Professional Documents
Culture Documents
Kevlar by Abhishek Jaguessar
Uploaded by
reedoye21Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Kevlar by Abhishek Jaguessar
Uploaded by
reedoye21Copyright:
Available Formats
KEVLAR BY ABHISHEK JAGUESSAR
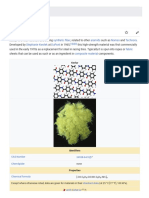
Kevlar is the registered trademark for a para-aramid synthetic fiber , related to other aramid s such as Nomex and Technora . Developed at DuPont in 1965, this high strength material was first commercially used in the early 1970s as a replacement for steel in racing tires. Typically it is spun into ropes or fabric sheets that can be used as such or as an ingredient in composite material components. Currently, Kevlar has many applications, ranging from bicycle tires and racing sails to body armor because of its high tensile strength -to-weight ratio; by this measure it is 5 times stronger than steel on an equal weight basis. When used as a woven material, it is suitable for mooring lines and other underwater applications. A similar fiber called Twaron with roughly the same chemical structure was developed by Akzo in the 1970s; commercial production started in 1986, and Twaron is now manufactured by Teijin .
History
Poly-paraphenylene terephthalamide - branded Kevlar - was invented by Stephanie Kwolek while working for DuPont. In anticipation of a gas shortage, in 1964 her group began searching for a new lightweight strong fiber to use for light but strong tires. The polymers she had been working with at the time, poly-p-Phenylene-terephthalate and polybenzamide, formed liquid crystal while in solution, something unique to those polymers at the time. The solution was "cloudy, opalescent upon being stirred, and of low viscosity" and usually was thrown
away. However, Kwolek persuaded the technician, Charles Smullen, who ran the "spinneret" to test her solution, and was amazed to find that the fiber did not break, unlike nylon. Both her supervisor and laboratory director understood the significance of her discovery and a new field of polymer chemistry quickly arose. By 1971, modern Kevlar was introduced. However, Kwolek was not very involved in developing the applications of Kevlar.
Production
Kevlar is synthesized in solution from the monomers 1,4-phenylene -diamine (para-phenylenediamine) and terephthaloyl chloride in a condensation reaction yielding hydrochloric acid as a byproduct. The result has liquid-crystalline behavior, and mechanical drawing orients the polymer chains in the fiber's direction. Hexamethylphosphoramide (HMPA) was the solvent initially used for the polymerization, but for safety reasons, DuPont replaced it by a solution of N-methyl-pyrrolidone and calcium chloride. As this process was patented by Akzo (see above) in the production of Twaron, a patent war ensued.
Kevlar (poly paraphenylene terephthalamide) production is expensive because of the difficulties arising from using concentrated sulfuric acid , needed to keep the water-insoluble polymer in solution during its synthesis and spinning . Several grades of Kevlar are available: 1. Kevlar K-29 in industrial applications, such as cables, asbestos replacement, brake linings, and body/vehicle armor.
2. 3. 4. 5. 6. 7. 8.
Kevlar K49 high modulus used in cable and rope products. Kevlar K100 colored version of Kevlar Kevlar K119 higher-elongation, flexible and more fatique resistant. Kevlar K129 higher tenacity for ballistic applications. Kevlar AP has 15% higher tenacity than K-29. Kevlar XP lighter weight resin and KM2 plus fiber combination. Kevlar KM2 enhanced ballistic resistance for armor applications
The ultraviolet component of sunlight degrades and decomposes Kevlar, a problem known as UV degradation , and so it is rarely used outdoors without protection against sunlight.
Structure and properties
When Kevlar is spun , the resulting fiber has a tensile strength
of about 3,620 MPa, and a relative density of 1.44. The polymer owes its high strength to the many inter-chain bonds. These inter-molecular hydrogen bonds form between the carbonyl groups and NH centers. Additional strength is derived from aromatic stacking interactions between adjacent strands. These interactions have a greater influence on Kevlar than the van der Waals interactions and chain length that typically influence the properties of other synthetic polymers and fibers such as Dyneema. The presence of salts and certain other impurities, especially calcium , could interfere with the strand interactions and caution is used to avoid inclusion in its production. Kevlar's structure consists of relatively rigid molecules which tend to form mostly planar sheet-like structures rather like silk protein.
Thermal properties
Kevlar maintains its strength and resilience down to cryogenic temperatures (196 C); in fact, it is slightly stronger at low temperatures. At higher temperatures the tensile strength is immediately reduced by about 1020%, and after some hours the strength progressively reduces further. For example at 160 C (320 F) about 10% reduction in strength occurs after 500 hours. At 260 C (500 F) 50% strength reduction occurs after 70 hours. Armor Kevlar is a well-known component of personal armor such as combat helmet s, Ballistic face mask s, and Ballistic vests. The PASGT helmet and vest used by United States military forces from the 1980s into 2005 both have Kevlar as a key component, as do their replacements. Other military uses include bulletproof facemasks used by sentries and spall liners used to protect the crews of armoured fighting vehicle s. Related civilian applications include Emergency Service's protection gear if it involves high heat (e.g., tackling a fire), and Kevlar body armor such as vests for police officers, security, and SWAT . Personal protection Kevlar is used to manufacture gloves, sleeves, jackets, chaps and other articles of clothing designed to protect users from cuts, abrasions and heat. Kevlar based protective gear is often considerably lighter and thinner than equivalent gear made of more traditional materials. Sports equipment It is used as an inner lining for some bicycle tire s to prevent punctures, and due to its excellent heat resistance, is used for fire poi wicks. In table tennis, plies of Kevlar are added to custom ply blades, or paddles, in order to increase bounce and reduce weight. It is used for motorcycle safety clothing , especially in the areas featuring padding such as shoulders and elbows. It was also used as speed control patches for certain Soap Shoes models. In Kyudo or Japanese archery , it may be used as an alternative to more expensive hemp for bow string s. It is one of the main materials used for paraglider suspension lines. It is also used in the laces for the adidas F50 adizero Prime football boot. It is even used in sails for high performance racing boats. Audio equipment
Kevlar has also been found to have useful acoustic properties for loudspeaker cones, specifically for bass and midrange drive units. Drumheads Kevlar is sometimes used as a material on marching snare drums. It allows for an extremely high amount of tension, resulting in a cleaner sound. There is usually some sort of resin poured onto the kevlar to make the head airtight, and a nylon top layer to provide a flat striking surface. This is one of the primary types of marching snare drum heads. Remo 's "Falam Slam" Patch is made with kevlar and is used to reinforce bass drum heads where the beater strikes. Woodwind reeds Kevlar is used in the woodwind reeds of Fibracell. The material of these reeds is a composite of aerospace materials designed to duplicate the way nature constructs cane reed. Very stiff but sound absorbing Kevlar fibers are suspended in a lightweight resin formulation. Frying pans Kevlar is sometimes used as a substitute for teflon in some non-stick frying pans. Rope, cable, sheath The fiber is used in woven rope and in cable, where the fibers are kept parallel within a polyethylene sleeve. The cables have been used in suspension bridge s such as the bridge at Aberfeldy in Scotland . They have also been used to stabilise cracking concrete cooling towers by circumferential application followed by tensioning to close the cracks. Kevlar is widely used as a protective outer sheath for optical fiber cable , as its strength protects the cable from damage and kinking. When used in this application it is commonly known by the trademarked name parafil. Electricity generation Kevlar was used by scientists at Georgia Institute of Technology as a base textile for an experiment in electricity-producing clothing. This was done by weaving zinc oxide nanowire s into the fabric. If successful, the new fabric would generate about 80 milliwatts per square meter. Building construction A retractable roof of over 60,000 square feet (5,575 square metres) of Kevlar was a key part of the design of Montreal's Olympic stadium for the 1976 Summer Olympics . It was spectacularly unsuccessful, as it was completed ten years late and replaced just ten years later in May 1998 after a series of problems. Brakes The chopped fiber has been used as a replacement for asbestos in brake pads. Dust produced from asbestos brakes is toxic, while aramids are a benign substitute. Expansion joints and hoses Kevlar can be found as a reinforcing layer in rubber bellows expansion joints and rubber hose s, for use in high temperature applications, and for its high strength. It is also found as a braid layer used on the outside of hose assemblies, to add protection against sharp objects.
Particle physics experiment A thin kevlar window has been used by the NA48 experiment at CERN to separate a vacuum vessel from a vessel at nearly atmospheric pressure, both 192 cm in diameter. The window has provided vacuum tightness combined with reasonably small amount of material (only 0.3% to 0.4% of radiation length ).
Composite materials
Aramid fibers are widely used for reinforcing composite materials, often in combination with carbon fiber and glass fiber. The matrix for high performance composites is usually epoxy resin. Typical applications include monocoque bodies for F1 racing cars, helicopter rotor blades, tennis , table tennis , badminton and squash racket s, kayak s, cricket bats, and field hockey , ice hockey and lacrosse sticks.
You might also like
- Consumer Chemistry (CH 419)Document9 pagesConsumer Chemistry (CH 419)Mihir Kumar MechNo ratings yet
- KevlarDocument6 pagesKevlarsunitbhaumikNo ratings yet
- KevlarDocument8 pagesKevlarapi-282592950No ratings yet
- Kevlar: Synthetic Fiber Aramids Nomex Technora Stephanie Kwolek Dupont Fabric Composite MaterialDocument12 pagesKevlar: Synthetic Fiber Aramids Nomex Technora Stephanie Kwolek Dupont Fabric Composite MaterialFaizan AlamNo ratings yet
- Kevlar Fiber: Devansh GuptaDocument23 pagesKevlar Fiber: Devansh Guptagopal rao sirNo ratings yet
- Kevlar ProjedtDocument11 pagesKevlar Projedtapi-345347907No ratings yet
- Kevlar-The Super Tough Fiber: Engr. Reashad Bin Kabir, Engr. Nasrin FerdousDocument5 pagesKevlar-The Super Tough Fiber: Engr. Reashad Bin Kabir, Engr. Nasrin FerdousBabtista EzraNo ratings yet
- KEVLAR2SUBBUDocument17 pagesKEVLAR2SUBBUSubbu SureshNo ratings yet
- KevlarDocument14 pagesKevlarKhaledIssamAl-qudah0% (1)
- Physics Presentation ResearchDocument3 pagesPhysics Presentation ResearchAngus LivingstoneNo ratings yet
- Kevlar Is A Heat-Resistant and Strong: paraDocument1 pageKevlar Is A Heat-Resistant and Strong: parasubhamNo ratings yet
- Polymeric Composite Materials-5.2Document23 pagesPolymeric Composite Materials-5.2HALİM BOZTEPENo ratings yet
- Du Pont Kevlar Aramid Und Us Trial Fiber Abridged)Document16 pagesDu Pont Kevlar Aramid Und Us Trial Fiber Abridged)canhconvsip100% (1)
- Uttara University: Submitted ToDocument8 pagesUttara University: Submitted ToMOJAHID HASAN Fall 19No ratings yet
- Synthesis of KevlarDocument5 pagesSynthesis of KevlarAimee Ruth Inuguidan Dagupon100% (2)
- Kevlar: From Wikipedia, The Free EncyclopediaDocument9 pagesKevlar: From Wikipedia, The Free EncyclopediaMust WatchNo ratings yet
- Aramid Fibres and Trade Names PDFDocument6 pagesAramid Fibres and Trade Names PDFKali MuthuNo ratings yet
- Types of Fiber ReinforcementDocument9 pagesTypes of Fiber ReinforcementNebyat YazachewNo ratings yet
- 8.kevlar-A New Fibre Reinforcement For Conveyor BeltingDocument11 pages8.kevlar-A New Fibre Reinforcement For Conveyor BeltingLejo VargheseNo ratings yet
- Aramid FiberDocument5 pagesAramid FiberRamesh Iyer100% (1)
- Chapter 03 Synthetic Fiber RopesDocument12 pagesChapter 03 Synthetic Fiber RopesCaptIsqanNo ratings yet
- Rope Materials in Chronological OrderDocument5 pagesRope Materials in Chronological Ordermick.pride81No ratings yet
- KevlarDocument11 pagesKevlarLisbeth Damian SamameNo ratings yet
- Project - Aramid FibersDocument22 pagesProject - Aramid FibersMohammed Anass EL MAROUANINo ratings yet
- Kevlar Bullet Proof: Kevlar Is A Material That Is Used in Making Bulletproof Jackets and VestsDocument2 pagesKevlar Bullet Proof: Kevlar Is A Material That Is Used in Making Bulletproof Jackets and Vestsashishtelang2013No ratings yet
- ScriptDocument4 pagesScriptJoel KhouriNo ratings yet
- Composite Material ApplicationsDocument35 pagesComposite Material ApplicationsTejas shastrakarNo ratings yet
- High Strength CordDocument8 pagesHigh Strength CordJonathan RamljakNo ratings yet
- Fishing Boat ProjectDocument7 pagesFishing Boat ProjectStephen DuamorNo ratings yet
- 14 Strongest MaterialsDocument13 pages14 Strongest MaterialsImam MaulanaNo ratings yet
- Aircraft Material and Processes 1006Document12 pagesAircraft Material and Processes 1006Gokul Raam GNo ratings yet
- Kevlar: Poly-Para-Phenylene-Terephthalamide (-CO-C H - CO-NH-C H - NH-) Let's Just Call It..Document24 pagesKevlar: Poly-Para-Phenylene-Terephthalamide (-CO-C H - CO-NH-C H - NH-) Let's Just Call It..Banka Banga0% (1)
- Chemical Formula of KevlarDocument8 pagesChemical Formula of KevlarChristian PamaylaonNo ratings yet
- Aramid FibresDocument40 pagesAramid FibresAnand Dubey100% (3)
- Composite MaterialDocument92 pagesComposite MaterialAparna RNo ratings yet
- Hi Performance FibersDocument13 pagesHi Performance Fibersahmer adnanNo ratings yet
- Wind Turbine Blade Production - New Products Keep Pace As Scale IncreasesDocument8 pagesWind Turbine Blade Production - New Products Keep Pace As Scale IncreasesSandeep BandyopadhyayNo ratings yet
- The Splicing Handbook Chapter 1Document17 pagesThe Splicing Handbook Chapter 1Archie AdkinsNo ratings yet
- CL 404 Material Science Group 2Document30 pagesCL 404 Material Science Group 2Akash PrasanthNo ratings yet
- Material Report: Carbon FiberDocument3 pagesMaterial Report: Carbon FiberOkasha ChhotaniNo ratings yet
- Praxis Business School: Carbon Fibre TechnologyDocument16 pagesPraxis Business School: Carbon Fibre Technologyankit1217No ratings yet
- Synthetic Slings PDFDocument3 pagesSynthetic Slings PDFNeemo FishNo ratings yet
- Common Fiber Reinforcement of A Glass, Carbon and Kevlar Common Fiber Reinforcement of A Glass, Carbon and KevlarDocument28 pagesCommon Fiber Reinforcement of A Glass, Carbon and Kevlar Common Fiber Reinforcement of A Glass, Carbon and KevlarCedrick LandichoNo ratings yet
- Report No. 1 - PROPELLER CONSTRUCTIONDocument10 pagesReport No. 1 - PROPELLER CONSTRUCTIONRev Xavier CruzNo ratings yet
- W2-Reinforcement Mater StuDocument34 pagesW2-Reinforcement Mater StuTuğbaNo ratings yet
- Thermal Resistant FibersDocument21 pagesThermal Resistant FibersNimra GhafoorNo ratings yet
- Added Layer of Non-Newtionian Fluid (Oobleck) in Level-IIA Kevlar Vests As An Impulse Reducer For Gunshot ImpactDocument8 pagesAdded Layer of Non-Newtionian Fluid (Oobleck) in Level-IIA Kevlar Vests As An Impulse Reducer For Gunshot ImpactZedwin Sta MonicaNo ratings yet
- Mineral FibrelDocument19 pagesMineral FibrelnitishkohliNo ratings yet
- CompositeDocument24 pagesCompositeKartik SharmaNo ratings yet
- Kevlar:DUPONT: Form Properties UsesDocument3 pagesKevlar:DUPONT: Form Properties UsesOmer HassanNo ratings yet
- Spectra and Plasma RopesDocument11 pagesSpectra and Plasma RopeslauraNo ratings yet
- Carbon and Graphite FibersDocument8 pagesCarbon and Graphite FibersEirick Wayne Zuñigga De-ItzelNo ratings yet
- Fiberglass: Fiberglass (American English), or Fibreglass (Commonwealth English) Is A Common Type of FiberDocument13 pagesFiberglass: Fiberglass (American English), or Fibreglass (Commonwealth English) Is A Common Type of FibersacNo ratings yet
- Additive Manufacturing AssignmentDocument4 pagesAdditive Manufacturing AssignmentArenNo ratings yet
- Carbon FibreDocument33 pagesCarbon FibrePrasad NbNo ratings yet
- Plastics in Building Structures: Proceedings of a Conference Held in London, 14-16 June 1965From EverandPlastics in Building Structures: Proceedings of a Conference Held in London, 14-16 June 1965No ratings yet
- Knots You Need to Know: Easy-to-Follow Guide to the 30 Most Useful KnotsFrom EverandKnots You Need to Know: Easy-to-Follow Guide to the 30 Most Useful KnotsNo ratings yet
- Geotextiles and Geomembranes HandbookFrom EverandGeotextiles and Geomembranes HandbookT.S. IngoldRating: 5 out of 5 stars5/5 (1)
- Maternal Mortality by Abhishek JaguessarDocument24 pagesMaternal Mortality by Abhishek Jaguessarreedoye21No ratings yet
- Epilepsy in Pregnancy by Abhishek JaguessarDocument57 pagesEpilepsy in Pregnancy by Abhishek Jaguessarreedoye21No ratings yet
- HR OBSTET by Abhishek JaguessarDocument22 pagesHR OBSTET by Abhishek Jaguessarreedoye21No ratings yet
- Abdominal Pain During Pregnancy by Abhishek JaguessarDocument27 pagesAbdominal Pain During Pregnancy by Abhishek Jaguessarreedoye21No ratings yet
- Homebirth by Abhishek JaguessarDocument27 pagesHomebirth by Abhishek Jaguessarreedoye21No ratings yet
- Forceps Delivery by Abhishek JaguessarDocument32 pagesForceps Delivery by Abhishek Jaguessarreedoye21No ratings yet
- The Adult Learner by Abhishek JaguessarDocument16 pagesThe Adult Learner by Abhishek Jaguessarreedoye21No ratings yet
- Christmas Symbols by Abhishek JaguessarDocument8 pagesChristmas Symbols by Abhishek Jaguessarreedoye21No ratings yet
- Dysfunctional Labour by Abhishek JaguessarDocument34 pagesDysfunctional Labour by Abhishek Jaguessarreedoye21No ratings yet
- Christmas Celebrations by Abhishek JaguessarDocument12 pagesChristmas Celebrations by Abhishek Jaguessarreedoye21No ratings yet
- December Hanukkah by Abhishek JaguessarDocument7 pagesDecember Hanukkah by Abhishek Jaguessarreedoye21No ratings yet
- Making and Naming Compounds by Abhishek JaguessarDocument9 pagesMaking and Naming Compounds by Abhishek Jaguessarreedoye21No ratings yet
- December Kwanzaa by Abhishek JaguessarDocument7 pagesDecember Kwanzaa by Abhishek Jaguessarreedoye21No ratings yet
- The An Trail by Abhishek JaguessarDocument15 pagesThe An Trail by Abhishek Jaguessarreedoye21No ratings yet
- Hnduism by Abhishek JaguessarDocument28 pagesHnduism by Abhishek Jaguessarreedoye21No ratings yet
- I Am That by Abhishek JaguessarDocument34 pagesI Am That by Abhishek Jaguessarreedoye21No ratings yet
- Mass Formula Atomic Mass and Empirical Formula by Abhishek JaguessarDocument12 pagesMass Formula Atomic Mass and Empirical Formula by Abhishek Jaguessarreedoye21No ratings yet
- Group 15 The Nitrogen Group by Abhsihek JaguessarDocument6 pagesGroup 15 The Nitrogen Group by Abhsihek Jaguessarreedoye21No ratings yet
- Group 16 The Oxygen Group by Abhishek JaguessarDocument7 pagesGroup 16 The Oxygen Group by Abhishek Jaguessarreedoye21No ratings yet
- Group 13 The Boron Family by Abhishek JaguessarDocument4 pagesGroup 13 The Boron Family by Abhishek Jaguessarreedoye21No ratings yet
- Group 14 The Carbon Group by Abhishek JaguessarDocument5 pagesGroup 14 The Carbon Group by Abhishek Jaguessarreedoye21No ratings yet
- Electrical ConduitsDocument8 pagesElectrical ConduitsFrisco Gabriel100% (1)
- Super ACE Brochure PDFDocument8 pagesSuper ACE Brochure PDFAdriawan AnnesNo ratings yet
- Heaven-Bryan Adams - LTR - Not PDFDocument3 pagesHeaven-Bryan Adams - LTR - Not PDFHR LuNo ratings yet
- Chapter 10-Rotation of A Rigid Object About A Fixed Axis: Multiple ChoiceDocument23 pagesChapter 10-Rotation of A Rigid Object About A Fixed Axis: Multiple ChoiceMerlin Trochez 504No ratings yet
- Refereeing Basics Course PDFDocument52 pagesRefereeing Basics Course PDFapi-496743421100% (1)
- 1Kklwtq Aygo Hatchback 5 Doors 1.0 L (68 HP) 5 Speed Multimode X-CiteDocument8 pages1Kklwtq Aygo Hatchback 5 Doors 1.0 L (68 HP) 5 Speed Multimode X-CiteRaduRouaNo ratings yet
- Plaster ManpowerDocument1 pagePlaster ManpowerAmr HamedNo ratings yet
- Assignment 2 On Respiratory PPEDocument11 pagesAssignment 2 On Respiratory PPEAngelo SaysonNo ratings yet
- UntitledDocument23 pagesUntitledAlmir Souza Jr.No ratings yet
- Samurai Warriors Prima Official EGuideDocument129 pagesSamurai Warriors Prima Official EGuidelac1981No ratings yet
- Wikipedia Sky DivingDocument2 pagesWikipedia Sky DivingBocah NdesoNo ratings yet
- Muscle Contraction: Energy SystemsDocument37 pagesMuscle Contraction: Energy SystemsRene John Bulalaque EscalNo ratings yet
- Memories Aboard A Paper Plane: Ninja World Tournament - Ally EventsDocument3 pagesMemories Aboard A Paper Plane: Ninja World Tournament - Ally EventsninosaurusNo ratings yet
- Home Workouts Encyclopaedia 60 Workout Programs To Stay Active From HomeDocument332 pagesHome Workouts Encyclopaedia 60 Workout Programs To Stay Active From HomeYmiuchin100% (2)
- Direct-Hit - Search - PDF 3Document1 pageDirect-Hit - Search - PDF 3Joycee Lázaro ReyesNo ratings yet
- Chris Bumstead PPL PDFDocument8 pagesChris Bumstead PPL PDFBrandon Foley94% (17)
- MLB Report CardDocument58 pagesMLB Report CardAustin DeneanNo ratings yet
- NLB Catálogo de AccesoriosDocument106 pagesNLB Catálogo de Accesoriosjaime farfanNo ratings yet
- EagleorniDocument1 pageEagleorniMayurVashishthNo ratings yet
- Sear Trigger TuningDocument4 pagesSear Trigger TuningDustin Gray100% (1)
- Body Parts WorksheetsDocument4 pagesBody Parts WorksheetsYuyu MomokoNo ratings yet
- Ford-LRG425 PARTDocument64 pagesFord-LRG425 PART닌닌No ratings yet
- Ejercicios en Ingles de Las Condicionales 1 y 2Document4 pagesEjercicios en Ingles de Las Condicionales 1 y 2AlbertoNo ratings yet
- MilindnilekaniresumeDocument2 pagesMilindnilekaniresumeapi-299872333No ratings yet
- Machinarium WalkthroughDocument16 pagesMachinarium WalkthroughKumar SanjuNo ratings yet
- Fluid Mechanics - 2009Document18 pagesFluid Mechanics - 2009Paragmoni KalitaNo ratings yet
- Enhancing Mitochondrial Function With D RiboseDocument6 pagesEnhancing Mitochondrial Function With D RiboseLeanne Montgomery100% (1)
- Audi LagiDocument3 pagesAudi LagiRuly KurniadiNo ratings yet
- Duckquest 3Document1 pageDuckquest 3No KubaNo ratings yet
- Selection - Schedule - 18 - 20 - PDF 2021 Sep 29 16 47 48Document11 pagesSelection - Schedule - 18 - 20 - PDF 2021 Sep 29 16 47 48Nhai VijayawadaNo ratings yet