solution
AT
C
=80 25 =55 C
K T
K T
o
k
o
k
99 ) 25 80 (
100
180
55 ) 25 80 (
100
100
= = A
= = A
AT
K
=AT
C
Example 1-3
A particular resistance thermometer has a resistance 30 O at the ice point,
41.58 O at the steam point and 34.59 O when immersed in a boiling liquid. A
constant volume gas thermometer gives readings of 1.333 10
5
Pa, 1.821 10
5
Pa
and 1.528 10
5
Pa at the same three temperatures. Calculate the temperature at
which the liquid is boiling. a) on the scale of the gas thermometer, b) on the scale of
resistance thermometer.
Solution
According to the gas thermometer
C 96 . 39 100
10 333 . 1 10 821 . 1
10 333 . 1 10 528 . 1
100
P P
P P
o
5 5
5 5
0 100
0
=
=
= u
u
According to the resistance scale
C 64 . 39 100
30 58 . 41
30 59 . 34
100
R R
R R
o
0 100
0
=
= u
u
Exercises ;
- What is 0 K on Celsius and Fahrenheit scale.
- What is the room temperature of 72 F in Celsius scale.
- Normal temperature of human body is 98.6 F. what is it on Celsius scale.
- What is the temperature change of 20 C in both F and K scale.
You might also like
- Heat and TemperatureDocument26 pagesHeat and TemperatureWanMardziyyahNo ratings yet
- Thermal PhysicsDocument24 pagesThermal PhysicsSuraj GopaulNo ratings yet
- Topic 1Document26 pagesTopic 1SJK(C) PHUI YINGNo ratings yet
- Thermometry Physics A LevelDocument16 pagesThermometry Physics A LevelNayana GaleaNo ratings yet
- Heat and ThermometryDocument8 pagesHeat and ThermometryElizabeth AnyegaNo ratings yet
- Understanding Thermal PrincipleDocument7 pagesUnderstanding Thermal PrincipleAngie Kong Su MeiNo ratings yet
- HEAT AND PROPERTIES OF MATTER Phy 103Document53 pagesHEAT AND PROPERTIES OF MATTER Phy 103junaidukabirnabadeNo ratings yet
- 05 Ley Cero Escalas TemperaturaDocument32 pages05 Ley Cero Escalas TemperaturaMichelle SerranoNo ratings yet
- SFG 3023 Chapter 1Document67 pagesSFG 3023 Chapter 1Nik AshrafNo ratings yet
- Physics I-21-22Document50 pagesPhysics I-21-22Ahmed BagradNo ratings yet
- Thermal Physics PDFDocument87 pagesThermal Physics PDFPriyanshu SharmaNo ratings yet
- TD Module 2Document47 pagesTD Module 2mujeebNo ratings yet
- Chap03 TemperatureNHeatDocument24 pagesChap03 TemperatureNHeatsamtomNo ratings yet
- Lesson 4.1 (Smtai 09) .Document5 pagesLesson 4.1 (Smtai 09) .Ilman MohamadNo ratings yet
- Thermal Properties of MatterDocument9 pagesThermal Properties of MatterTrillionare HackNo ratings yet
- Fundamentals of ThermometryDocument18 pagesFundamentals of Thermometryikaro181083No ratings yet
- Lec. 2Document32 pagesLec. 2Ali. AboudNo ratings yet
- Phys2 Ch2 Heat Temp Law0Document56 pagesPhys2 Ch2 Heat Temp Law0Trung Trần100% (1)
- Section III 12 TemperatureDocument27 pagesSection III 12 Temperaturedanwilliams85No ratings yet
- Phys 2 Lecture 01 Thermodynamics-1Document19 pagesPhys 2 Lecture 01 Thermodynamics-1Maruja TheaNo ratings yet
- Chapter-6 Temperature - HeatDocument8 pagesChapter-6 Temperature - Heat2220678No ratings yet
- Chapter-6 Temperature & Heat PDFDocument5 pagesChapter-6 Temperature & Heat PDFKoushik DewriNo ratings yet
- Lecture NotesDocument80 pagesLecture Notes8ienneNo ratings yet
- IIT 23 Phy CH 13 Thermal Physics 1 1634654541102Document71 pagesIIT 23 Phy CH 13 Thermal Physics 1 1634654541102Swaroop NaikNo ratings yet
- Heat Model1Document115 pagesHeat Model1deoNo ratings yet
- Lecture 01 Temperature MeasurementDocument13 pagesLecture 01 Temperature MeasurementcaptainamericaNo ratings yet
- Keph 203Document24 pagesKeph 203Ayush JaiswalNo ratings yet
- Science 8: Most Essential Learning CompetenciesDocument24 pagesScience 8: Most Essential Learning CompetenciesVeronica KimNo ratings yet
- P103 Chapter10 TAT wk3wk4Document62 pagesP103 Chapter10 TAT wk3wk4Muhammad SaeedNo ratings yet
- 06082021024459am - S.1 Physics Heat IiDocument41 pages06082021024459am - S.1 Physics Heat Iimarionmbambu9No ratings yet
- Physics IntroductionDocument25 pagesPhysics IntroductionhannaNo ratings yet
- NCERT PH 2 Thermal Properties of MatterDocument24 pagesNCERT PH 2 Thermal Properties of MatterkdsiddhantNo ratings yet
- CHEMISTRYDocument12 pagesCHEMISTRYmurtada18851No ratings yet
- Chapter 3 Temperature and HeatDocument7 pagesChapter 3 Temperature and Heatmechmuthu1No ratings yet
- Chapter Ten Lecture Ten Thermodynamics: TemperatureDocument16 pagesChapter Ten Lecture Ten Thermodynamics: TemperatureTony AtefNo ratings yet
- Form Four Notes PDFDocument23 pagesForm Four Notes PDFSanti NgoranNo ratings yet
- Phys2 Ch2 Heat Temp Law0Document56 pagesPhys2 Ch2 Heat Temp Law0nanduNo ratings yet
- Chapter-6 Temperature Heat PDFDocument8 pagesChapter-6 Temperature Heat PDFMohammad Fuad HasanNo ratings yet
- g484 Module 3 4 3 2 TemperatureDocument6 pagesg484 Module 3 4 3 2 Temperatureapi-236179294No ratings yet
- Phy CH 8 Final 9thDocument28 pagesPhy CH 8 Final 9thmastersahb302No ratings yet
- Hermal Roperties OF Atter: Hapter ENDocument24 pagesHermal Roperties OF Atter: Hapter ENChethan KumarNo ratings yet
- Lecture 3Document43 pagesLecture 3Farhan Mukhtiar YousafzaiNo ratings yet
- Temperature: Can Be Thought of AsDocument5 pagesTemperature: Can Be Thought of AsTarek Mohamed Ahmed AhmedNo ratings yet
- CalorDocument14 pagesCaloreka123No ratings yet
- PHY 103: Basic Principle of Physics II: Heat and ThermodynamicsDocument32 pagesPHY 103: Basic Principle of Physics II: Heat and ThermodynamicsLawal HakeemNo ratings yet
- 01-Thermal Expansion (Theory)Document24 pages01-Thermal Expansion (Theory)music_is_mypassion60% (5)
- Chapter - 11 HeatDocument22 pagesChapter - 11 HeatMuhammad Arif RattarNo ratings yet
- Kuliah TemperaturDocument19 pagesKuliah TemperaturDedy Setiawan ستياوانNo ratings yet
- Bansal CLasses Physics Study Material For IIT JEuEDocument630 pagesBansal CLasses Physics Study Material For IIT JEuESuman KunduNo ratings yet
- Lab Session 9, Experiment 8: Calorimetry, Heat of ReactionDocument7 pagesLab Session 9, Experiment 8: Calorimetry, Heat of ReactionFatin IziantiNo ratings yet
- Heat Capacity: Textbook of Heat. Copies of The Relevant Sections Are Available atDocument14 pagesHeat Capacity: Textbook of Heat. Copies of The Relevant Sections Are Available atSunday Glo M. CabuyaoNo ratings yet
- TemperatureDocument6 pagesTemperatureNur Khairiah Daimah SanupinNo ratings yet
- PHYSICS XI CH-11 (Thermal Properties of Matter)Document28 pagesPHYSICS XI CH-11 (Thermal Properties of Matter)Nandita JainNo ratings yet
- 3.0 Laws of ThermodynamicsDocument41 pages3.0 Laws of ThermodynamicsBrandon LubegaNo ratings yet
- Thermal ExpansionDocument27 pagesThermal ExpansionVijay AgarwalNo ratings yet
- Experiment 5 Newton's Law of CoolingDocument7 pagesExperiment 5 Newton's Law of Coolingatif irshadNo ratings yet
- Heat Lecture NotesDocument62 pagesHeat Lecture NotesAS HUMBLE PIANONo ratings yet
- Hearter Selection GuideDocument30 pagesHearter Selection GuidesoayNo ratings yet
- Wdghwahdg PDFDocument2 pagesWdghwahdg PDFKim Howard CastilloNo ratings yet
- Conversion of Units) Temp, Mass, Volume, Length, Density) G7Document65 pagesConversion of Units) Temp, Mass, Volume, Length, Density) G7Melanie Niña Cullar100% (1)
- RK330-01 Atmospheric Temperature, Humidity & Pressure SensorDocument2 pagesRK330-01 Atmospheric Temperature, Humidity & Pressure SensorRach ToyNo ratings yet
- Temperature Performance Study-SilviaDocument11 pagesTemperature Performance Study-SilviadalheimerNo ratings yet
- Introduction To Temperature MeasurementDocument20 pagesIntroduction To Temperature MeasurementjimsistiNo ratings yet
- Gr4 Wk35 Measuring TemperatureDocument2 pagesGr4 Wk35 Measuring TemperaturerosinaNo ratings yet
- Temperature and Heart Attack Detection Using IOT (Arduino and ThingSpeak)Document8 pagesTemperature and Heart Attack Detection Using IOT (Arduino and ThingSpeak)WARSE Journals100% (1)
- Anexo 1 Bateria 100ah SCIFP48100ADocument2 pagesAnexo 1 Bateria 100ah SCIFP48100Asergiob63No ratings yet
- Manual Midtronics Cte 1000 PDFDocument44 pagesManual Midtronics Cte 1000 PDFFile WalkerNo ratings yet
- Phy. 12. End of Term 1. 09Document18 pagesPhy. 12. End of Term 1. 09Tabo TaizyaNo ratings yet
- Full Download Test Bank For Principles of Physics A Calculus Based Text 5th Edition PDF Full ChapterDocument36 pagesFull Download Test Bank For Principles of Physics A Calculus Based Text 5th Edition PDF Full Chapterproponesteerage.h4oal1100% (19)
- Ascot AUKWS11 Weather StationDocument6 pagesAscot AUKWS11 Weather StationZiggy BussyNo ratings yet
- Rak Therm - CatalogueDocument52 pagesRak Therm - CatalogueHussainhabeebi100% (1)
- 0580 s08 QP 1Document25 pages0580 s08 QP 1Hubbak Khan100% (1)
- DTW Tutorials 135 Physics Formula SheetDocument16 pagesDTW Tutorials 135 Physics Formula Sheetggold0934No ratings yet
- Ut 303 PDFDocument2 pagesUt 303 PDFCalibration Abu DhabiNo ratings yet
- E633-13 Standard Guide For Use of Thermocouples in Creep and Stress-Rupture Testing To 1800 - F (1000 - C) in AirDocument8 pagesE633-13 Standard Guide For Use of Thermocouples in Creep and Stress-Rupture Testing To 1800 - F (1000 - C) in AirislamakthamNo ratings yet
- Manual Infusion Pump BYS-820 PDFDocument16 pagesManual Infusion Pump BYS-820 PDFJajang67% (3)
- Pro 200 Series Manual 0717Document32 pagesPro 200 Series Manual 0717Victor MondragonNo ratings yet
- Heat Thermal Contact Thermal EquilibriumDocument7 pagesHeat Thermal Contact Thermal EquilibriumfariaienNo ratings yet
- Physics of Radiology and ImagingDocument426 pagesPhysics of Radiology and ImagingFlorentina Andronescu100% (1)
- Chap 1 ThermodynamicsDocument41 pagesChap 1 ThermodynamicsAsmawi Mohd KhailaniNo ratings yet
- Chapter 2 Instrument ParametersDocument10 pagesChapter 2 Instrument Parameterswan nur mursyidahNo ratings yet
- SCADADocument14 pagesSCADANunna BaskarNo ratings yet
- Holt Algebra 1 - Chapter 01 Test PDFDocument8 pagesHolt Algebra 1 - Chapter 01 Test PDFStanleyNo ratings yet
- Audels Engineers and Mechanics Guide Volume 5 From WWW Jgokey ComDocument556 pagesAudels Engineers and Mechanics Guide Volume 5 From WWW Jgokey Comjdsa123No ratings yet
- Gas LawsDocument16 pagesGas LawsJeremy Villadiego Baybay100% (1)
- Ulpsm O3Document7 pagesUlpsm O3Imas YunarsihNo ratings yet
- Heat 2011-1-1Document134 pagesHeat 2011-1-1Lyaz AntonyNo ratings yet