Professional Documents
Culture Documents
Fever and Abd - Pain
Uploaded by
Av RienOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fever and Abd - Pain
Uploaded by
Av RienCopyright:
Available Formats
The
n e w e ng l a n d j o u r na l
of
m e dic i n e
case records of the massachusetts general hospital
Founded by Richard C. Cabot Nancy Lee Harris, m.d., Editor Eric S. Rosenberg, m.d., Associate Editor Jo-Anne O. Shepard, m.d., Associate Editor Alice M. Cort, m.d., Associate Editor Sally H. Ebeling, Assistant Editor Christine C. Peters, Assistant Editor
Case 29-2010: A 29-Year-Old Woman with Fever and Abdominal Pain
Daniel P. Hunt, M.D., Ashraf Thabet, M.D., and Eric S. Rosenberg, M.D.
Pr e sen tat ion of C a se
From the Departments of Medicine (D.P.H., E.S.R.), Radiology (A.T.), and Pathology (E.S.R.), Massachusetts General Hospital; and the Departments of Medicine (D.P.H., E.S.R.), Radiology (A.T.), and Pathology (E.S.R.), Harvard Medical School both in Boston.
N Engl J Med 2010;363:1266-74.
Copyright 2010 Massachusetts Medical Society.
Dr. Vernon A. Rayford (MedicinePediatrics): A 29-year-old woman was admitted to the hospital because of fever and increasing abdominal pain. The patient had spastic quadriplegia due to cerebral palsy but had been in her usual state of health until approximately 2 weeks before admission, when intermittent fevers, with temperatures up to 37.7C, developed. One day before admission, pain in the left flank and left lower quadrant developed, and she noted foul-smelling urine. During the night before admission, the oral temperature rose to 39.1C, associated with nausea and pain in the chest, both legs, and the abdomen, which radiated to the back, both flanks, and the midscapular region. She took ibuprofen, and her parents brought her to the emergency department at this hospital in the early afternoon. The patient rated the pain at 8 on a scale of 1 to 10, with 10 indicating the most severe pain. She had cerebral palsy with spastic quadriplegia, obesity, iron-deficiency anemia, polycystic ovary syndrome with irregular menses, recurrent urinary tract infections, and nephrolithiasis. A ureteral stent had been placed temporarily 10 years earlier because of an obstructing stone in the left ureter. She drank alcohol socially and did not smoke or use illicit drugs. She lived with her parents and a sibling in an urban area, and she had recently broken up with her boyfriend. She used a wheelchair and required assistance cutting food. She followed a low-oxalate diet. She reported no contact with sick persons and no exposure to ticks, and she was not sexually active. Her father and paternal grandfather had diabetes mellitus, her father had reactive arthritis (formerly known as Reiters syndrome), one grandfather had relapsing polychondritis, and two grandparents had coronary artery disease. On examination, the patient, who was in a wheelchair, was alert and communicative. The temperature was 37.5C, the blood pressure 119/63 mm Hg, the pulse 108 beats per minute, and the oxygen saturation 96% while she was breathing ambient air. There was mild tenderness of the sternum, which was reproduced with deep inspirations, and tenderness of the left costovertebral angle. The abdomen was soft and tender to palpation on the left side, with the most severe tenderness in the left lower quadrant; there was no rebound or guarding. Radial pulses were 2+. The remainder of the examination was consistent with spastic quadriplegia.
1266
n engl j med 363;13
nejm.org
september 23, 2010
The New England Journal of Medicine Downloaded from nejm.org on March 5, 2012. For personal use only. No other uses without permission. Copyright 2010 Massachusetts Medical Society. All rights reserved.
case records of the massachusetts gener al hospital
The platelet count and levels of serum electrolytes, glucose, calcium, phosphorus, magnesium, total protein, albumin, globulin, amylase, and lipase were normal, as were tests of renal function; other test results are shown in Table 1. Review of the peripheral-blood smear revealed anisocytosis (2+), polychromatocytosis (1+), hypochromatocytosis (2+), and microcytosis (3+). Urinalysis revealed clear amber urine with a specific gravity of 1.025, a pH of 6.0, 2+ bilirubin, 1+ protein, and trace amounts of ketones and urobilinogen; a culture was sterile. While the patient was in the emergency department, narcotic analgesia was administered intravenously, and her pain decreased to a score of 7 out of 10. Eight hours after arrival, the patient vomited once; ondansetron was administered. Computed tomography (CT) of the abdomen was performed after the oral and intravenous administration of contrast material, but it was complicated by extravasation of the contrast material at the intravenous site in the right arm. The study showed malrotation of the left extrarenal pelvis, multiple cortical defects in the left kidney that were consistent with scarring, and a urinary catheter in the urethra. The spleen was mildly enlarged (14.8 cm in the craniocaudal dimension; upper limit of the normal range, 12 to 13). There were prominent periportal, mesenteric, inguinal, and retroperitoneal lymph nodes, up to 1.4 cm in diameter, with trace free fluid in the pelvis. The patient was admitted to the hospital early the next morning. On the day of admission, the temperature was 38.1C. The pain (rated as 8 out of 10) persisted, and narcotic analgesia was administered intravenously. Nausea and vomiting recurred but lessened after the administration of prochlorperazine. Treatment with dalteparin sodium was begun. A repeat culture of the urine grew rare mixed bacteria. The chest radiograph showed low lung volumes and no opacities that were suggestive of pneumonia. The next day, ultrasonography of the kidneys and the venous system of the lower extremities was normal, with no evidence of hydronephrosis or deep venous thrombosis. During the third, fourth, and fifth hospital days, the serum iron-binding capacity and levels of iron, ferritin, folate, and vitamin B12 were normal; other laboratory-test results are shown in Table 1. On the third day, the temperature rose to
38.5C. Urinalysis revealed leukocytes (>100 white cells per high-power field), and a urine culture grew Proteus mirabilis and Escherichia coli; blood cultures remained sterile. Ciprofloxacin was administered. The next day, a cherry-red rash developed on the patients feet and resolved spontaneously after several hours. Ultrasonography of the abdomen was normal. Low-grade fevers occurred intermittently thereafter, and severe abdominal pain (8 out of 10) persisted; it was greatest in the left upper quadrant, with radiation to the left flank, and was associated with nausea and intermittent vomiting. On the fifth day, testing for antibodies to Borrelia burgdorferi, cytomegalovirus (CMV), and hepatitis B and C viruses was negative, as were tests for antinuclear antibody, CMV antigenemia, and heterophile antibody; other test results are shown in Table 1. A CT scan of the abdomen, after the intravenous administration of contrast material, showed persistent mild splenomegaly with peripheral wedge-shaped areas of hypoattenuation that were consistent with infarcts; other findings were unchanged from the CT performed on admission. Tests for malaria and antibodies to the human immunodeficiency virus (HIV) and heparin platelet factor 4 were negative, as were nucleic acid testing for ehrlichia, Coombs direct antibody test, cold-agglutinin screening, and testing for lupus anticoagulant; hemoglobin electrophoresis and levels of fibrinogen, homocysteine, lipoprotein(a), 2-glycoprotein I, antithrombin III, and protein C (functional) were normal. Other test results are shown in Table 1. Blood cultures remained sterile. Transthoracic echocardiography was normal, with no evidence of valvular vegetations. On the 10th day, diagnostic test results were received.
Differ en t i a l Di agnosis
Dr. Daniel P. Hunt: I am aware of the diagnosis. My differential diagnosis will focus on clues early in the course of this patients illness that might lead to an early presumptive diagnosis that could be efficiently confirmed with minimal laboratory testing. Two weeks before admission, fever developed in this patient, with no other reported symptoms. It would be essential for the admitting physician
n engl j med 363;13
nejm.org
september 23, 2010
1267
The New England Journal of Medicine Downloaded from nejm.org on March 5, 2012. For personal use only. No other uses without permission. Copyright 2010 Massachusetts Medical Society. All rights reserved.
The
n e w e ng l a n d j o u r na l
of
m e dic i n e
Table 1. Laboratory Data. Variable Hematocrit (%) Hemoglobin (g/dl) White-cell count (per mm3) Differential count (%) Neutrophils Band forms Lymphocytes Atypical lymphocytes Monocytes Metamyelocytes Eosinophils Myelocytes Reticulocytes (%) Mean corpuscular volume (m3) Red-cell distribution width (%)
d-Dimer
Reference Range, Adults* 36.046.0 (women) 12.016.0 (women) 450011,000 4070 010 2244 0 411 0 08 0 0.52.5 80100 11.514.5 <500 10.813.4 21.033.0 0.01.0 0.00.4 730 932 30100 110210 <8.0 2750 Negative at 1:20 dilution 015 015
On Admission 35.4 11.4 6500 51 3 35 4 6 1 0 0 72 18.2
3rd Day 27.5 9.0 5200 54 4 29 2 10 1 0 0 2.3 73 18.3
4th Day 27.1 8.6 5000
5th Day 26.8 8.4 7400
7th Day 25.8 8.5 9600 27 1 58 3 8 0 2 1 3.7
74 18.9 3108
75 19.2 15.8
75 20.1 14.7 51.0
(ng/ml)
Prothrombin time (sec) Activated partial-thromboplastin time (sec) Bilirubin (mg/dl) Total Direct Alanine aminotransferase (U/liter) Aspartate aminotransferase (U/liter) Alkaline phosphatase (U/liter) Lactate dehydrogenase (U/liter) C-reactive protein (mg/liter) Ceruloplasmin (mg/dl) Antibody to smooth muscle IgG anticardiolipin antibody IgM anticardiolipin antibody
1.1 0.4 38 43 120
2.0 1.0 34 56 105 612
3.5 2.1 77 129 120
3.7 2.5 131 215 127 75.6 53 Positive at 1:40 dilution
3.1 2.0 202 304 170 598
19.4 23.7
* Reference values are affected by many variables, including the patient population and the laboratory methods used. The ranges used at Massachusetts General Hospital are for adults who are not pregnant and do not have medical conditions that could affect the results. They may therefore not be appropriate for all patients. To convert the values for bilirubin to micromoles per liter, multiply by 17.1.
to explore symptoms that might localize a source for the fever. Specifically, we should be sure that she had not noted symptoms of respiratory or urinary infection. In view of the history of recurrent urinary tract infections, we should also be sure that she had not initiated treatment with antibiotics before she presented at the hospital.
1268
n engl j med 363;13
After this smoldering illness, she had a relatively sudden onset of acute abdominal pain, flank pain, and foul-smelling urine. The initial examination revealed fever, mild tachycardia, tenderness of the sternum and left costovertebral angle, and leftsided abdominal tenderness, particularly in the left lower quadrant.
september 23, 2010
nejm.org
The New England Journal of Medicine Downloaded from nejm.org on March 5, 2012. For personal use only. No other uses without permission. Copyright 2010 Massachusetts Medical Society. All rights reserved.
case records of the massachusetts gener al hospital
Abdominal pain
In patients presenting to an outpatient clinic with a new onset of abdominal pain, the location of the pain may or may not be predictive of the underlying pathologic features.1 In one study, sensitivity was high for pain in the epigastric region, indicating gastroduodenal processes; for right subcostal pain, indicating hepatobiliary diseases; and for mid-to-lower abdominal pain, indicating gynecologic diseases among women.1 This study suggests that we probably should not focus our differential diagnosis on organs in the left lower quadrant to the exclusion of other abdominal organs. However, we need to start somewhere, so we will begin with the most likely causes of pain in the left lower quadrant in a young woman: salpingitis, ectopic pregnancy, irritable bowel syndrome, inflammatory bowel disease, inguinal hernia, nephrolithiasis, and diverticulitis. The medical history indicates that the patient is not sexually active, making salpingitis and ectopic pregnancy unlikely. The absence of clinically significant bowel symptoms lessens the likelihood of irritable bowel syndrome or inflammatory bowel disease. An inguinal hernia would have to be incarcerated to generate the described degree of pain, and this should have been evident on examination. We are left with diverticulitis and nephrolithiasis, and diverticulitis is less likely than nephrolithiasis in a relatively young woman. The presence of fever, tenderness in the left costovertebral angle, and left-sided abdominal tenderness, particularly in the left lower quadrant, suggests a recurrent ureteral stone with associated pyelonephritis, particularly in view of the patients history. It would be helpful to know whether the pain was colicky, as it had been during her previous episodes of nephrolithiasis, and whether it had been present in a lesser degree during her 2-week illness. A kidney stone with pyelonephritis is unlikely because of the normal white-cell count and the absence of dysuria. The results of the initial urinalysis argue against a urinary tract infection, but complete obstruction of the left ureter needs to be ruled out, since obstruction might prevent the appearance of cells or bacteria in the urine. We will need to examine the initial abdominal CT scan carefully for evidence of a ureteral stone. In patients presenting to the emergency department with acute abdominal pain, early abdomin engl j med 363;13
nal CT scanning provides better diagnostic accuracy than does the standard practice of supine abdominal and erect chest imaging2 and less frequently misses unexpected and serious conditions.3 When different diagnostic strategies are compared in the evaluation of acute abdominal pain, CT has a sensitivity of 89% and a specificity of 77% in serious conditions.4 In cases in which ultrasonography is negative, ultrasonography followed by CT has the best sensitivity (94%) with the fewest missed serious conditions but has a slightly lower specificity (68%). Therefore, on the patients presentation to the emergency department, it would be appropriate to obtain an abdominal CT scan. Dr. Thabet, may we review the imaging studies? Dr. Ashraf Thabet: On admission, CT of the abdomen and pelvis was performed with oral contrast material alone, after administration of intravenous contrast material was discontinued owing to extravasation. Images of the kidneys show cortical divots within the left kidney (Fig. 1A), which are consistent with scarring. There is no nephrolithiasis or hydronephrosis present to suggest obstructive uropathy. There is splenomegaly, with the spleen measuring 14.8 cm in the craniocaudal dimension. A mildly enlarged portacaval lymph node (Fig. 1B) is also shown. There is no evidence of bowel obstruction, bowel inflammation, or large fluid collections. Dr. Hunt: In this case, CT of the abdomen was very informative. Since the scan did not show diverticular disease, we could essentially rule out the diagnosis of diverticulitis. The scarring of the left kidney is consistent with previous upper urinary tract infection but does not explain the patients acute symptoms on presentation. The severity of this young womans pain is of great concern, since it apparently was difficult to control with narcotic analgesic agents. We would be well advised to consider an admonition about severe abdominal pain from Copes classic text, which says that the majority of severe abdominal pains which ensue in patients who have been previously fairly well, and which last as long as six hours, are caused by conditions of surgical import.5 Intraabdominal processes that would generate very severe pain include bowel perforation, obstruction, or infarction; aortic dissection or rupture; and an acute inflammatory process (e.g., pancreatitis). There is no evidence for the
september 23, 2010
nejm.org
1269
The New England Journal of Medicine Downloaded from nejm.org on March 5, 2012. For personal use only. No other uses without permission. Copyright 2010 Massachusetts Medical Society. All rights reserved.
The
n e w e ng l a n d j o u r na l
of
m e dic i n e
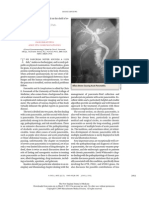
Figure 1. CT Image of the Abdomen and Pelvis. The CT scan was obtained with only oral contrast material. A coronal image (Panel A) shows cortical divots (arrowhead) within the left kidney, features that are consistent with scarring. No renal calculus or hydronephrosis is shown. There is splenomegaly, with the spleen 14.8 cm in the craniocaudal dimension (arrows). An axial image (Panel B) shows a mildly enlarged portacaval lymph node (arrow), 1.4 cm in diameter in the short-axis view. On the fifth hospital day, repeat CT of the abdomen and pelvis was performed after the administration of both oral and intravenous contrast material. On a coronal image (Panel C), a peripheral, wedgeshaped area of hypoenhancement is seen in the spleen (arrow), as is renal scarring (arrowhead). Axial images (Panels D and E) show multiple wedge-shaped areas of hypoenhancement (arrows) in the periphery of the spleen, features that are consistent with splenic infarcts. Splenomegaly is unchanged.
first three processes on physical examination of the patient or on CT scans, and the normal whitecell and differential counts seem incongruous with the severity of pain and the implied urgency of an intraabdominal process. The normal amylase and lipase levels and the normal appearance of the pancreas on the CT scan argue strongly against pancreatitis. Given the lack of intravenous contrast material on the initial CT scan, I am concerned that we may not be able to rule out a vascular process or an infarction of an intraabdominal organ.
Splenomegaly and lymphadenopathy
The finding of splenomegaly and lymphadenopathy in a patient with a protracted, intermittent fever expands our list of diagnostic considerations (Table 2). Of these, the most likely in our patient would be infectious mononucleosis due to Epstein Barr virus (EBV) or CMV. With this consideration, we should review the hematologic studies. The presence of atypical lymphocytes is intriguing, and the blood smear should be reviewed. If the percentage of atypical lymphocytes was substantially higher than reported, infectious mononucleosis would lead the diagnostic possibilities.6 Although the patients elevated aminotransferase levels are evidence of hepatic involvement, the levels are lower than would be expected in a case of hepatitis A, B, or C, and viral hepatitis is unlikely to cause splenomegaly and lymphadenopathy. Bacterial infections are less likely in view of the normal white-cell count and the course of the illness. Infective endocarditis seems unlikely be1270
RETAKE AUTHOR ICM cause of the absence of cardiac findings, 1st the fi a 2nd REG F FIGURE a-e negative echocardiogram, and negative blood 3rd CASE TITLE cultures. Since the patient had4-C traveled or not Revised EMail Line SIZE been Enon ARTIST: mleahy or H/T persons, mycobacexposed to ticks sick H/T 16p6 FILL Combo terial, parasitic, rickettsial, and fungal illnesses AUTHOR, PLEASE NOTE: are unlikely. Although systemic lupus erytheFigure has been redrawn and type has been reset. matosus may be Please check carefully.many ways, and manifested in we should keep this diagnosis on the list, there is
hunt
JOB:
36313
ISSUE:
09-23-10
n engl j med 363;13
nejm.org
september 23, 2010
The New England Journal of Medicine Downloaded from nejm.org on March 5, 2012. For personal use only. No other uses without permission. Copyright 2010 Massachusetts Medical Society. All rights reserved.
case records of the massachusetts gener al hospital
little to suggest it. Lymphoma remains a possibility, and other hematologic processes may be associated with splenomegaly, but these would be less likely to account for the fever in the absence of a second process. Tests for EBV, CMV, and HIV should be performed in cases such as these. An antinuclear-antibody test would be reasonable, but we would need to interpret a positive result with caution.
Anemia
Table 2. Causes of Splenomegaly and Subacute Fever. Infection Viral (hepatitis, infectious mononucleosis, cytomegalovirus, human immunodeficiency virus) Bacterial (salmonella, brucella, pyogenic abscess, endocarditis) Mycobacterial (tuberculosis, atypical mycobacterial infections) Parasitic (malaria, toxoplasmosis) Rickettsial (Rocky Mountain spotted fever) Fungal (histoplasmosis) Inflammation Systemic lupus erythematosus Rheumatoid arthritis Sarcoidosis Serum sickness Malignant condition Lymphomas Acute and chronic leukemias Other hematologic disorders (acute and chronic autoimmune hemolytic anemias)
We should briefly consider the anemia that worsened during the early hospital stay, coincident with a rising bilirubin level and an elevated lactase dehydrogenase level. The direct bilirubin level was more than 50% of the total, which argues for hepatic or posthepatic processes as the major cause of the patients hyperbilirubinemia.7 The elevated red-cell distribution width combined with a low mean corpuscular volume prompts consideration of a relatively short list of causes, including iron deficiency, -thalassemia, anemia of inflammation (in some patients), deficiency in glucose-6phosphate dehydrogenase, and fragmented red cells.7 Although it is likely that several processes contributed to her anemia, the available iron studies suggest iron deficiency, the history suggests the possibility of anemia of inflammatory disease, and the lactase dehydrogenase level suggests possible coincident hemolysis. By day 3 of the patients hospitalization, the abdominal pain was localized to the left upper quadrant and left flank. This refocuses our differential diagnosis for the cause of her abdominal pain. Processes that have not yet been eliminated from our differential diagnosis include gastric ulcer, gastritis, splenic abscess, and splenic infarction. Intrathoracic or systemic processes might cause pain in the left upper quadrant and must be kept in mind, but knowing that the patient has splenomegaly should focus our attention on that organ. Repeat CT imaging after the intravenous administration of contrast material would be appropriate. On day 5, the results of a number of diagnostic studies became available and should refine our working differential diagnosis. The negative tests for CMV, hepatitis, and antinuclear antibodies effectively reduce if not eliminate the associated diagnoses from consideration. My leading consideration is infectious mononucleosis, but the heterophile-antibody test was negative. The sensitivn engl j med 363;13
ity of heterophile-antibody testing in the diagnosis of infectious mononucleosis ranges from 81 to 95%, with excellent specificity.8 Early in the course of illness, the heterophile-antibody test may be negative.9 At this point in the course of the patients illness, I think that infectious mononucleosis remains the leading diagnostic consideration, despite the negative heterophile-antibody test. Dr. Thabet, may we review the second CT scan? Dr. Thabet: A subsequent CT scan of the abdomen and pelvis, obtained after the administration of intravenous and oral contrast material, shows persistent splenomegaly (Fig. 1C, 1D, and 1E). In addition, multiple wedge-shaped areas of hypoenhancement are shown in the periphery of the spleen, features that are consistent with splenic infarcts. Other findings were unchanged. Dr. Hunt: There are many causes of splenic infarction (Table 3), and it is tempting to broaden the differential diagnosis again to include other causes, but I think this would be an error. Before extensive and expensive additional testing is ordered, we should determine whether splenic infarction is consistent with our leading diagnosis.
Splenic infarction
The majority of patients with splenic infarction present with pain in the left upper quadrant or
september 23, 2010
nejm.org
1271
The New England Journal of Medicine Downloaded from nejm.org on March 5, 2012. For personal use only. No other uses without permission. Copyright 2010 Massachusetts Medical Society. All rights reserved.
The
n e w e ng l a n d j o u r na l
of
m e dic i n e
Table 3. Differential Diagnosis of Splenic Infarction.* Myeloid disorders Myeloproliferative neoplasms (myelofibrosis, polycythemia vera, essential thrombocythemia) Myelodysplastic syndromes Acute leukemias Thromboembolic events Cardioembolic origin Hypercoagulable states Antiphospholipid syndrome Malignant conditions Lymphomas Hemoglobinopathy Conditions with marked splenomegaly Trauma Wandering spleen Infection Infective endocarditis Infectious mononucleosis Cytomegalovirus Malaria * Data are from Antopolsky et al.,10 Goerg and Schwerk,11 and Nores et al.12
Diagnosing this patients illness is challenging and underscores the importance of appropriately framing the most prominent features of the illness, carefully reviewing all the imaging and laboratory data, and focusing further testing on the diagnoses of greatest clinical probability. I think that a diagnosis of infectious mononucleosis could have been made in this case by no later than the third hospital day, on the basis of a subacute illness in a 30-year-old woman with fever, splenomegaly, lymphadenopathy, hepatitis, and a normal white-cell count with evolving lymphocytosis, despite the negative heterophile-antibody test. Testing for antibodies to EBV-specific viral capsid antigen and EBV nuclear antigen proteins should be performed; the presence of IgM antibodies to EBV-specific viral capsid antigen would be diagnostic.
Cl inic a l Di agnosis
Acute EpsteinBarr viral infection.
Dr . Da niel Hun t s Di agnosis
Acute EpsteinBarr viral infection.
left flank or both, although a surprising percentage of patients are asymptomatic.10 The majority of patients with splenic infarction have elevated lactate dehydrogenase levels, and about a quarter of them have fever and chills. Some patients have splenic infarction with an infectious cause, such as EBV.10 An understanding that infectious mononucleosis has been associated with splenic infarction13-16 should prompt repeated or more sensitive testing for mononucleosis before alternative diagnoses are pursued.
Infectious Mononucleosis
Pathol o gic a l Discussion
Dr. Eric S. Rosenberg: There are three testing strategies for the diagnosis of acute EBV infection. The most common is to test for the presence of heterophile antibodies. These heterogeneous, predominantly IgM antibodies do not directly target EBV but, for reasons not completely understood, can be detected at some time in approximately 90% of cases of acute EBV infection.20 Heterophile antibodies may persist for several months but typically wane within 1 year. Testing for heterophile antibodies was negative in this patient, suggesting one of three possibilities: she does not have acute EBV infection, she has EBV infection but heterophile antibodies have not yet been generated, or she is one of the 10% of persons with EBV infection who do not have a heterophile response. Since the heterophile-antibody test was negative but suspicion for EBV infection was high, the next tests for EBV involved measuring EBVspecific antibodies and EBV DNA. On the sixth hospital day, EBV-specific serologic samples were sent out, and the results were reported 4 days
If the diagnosis is infectious mononucleosis, does this account for the other features of this patients illness? Elevation of aminotransferase levels occurs in 50 to 80% of patients with mononucleosis17 and in up to 3% of patients with hemolytic anemia. In one series, anticardiolipin antibodies, noted in this patient, were detected in the serum of 30% of patients with infectious mononucleosis.18 Furthermore, splenic infarction has been associated with anticardiolipin antibodies and infectious mononucleosis.19
1272
n engl j med 363;13
nejm.org
september 23, 2010
The New England Journal of Medicine Downloaded from nejm.org on March 5, 2012. For personal use only. No other uses without permission. Copyright 2010 Massachusetts Medical Society. All rights reserved.
case records of the massachusetts gener al hospital
later. IgM antibody against the viral capsid antigen was present, and IgG antibody against the viral capsid antigen was absent. IgM antibody against viral capsid antigen is typically present for 4 to 8 weeks after infection, and the detection of IgM is diagnostic of acute EBV infection.20 Testing for antibody against EBV nuclear antigen was negative, which is also consistent with acute EBV infection, since this antibody is typically generated several weeks into the course of infection. EBV nucleic acid was detectable (1000 DNA copies per milliliter of plasma), further supporting the diagnosis of acute EBV infection. Ten months after presentation, repeat serologic testing was performed, and IgG antibodies against viral capsid antigen and EBV nuclear antigen were present, indicating that seroconversion had occurred and confirming the diagnosis of acute EBV infection. Dr. Hawthorne, what happened with this patient? Dr. Katie M. Hawthorne (Medicine): After the diagnosis of infectious mononucleosis was made, the patient reported that she had a new boyfriend, which is the most likely explanation for her acquisition of EpsteinBarr virus infection. She remained afebrile after hospital day 2, but the pain in her left upper quadrant persisted. During the next several days, the results of liver-function tests peaked and then began a downward trend. The pain diminished slightly, and the patient was discharged with narcotics for pain control. As an outpatient, she continued to have pain in the left upper quadrant but was able to switch from oxycodone to ibuprofen for pain relief. A repeat CT scan obtained 3 months after discharge showed that the spleen had returned to a normal size, with almost complete resolution of all focal
References
1. Yamamoto W, Kono H, Maekawa M,
defects. Results of liver-function tests and the hematocrit have subsequently returned to normal. A Physician: How often do you see acute EBV infection without atypical lymphocytes? Dr. Hunt: One study showed that atypical lymphocytosis, defined as the presence of more than 10% atypical lymphocytes, was absent in 47% of patients who had infectious mononucleosis and a positive test for heterophile antibodies.21 A Physician: If the heterophile-antibody test is negative, do you recommend performing it again? Do you recommend performing EBV-specific serologic testing? Dr. Rosenberg: In patients with an uncomplicated mononucleosis syndrome, I would recommend waiting a week and then repeating the heterophile test. In patients with more serious illness, it is appropriate to perform EBV-specific serologic testing. A Physician: Since the patient had detectable anticardiolipin antibodies and splenic infarcts, would you recommend anticoagulation? Dr. Hunt: No. We do not know enough about this condition to say that there is a substantial risk of thrombosis, so I would not recommend anticoagulation for this patient.
PATHOL O GIC A L DI AGNOSIS
Acute EpsteinBarr viral infection (infectious mononucleosis).
This case was presented at the Medical Case Conference, December 18, 2009. Dr. Rosenberg reports serving on the paid advisory board of Viral Genetics, as a paid consultant to T2 Biosystems, and as a scientific advisor to TBS Technologies and having equity ownership in TBS Technologies. No other potential conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Fukui T. The relationship between abdominal pain regions and specific diseases: an epidemiologic approach to clinical practice. J Epidemiol 1997;7:27-32. 2. Sala E, Watson CJ, Beadsmoore C, et al. A randomized, controlled trial of routine early abdominal computed tomography in patients presenting with non-specific acute abdominal pain. Clin Radiol 2007;62:961-9. 3. Ng CS, Watson CJE, Palmer CR, et al. Evaluation of early abdominopelvic computed tomography in patients with acute abdominal pain of unknown cause: pro-
spective randomized study. BMJ 2002;325: 1387-90. 4. Lamris W, van Randen A, van Es HW, et al. Imaging strategies for detection of urgent conditions in patients with acute abdominal pain: diagnostic accuracy study. BMJ 2009;338:b2431. 5. Silen W. Copes early diagnosis of the acute abdomen, 15th ed. New York: Oxford University Press, 1979. 6. Bell AT, Fortune B, Sheeler R. Clinical inquiries: what test is the best for diagnosing infectious mononucleosis? J Fam Pract 2006;55:799-802. 7. Wallach JB. Interpretation of diagnos-
tic tests. 8th ed. Philadelphia: Lippincott Williams & Wilkins, 2007. 8. Bruu AL, Hjetland R, Holter E, et al. Evaluation of 12 commercial tests for detection of Epstein-Barr virus-specific and heterophile antibodies. Clin Diagn Lab Immunol 2000;7:451-6. 9. Gulley ML, Tang W. Laboratory assays for Epstein-Barr virus-related disease. J Mol Diagn 2008;10:279-92. 10. Antopolsky M, Hiller N, Salameh S, Goldshtein B, Stalnikowicz R. Splenic infarction: 10 years of experience. Am J Emerg Med 2009;27:262-5. 11. Goerg C, Schwerk WB. Splenic infarc-
n engl j med 363;13
nejm.org
september 23, 2010
1273
The New England Journal of Medicine Downloaded from nejm.org on March 5, 2012. For personal use only. No other uses without permission. Copyright 2010 Massachusetts Medical Society. All rights reserved.
case records of the massachusetts gener al hospital
tion: sonographic patterns, diagnosis, follow-up, and complications. Radiology 1990; 174:803-7. 12. Nores M, Phillips EH, Morgenstern L, Hiatt JR. The clinical spectrum of splenic infarction. Am Surg 1998;64:182-8. 13. Breuer C, Janssen G, Laws HJ, et al. Splenic infarction in a patient with hereditary spherocytosis, protein C deficiency and acute infectious mononucleosis. Eur J Pediatr 2008;167:1449-52. 14. Suzuki Y, Shichishima T, Mukae M, et al. Splenic infarction after Epstein-Barr virus infection in a patient with hereditary spherocytosis. Int J Hematol 2007;85:380-3.
15. Symeonidis A, Papakonstantinou C,
Seimeni U, et al. Non hypoxia-related splenic infarct in a patient with sickle cell trait and infectious mononucleosis. Acta Haematol 2001;105:53-6. 16. Trevenzoli M, Sattin A, Sgarabotto D, Francavilla E, Cattelan AM. Splenic infarct during infectious mononucleosis. Scand J Infect Dis 2001;33:550-1. 17. Jenson HB. Acute complications of Epstein-Barr virus infectious mononucleosis. Curr Opin Pediatr 2000;12:263-8. 18. Sorice M, Pittoni V, Griggi T, et al. Specificity of anti-phospholipid antibodies in infectious mononucleosis: a role for
anti-cofactor protein antibodies. Clin Exp Immunol 2000;120:301-6. 19. van Hal S, Senanayake S, Hardiman R. Splenic infarction due to transient antiphospholipid antibodies induced by acute Epstein-Barr virus infection. J Clin Virol 2005;32:245-7. 20. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med 2010;362: 1993-2000. 21. Ventura KC, Hudnall SD. Hematologic differences in heterophile-positive and heterophile-negative infectious mononucleosis. Am J Hematol 2004;76:315-8.
Copyright 2010 Massachusetts Medical Society.
Lantern Slides Updated: Complete PowerPoint Slide Sets from the Clinicopathological Conferences
Any reader of the Journal who uses the Case Records of the Massachusetts General Hospital as a teaching exercise or reference material is now eligible to receive a complete set of PowerPoint slides, including digital images, with identifying legends, shown at the live Clinicopathological Conference (CPC) that is the basis of the Case Record. This slide set contains all of the images from the CPC, not only those published in the Journal. Radiographic, neurologic, and cardiac studies, gross specimens, and photomicrographs, as well as unpublished text slides, tables, and diagrams, are included. Every year 40 sets are produced, averaging 50-60 slides per set. Each set is supplied on a compact disc and is mailed to coincide with the publication of the Case Record. The cost of an annual subscription is $600, or individual sets may be purchased for $50 each. Application forms for the current subscription year, which began in January, may be obtained from the Lantern Slides Service, Department of Pathology, Massachusetts General Hospital, Boston, MA 02114 (telephone 617-726-2974) or e-mail Pathphotoslides@partners.org.
1274
n engl j med 363;13
nejm.org
september 23, 2010
The New England Journal of Medicine Downloaded from nejm.org on March 5, 2012. For personal use only. No other uses without permission. Copyright 2010 Massachusetts Medical Society. All rights reserved.
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Rectal VaricesDocument4 pagesRectal VaricesAv RienNo ratings yet
- PMN ApoptosisDocument9 pagesPMN ApoptosisAv RienNo ratings yet
- Hematemesis PDFDocument7 pagesHematemesis PDFkevin_jawanNo ratings yet
- Hematemesis PDFDocument7 pagesHematemesis PDFkevin_jawanNo ratings yet
- Acute Pancreatitis: To The EditorDocument1 pageAcute Pancreatitis: To The EditorAv RienNo ratings yet
- Necrotizing PancreatitisDocument2 pagesNecrotizing PancreatitisAv RienNo ratings yet
- Acute Pancreatitis: To The EditorDocument1 pageAcute Pancreatitis: To The EditorAv RienNo ratings yet
- Case 35-2003: A 75-Year-Old Man With A Cystic Lesion of The PancreasDocument8 pagesCase 35-2003: A 75-Year-Old Man With A Cystic Lesion of The PancreasAv RienNo ratings yet
- Acute Pancreatitis: To The EditorDocument1 pageAcute Pancreatitis: To The EditorAv RienNo ratings yet
- Acute Pancreatitis: To The EditorDocument1 pageAcute Pancreatitis: To The EditorAv RienNo ratings yet
- Case 35-2003: A 75-Year-Old Man With A Cystic Lesion of The PancreasDocument8 pagesCase 35-2003: A 75-Year-Old Man With A Cystic Lesion of The PancreasAv RienNo ratings yet
- Necrotizing PancreatitisDocument2 pagesNecrotizing PancreatitisAv RienNo ratings yet
- Komplikasi CharlesDocument2 pagesKomplikasi CharlesAv RienNo ratings yet
- Recurrent PancreatitisDocument9 pagesRecurrent PancreatitisAv RienNo ratings yet
- Komplikasi CharlesDocument2 pagesKomplikasi CharlesAv RienNo ratings yet
- Fever and Abd - PainDocument9 pagesFever and Abd - PainAv RienNo ratings yet
- Necrotizing PancreatitisDocument2 pagesNecrotizing PancreatitisAv RienNo ratings yet
- AnemiaDocument11 pagesAnemiaAv RienNo ratings yet
- Pancreatitic CADocument13 pagesPancreatitic CAAv RienNo ratings yet