Professional Documents
Culture Documents
Food Research International: Luis C. Carrillo, Julián Londoño-Londoño, Andrés Gil
Uploaded by
Laura NogueraOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Food Research International: Luis C. Carrillo, Julián Londoño-Londoño, Andrés Gil
Uploaded by
Laura NogueraCopyright:
Available Formats
FRIN-04694; No of Pages 8
Food Research International xxx (2013) xxxxxx
Contents lists available at SciVerse ScienceDirect
Food Research International
journal homepage: www.elsevier.com/locate/foodres
Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia
Luis C. Carrillo, Julin Londoo-Londoo, Andrs Gil
Grupo de Investigacin en Sustancias Bioactivas, Sede de Investigacin Universitaria, Universidad de Antioquia, Carrera 53 # 61-30 Lab 229, A.A. 1226 Medelln, Colombia
a r t i c l e
i n f o
a b s t r a c t
The aims of the current work were to study the inuence of geographical areas on polyphenol and methylxanthine contents and the antioxidant activity in cocoa beans from different cocoa-growing areas of Colombia, and to evaluate the possibility of establishing a classication based on the geographical areas of the cocoagrowing regions. Eighteen cocoa farms located in eleven cocoa-growing areas were analyzed. The statistical analysis showed signicant differences (p b 0.05) in total polyphenol content (TPC), avan-3-ol, epicatechin, catechin, caffeine and theobromine contents as well as the theobromine/caffeine ratio and the antioxidant capacity between some of the different sampled farms, showing a signicant effect of the cocoa-producing region on these parameters. Generally, a proportional relationship has been proposed to exist between the polyphenol content with changes in altitude of plant crops, despite this, our results suggest that the lower the altitude, the more polyphenols, avan-3-ols and epicatechin are produced by the cocoa plant. Results of the principal component analysis (PCA) indicate that caffeine content and the theobromine/caffeine ratio could serve as parameters to establish a cocoa bean classication according to the geographical area of the cocoa-growing regions. 2013 Elsevier Ltd. All rights reserved.
Article history: Received 1 April 2013 Received in revised form 27 May 2013 Accepted 20 June 2013 Available online xxxx Keywords: Principal component analysis Growing areas Polyphenols Methylxanthines Antioxidant activity
1. Introduction New trends in food market show a steady increase in the consumption of cocoa products, since they have wide acceptance by consumers and also present multiple health benets due to the presence of polyphenols that possess high antioxidant capacity (Belscak, Komes, Horzic, Ganic, & Karlovic, 2009). Three main polyphenol groups can be distinguished in cocoa: catechins, anthocyanins and procyanidins, whose common characteristic is a avonoid-type structure (Wollgast & Anklam, 2000). These compounds have generally received special attention because of their physiological functions, that include modulation of eicosanoid synthesis, increase in nitric oxide synthesis, inhibition of oxidation of low density lipoprotein (LDL), inhibition of platelet activation, stimulation of anti-inammatory cytokine production, and inhibition of pro-inammatory cytokine production (Niemenak, Rohsius, Elwers, Ndoumou, & Lieberei, 2006; Othman, Ismail, Ghani, & Adenan, 2007). In addition, some epidemiological studies have established an inverse correlation between avonoid intake and the appearance of chronic diseases, such as cardiovascular and cerebrovascular diseases, cancer and other disorders such as diabetes and rheumatoid arthritis (Othman et al., 2007; Wollgast & Anklam, 2000). Although most of the studies indicate that the health benets of cocoa or
Corresponding author at: Grupo de Investigacin en Sustancias Bioactivas, Sede de Investigacin Universitaria, Universidad de Antioquia, Carrera 53 # 61-30 Lab 229, A.A. 1226 Medelln, Colombia. Tel.: +57 4 219 6591; fax: +57 4 2196590. E-mail address: angilqu@gmail.com (A. Gil). 0963-9969/$ see front matter 2013 Elsevier Ltd. All rights reserved. http://dx.doi.org/10.1016/j.foodres.2013.06.019
cocoa by-products are attributable to polyphenols, it should be noted that these are not only rich in polyphenols, but are also rich in methylxanthines, that account for about 3.2% of defatted unsweetened chocolate composition (Belscak et al., 2009). The main methylxanthines of cocoa are theobromine (3.7% on a fat-free basis) and caffeine (about 0.2%) and they have been associated with some physiological effects on various body systems, including the central nervous, gastrointestinal, respiratory, and renal systems (Li et al., 2012). Nevertheless, the possible synergistic interactions between avonoids and methylxanthines in cocoa products regarding health benets remain unclear and demand further studies. However, it is clear that the polyphenol and methylxanthine compounds are responsible for the astringent and bitter taste and affect the stability and digestibility of cocoa (Brunetto et al., 2007; Li et al., 2012). Cocoa is one of the crops of top economic interest in several countries such as the Ivory Coast, Ghana, Cameroon, Indonesia, Colombia, Venezuela and Ecuador. It is native of the New World, with probable origin lying in the upper basin of the Amazon River (Cuatrecasas, 1964; Motamayor et al., 2002). In recent years, confusion has arisen in the taxonomic classication of commercial cocoa, due to its genetic variability related to phenotypical characteristics such as color, shape and dimension of various parts of the ower, fruit and seed. Nevertheless, it is accepted that most of the commercial cocoa belongs to a single species (Theobroma cacao L.), which includes three genetic complexes: Criollo, Forastero/Amaznico, and Trinitario (Cuatrecasas, 1964; Motamayor et al., 2002; N'Goran, Laurent, Risterucci, & Lanaud, 1994). Currently, the cocoa crop in Colombia has a high genetic variability due to the sowing of ecotypes derived from crosses between Forastero/
Please cite this article as: Carrillo, L.C., et al., Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia, Food Research International (2013), http://dx.doi.org/10.1016/j.foodres.2013.06.019
L.C. Carrillo et al. / Food Research International xxx (2013) xxxxxx Table 1 Cocoa producer, geographic location, and regional climate conditions from different cocoa-growing regions. Cultivar code aL_1 aL_2 aL_3 aSH aVC aCA aGT bS_1 bS_2 bAR bTA_1 bTA_2 bVM bGM_1 bGM_2 cCT_1 cCT_2 cCT_3
a
Amaznico and Trinitario clones. Moreover, ecological conditions vary greatly in Colombia and the cocoa-growing regions are very scattered through its territory. In addition, the wide variety of microclimates around the country, leads to cocoa production with high variability in its chemical composition. These phenomena have been observed in other food products such as olive oil, honey, and essential oils of pine (Baltrusaityte, Venskutonis, & Ceksteryte, 2007; Semiz, Heijari, Isik, & Holopainen, 2007; Temime, Campeol, Cioni, Daoud, & Zarrouk, 2006), in which the chemical composition is inuenced by changes in the environmental culture conditions (climatic and edaphological). This means that both the production area and the genotypic variations are largely responsible for the quality characteristics of the nal food products, delivered to the consumer (Criado, Morello, Motilva, & Romero, 2004) including the cocoa by-products. The latter characteristics provide Colombia with the capability of producing various ecotypes of cocoa with different bioactive and avor proles. Nonetheless, to date, the ideal edapho-climatic conditions for the production of high quality cocoa beans, in terms of chemical composition and organoleptic properties remain unknown. The establishment of these conditions could contribute to strengthening the Denomination of Origin concept in cocoa by-products. Therefore, the objectives of the current work were to study the inuence of geographical areas on the behavior of the polyphenol and methylxanthine contents as well as the antioxidant activity in cocoa beans of different cocoa-growing areas, and to evaluate the possibility of establishing a classication according to the geographical area of the cocoa-growing regions, thus trying to contribute to a Colombian cocoa traceability. 2. Materials and methods 2.1. Chemicals and reagents All solvents were of analytical or HPLC grade and supplied by Merck Chemicals (Darmstadt, Germany). Deionized water was obtained with a Milli-Q water purication system (Millipore, Bedford, MA, USA). Standard compounds such as (+)-catechin, ()-epicatechin, and theobromine were purchased from ChromaDex (Irvine, California, USA); caffeine, gallic acid and Trolox (6-hydroxy-2,5,7,8tetramethylchromane-2-carboxylic acid) were obtained from SigmaAldrich (St. Louis, MO, USA), as well as other reagents such as uorescein, DPPH (2,2-diphenyl-1-picrylhydrazyl), AAPH (2,2-azobis (2-methylpropionamidine)dihydrochloride) and FolinCiocalteu's reagent. Vanillin and sulfuric acid were also purchased from Merck Chemicals (Darmstadt, Germany). 2.2. Samples For this study, commercially available sun-dried cocoa beans were used. Random sampling in eighteen farms (samples) with similar agricultural practices was carried out. The farms are located in eleven cocoa-growing areas scattered in three of the main cocoa-producing regions throughout Colombia (Table 1 and Fig. 1). These geographical zones (Andina (a), Orinoco (b) and Pacco (c) region) were chosen because they present clear differences in their edaphological and climatic conditions; according to the Holdridge Life Zones System (a global bioclimatic scheme for the classication of land areas) these zones can be classied as tropical wet forest, tropical moist forest and tropical rain forest, respectively (Lugo, Brown, Dodson, Smith, & Shugart, 1999). Only healthy beans in the same sun-drying processing stage, without any infection or physical damage, were processed. During sampling, the beans were washed, ground in a rotating blade mill and the powder obtained was nally stored at 20 C until sample preparation. In this study, each sample was carried out in triplicate using the procedures mentioned below, and each value
Zone
Latitude
Longitude
Altitude (masl)a 968 968 968 1426 750 1271 289 800 800 165 340 340 410 410 410 2 2 2
Average temperature (C) 20 20 20 20 26 23 33 25.6 25.6 29 28 28 27 25 25 29 29 29
a a a a a a a b b b b b b b b c c c
6 13 26 N 6 13 26 N 6 13 26 N 6 20 0 N 05 19 0 N 5 21 0 N 4 18 0 N 6 53 0 N 6 53 0 N 7 1 0 N 6 27 0 N 6 27 0 N 4 9 0 N 3 33 0 N 3 33 0 N 1 48 0 N 1 48 0 N 1 48 0 N
073 48 46 W 073 48 46 W 073 48 46 W 73 36 0 W 74 54 0 W 74 30 0 W 74 48 0 W 71 54 0 W 71 54 0 W 71 25 0 W 71 44 0 W 71 44 0 W 73 38 0 W 73 42 0 W 73 42 0W 78 45 0 W 78 45 0 W 78 45 0 W
Meters above sea level.
presented in the Results and discussion section is the mean of six to nine data standard deviation (SD). 2.3. Sample preparation The powdered cocoa samples were extracted as follows. The dried and ground sample was weighed (100 mg), degreased by adding 2 mL of hexane and centrifuged at 1565 g for 10 min, decanted and another 2 mL of hexane was added repeating the procedure three times. The fat-reduced cocoa powder was then extracted at room temperature with 1.5 mL of aqueous 2-propanol (60%, pH 9.0) for 60 min in an ultrasonic bath. After extraction, the mixture was centrifuged for 10 min at 391 g at 4 C. The supernatant was decanted and ltered through a nylon membrane (0.45 m). Finally, the supernatant was placed in a ask and lled to obtain 2 mL of extract, and stored in the dark at 20 C until used. 2.4. Determination of total polyphenol content The total polyphenol content (TPC) of cocoa extracts was determined spectrophotometrically according to a modied method proposed by Londoo, Montoya, Guerrero, Aristizabal, and Arango (2006). Briey, 30 L of previously prepared extract, 15 L Folin Ciocalteu's reagent, 225 L distilled water and 30 L of 20% Na2CO3 were mixed. After 1 h the absorbance of the blue color was measured at 760 nm against a blank sample. A calibration curve with gallic acid as standard was produced. The TPC is expressed as milligram equivalent of gallic acid (mg GAE) per gram of dried sample material (mg GAE/g dried sample). All measurements were performed in triplicate. 2.5. Flavan-3-ol content by vanillin assay Cocoa extracts were analyzed for their avan-3-ol content using a method described by Belscak et al. (2009), with some modications. Previously prepared extracts (200 L) were lled up to 1 mL with methanol. The total volume of the reaction medium was xed at 1 mL, comprising 166.7 L of sample (cocoa extract) or standard, 416.7 L of 1% vanillin working solution (prepared daily in methanol) and 416.7 L of H2SO4 9.0 N in methanol. The mixture was allowed to react for 5 min, in an ice bath, after which absorbance readings were taken at 500 nm. Two blanks were prepared: 1) replacing the sample
Please cite this article as: Carrillo, L.C., et al., Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia, Food Research International (2013), http://dx.doi.org/10.1016/j.foodres.2013.06.019
L.C. Carrillo et al. / Food Research International xxx (2013) xxxxxx
Fig. 1. Map of sampling zones a, b and c for cocoa beans.
by more 1% vanillin, and 2) replacing the vanillin solution by methanol. Absorbance of the blanks was subtracted from the absorbance of the corresponding extract and vanillin-containing sample. A calibration curve with catechin as standard was produced. The content of avan-3-ols is expressed as milligram equivalent of catechin per gram of dried sample (mg CE/g dried sample). All measurements were performed in triplicate. 2.6. HPLC analysis of catechins and methylxanthines HPLC analyses were carried out using an Agilent 1200 Series Rapid Resolution Liquid Chromatography system (Agilent Technologies, Palo Alto, CA, USA), equipped with a vacuum degasser, an autosampler, a quaternary pump and a Diode-Array Detector (DAD). The samples were centrifuged for 15 min at 10581 g and 4 C and then ltered through a 0.45 m lter (Nylon Membranes, Supelco, Bellefonte, USA) before HPLC analysis. To separate the compounds, the ow rate used varied between 0.75 and 1.0 mL/min and the analysis was carried out at 30 C. A Supelco Ascentis C18 (25 cm 4.6 mm, with a 5 m particle size) column was used for the chromatographic separation. The mobile phase used was water with 0.1% acetic acid as eluent A and methanol as eluent B. The optimal chromatographic method consisted in the following linear gradient: 0 min, 6% A; 7 min, 25% A; following isocratically until 30 min, and nally a conditioning cycle of 3 min with the initial conditions for the next analysis. The injection volume in the HPLC system was 10 L. The compounds separated were monitored with DAD set at 280 nm and peak spectra were recorded between 200 and 400 nm. Catechins (catechin and epicatechin) and methylxanthines (theobromine and caffeine) were identied by comparing the retention times and spectral data to those of standards. All analyses were repeated three times. 2.7. Determination of antioxidant capacity by ORAC The Oxygen Radical Absorbance Capacity (ORAC) assay was carried out according to a procedure described by Ou, Hampsch-Woodill, and Prior (2001), slightly modied. For ORAC assessment, all reagents were prepared in 10 mM phosphate buffer (pH 7.4), as well as the original cocoa extracts that were diluted as appropriate. Sample (25 L) and uorescein (150 L; 1 M, nal concentration) solutions were placed in the well of the microplate. The mixture was preincubated for 30 min at
37 C. AAPH solution (25 L; 250 mM, nal concentration) was rapidly added using a multichannel pipette. The microplate was immediately placed in the reader and the uorescence recorded every 2 min for 120 min at excitation and emission wavelengths of 485 and 520 nm, respectively. The microplate was automatically shaken prior to each reading. A blank (uorescein + AAPH) using phosphate buffer instead of the sample solution and ve calibration solutions using Trolox (0200 M, nal concentration) as antioxidant were also carried out in each assay. Regression equations between the area under the uorescence decay curve (AUC) and antioxidant concentration were calculated for all the Trolox standard solutions. All the reaction mixtures were prepared in duplicate, and at least three independent assays were performed for each sample. Final ORAC values are expressed as Trolox equivalents using the standard Trolox curve calculated for each assay (mol of Trolox equivalent/100 g of dried cocoa sample).
2.8. Statistical analysis and chemometric application All tests were performed in triplicate. Data are presented as means SD. The statistical signicance of differences between groups was evaluated by one-way ANOVA using the GraphPad Prism version 5.0 for Windows (GraphPad Software, San Diego, CA, USA). The differences between the means were assessed using the NewmanKeuls multiple comparison post-test and the signicance was identied at 5% level. The variables taken into account during the development of this study were subjected to multivariate analysis (principal component analysis (PCA)) performed using Statgraphics Centurion XVI Software (Version 16.0.07 for Windows, Statpoint Technologies, Inc). PCA was applied to separate the samples (n = 18) according to their values of total polyphenol content (TPC), avan-3-ols (FLV), epicatechin (EPI), catechin (CAT), caffeine (CAF) and theobromine (TEO) contents, ORAC values, as well as the theobromine/caffeine ratio (TE/CA) and the agroclimatic conditions of temperature (TEMP) and altitude (ALT) of the crops. In the PCA, the data set consisted of a 18 10 matrix, where the results obtained for each parameter were adopted as columns and the cocoa farms (samples) were used as the rows. Analyses were based on correlations and variances were computed as SS/(n 1). Eigenvalues higher than 1.0 were adopted to explain the projection of the assays on the factor-plane. All data were autoscaled before the analysis, which means that each column data matrix was mean-centered
Please cite this article as: Carrillo, L.C., et al., Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia, Food Research International (2013), http://dx.doi.org/10.1016/j.foodres.2013.06.019
L.C. Carrillo et al. / Food Research International xxx (2013) xxxxxx
and scaled to unit variance and avoid the effect of different scales of the variables (Cruz et al., 2013). 3. Results and discussion 3.1. Total polyphenol content (TPC) and avan-3-ol content of cocoa extracts The sample preparation was designed to obtain an enriched fraction of polyphenol compounds and methylxanthines, according to Niemenak et al. (2006). These authors established that the use of acetonewater at 60:40 v/v as solvent makes it possible to obtain maximum recovery of polyphenols and xanthines present in cocoa beans. Our extraction step was then optimized in order to be exhaustive and to simultaneously recover the two classes of metabolites. Some changes such as the type of solvent, the pH of the medium, the amount of sample as well as the duration of the extraction procedure and the use of ultrasonic-assisted extraction, allowed us to reach the highest concentrations of polyphenols and xanthines present in extracts of cocoa beans and cocoa by-products (data not shown). Table 2 presents the TPC and avan-3-ol content of defatted cocoa powder determined in aqueous 2-propanol extracts of eighteen cocoa farms tested. The TPC of extracts ranged from 44.940 1.174 to 70.090 1.988 mg GAE/g, while the avan-3-ol content of the same cocoa extracts ranged from 2.496 0.388 to 11.240 0.825 mg CE/g. The TPC and avan-3-ol content in the different cocoa farms decreased in the order: aL_1 N bAR N cCT_1 N cCT_2 N aVC N bS_2 N cCT_3 N bGM_2 N aGT N aL_3 N aSH N aL_2 N bTA_2 N bS_1 N bVM N bGM_1 N aCA N bTA_1 and bAR N aL_1 N cCT_2 N cCT_1 N bTA_2 N bS_1 N bS_2 N aGT N cCT_3 N bGM_2 N aVC N aL_3 N aSH N bVM N aL_2 N bGM_1 N bTA_1 N aCA for TPC and avan-3-ol content, respectively (Table 2), showing that both variables are highly correlated (Pearson r = 0.897). In addition, statistical analyses showed a signicant difference (p b 0.05) in TPC and avan-3-ol content between some of the farms sampled. These results are consistent with those reported by Othman et al. (2007), who found a signicant effect of the cocoa-producing region on the content of the polyphenol compounds that was highly correlated with its antioxidant activity. Another study discussed the different polyphenol contents in cocoa from the Ivory Coast, Equatorial Guinea, Venezuela, Peru, the Dominican Republic, Ecuador and Colombia (Toms-Barbern et al., 2007). These authors found that Colombian cocoa was the third richest in TPC (81.4 mg GAE/g) among the countries under study. This value is higher
than the ones obtained in our study, but it is known that TPC varies depending on the variety of the cocoa beans, degree of ripeness (harvest season) and more importantly, the geographical origin and post-harvest conditions, such as fermentation, drying, roasting, processing and storage (Wollgast & Anklam, 2000), that contribute to major discrepancies between results. Some authors have proposed that a proportional relationship exists between the content and the activity of compounds that are part of plant's antioxidant defense system (polyphenols) with changes depending on the altitude of crop growth (Criado et al., 2004), variations that have also been shown to enhance synthesis of phenylalanine ammonia lyase (PAL) in plants, leading to increased production of phenolics, including avonoids (Kishore, Ranjan, Pandey, & Gupta, 2010). As far as we are concerned, these arguments do not seem to apply to cocoa beans, because our results (Table 2) suggest that the lower the altitude, the more polyphenols and avan-3-ols are produced by the plant. This subject is discussed below. 3.2. High-performance liquid chromatography (HPLC) of cocoa extracts HPLC with UV detection at 280 nm has been the primary analytical method for the quantication of catechins and procyanidins in cocoa samples (Wollgast & Anklam, 2000). According to the literature data (Liu et al., 2006; Markham & Bloor, 1998), the addition of acids to the mobile HPLC phase improves the separation of the polyphenols since it reduces the ionization of both phenolic hydroxyl and carboxyl groups. In this study, using methanol and diluted acetic acid as solvent, two avan-3-ol compounds and two methylxanthines were identied by their retention times, HPLCUV spectra (280 nm), and chromatographic comparisons with primary standards (Fig. 2). The results showed no qualitative differences in chromatographic proles between cocoa extracts from different geographical areas of growth. However, signicant quantitative differences in catechins and methylxanthines were observed. As a whole, methylxanthines (theobromine and caffeine) were found in higher concentration than catechins, theobromine being the predominant compound in the extracts (Table 2). The highest content of theobromine was 9.679 0.273, and the lowest was 7.062 0.123 mg/g of defatted cocoa powders. The theobromine content in the different cocoa-growing farms decreased in the order of bAR N cCT_2 N bTA_1 N aCA N aL_1 N bGM_1 N aL_3 N bGM_2 N aGT N aL_2 N cCT_1 N aSH N bTA_2 N bVM N aVC N cCT_3 N bS_2 N bS_1. In comparison with theobromine, the content of caffeine was lower,
Table 2 Antioxidant capacity and content of methylxanthine and polyphenol compounds in cocoa beans from different cultivars. Cultivar code aL_1 aL_2 aL_3 aSH aVC aCA aGT bS_1 bS_2 bAR bTA_1 bTA_2 bVM bGM_1 bGM_2 cCT_1 cCT_2 cCT_3 TPC (mg GAE/g) 70.090 52.880 53.410 53.170 56.540 45.320 53.780 52.690 56.340 65.050 44.940 52.860 47.590 47.000 53.820 61.930 61.650 53.960 1.988a 2.062def 3.679de 0.165de 1.507cde 1.152efg 1.434de 3.325def 3.399cde 1.314abc 1.174fg 1.194def 1.947efg 2.319efg 0.470de 3.558bcd 0.778bcd 5.778de Flavan-3-ols (mg CE/g) 11.240 3.842 5.245 4.405 5.456 2.496 6.781 7.407 6.856 11.290 3.763 7.439 4.121 3.765 6.058 7.775 7.841 6.168 0.825a 0.739cd 0.520bcd 0.714cd 0.346bcd 0.388cd 0.506bc 0.945bc 0.833bc 0.451a 0.411cd 0.692bc 0.886cd 0.553cd 0.439bc 1.399bc 0.322bc 3.019bc Epicatechin (mg/g) 3.562 2.284 2.019 1.970 2.516 1.405 2.395 2.342 2.414 3.787 1.497 2.516 2.150 2.388 2.570 2.862 2.831 2.105 0.016a 0.112cde 0.064de 0.071de 0.156bcd 0.080f 0.057cd 0.126cde 0.130cd 0.070a 0.015f 0.112bcd 0.141cde 0.205cd 0.114bc 0.190bc 0.044bc 0.289de Catechin (mg/g) 1.297 0.377 0.245 0.346 0.286 0.323 0.370 0.431 0.487 0.493 0.226 0.615 0.569 0.391 0.350 0.355 0.342 0.468 0.031a 0.033cd 0.008d 0.013d 0.016d 0.012d 0.010cd 0.023cd 0.044bcd 0.031bc 0.006de 0.022bc 0.027bc 0.021cd 0.023d 0.033cd 0.024d 0.158cd ORAC valuesa 61812 40390 42020 51940 54027 50029 50002 52718 54020 63951 39130 49786 57189 38729 54339 61511 50837 43879 5042ab 3784b 6553b 7472ab 5753ab 6453ab 6868ab 3508ab 6116ab 7384ab 3562b 8320ab 3555ab 1084b 4357ab 7547ab 4677ab 4305b Caffeine (mg/g) 1.330 1.230 1.096 1.184 1.073 1.879 1.435 1.014 0.724 1.148 1.311 1.590 1.126 1.517 1.435 1.630 1.730 1.592 0.028gh 0.021hij 0.038jkl 0.040ij 0.009jkl 0.028a 0.021ef 0.075kl 0.025m 0.016ijk 0.023ghi 0.019cde 0.042jk 0.024def 0.023ef 0.022cd 0.040b 0.009cde Theobromine (mg/g) 8.863 8.581 8.777 8.306 8.242 9.076 8.639 7.062 8.024 9.679 9.109 8.274 8.245 8.824 8.734 8.354 9.510 8.217 0.119cd 0.138de 0.142cd 0.119def 0.057def 0.049bcd 0.094cde 0.123g 0.177ef 0.273ab 0.149bc 0.209def 0.076def 0.155cd 0.127cd 0.217def 0.237abc 0.048def
Data are presented as mean SD (n = 3). Values within a column followed by the same superscript letters are not signicantly different at the 0.05 level according to ANOVA (P b 0.05). a Expressed as (mol TroloxEq / 100 g).
Please cite this article as: Carrillo, L.C., et al., Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia, Food Research International (2013), http://dx.doi.org/10.1016/j.foodres.2013.06.019
L.C. Carrillo et al. / Food Research International xxx (2013) xxxxxx
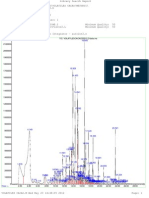
Fig. 2. HPLC chromatogram of cocoa bean extracts, rich in polyphenols and methylxanthines. Retention times of 13.13, 17.40, 23.59 and 31.05 min, correspond to theobromine, (+)-catechin, caffeine and ()-epicatechin respectively. For chromatographic conditions, see Section 2.6.
ranging from 1.879 0.028 to 0.724 0.025 mg/g of defatted cocoa powders, in the following decreasing order aCA N cCT_2 N cCT_1 N cCT_3 N bTA_2 N bGM_1 N aGT = bGM_2 N aL_1 N bTA_1 N aL_2 N aSH N bAR N bVM N aL_3 N aVC N bS_1 N bS_2. It has been proposed that the production area and the genotypic variations, are largely responsible for the quality characteristics of the nal cocoa by-products, in terms of bioactive compounds (Belscak et al., 2009). Regarding this possibility, the high genetic variability of Colombian cocoa due to sowing of ecotypes derived from crosses between Forastero/Amaznico and Trinitario clones became an important issue concerning the classication of cocoa beans used during this study. Nevertheless, it is important to highlight that previously, studies carried out by Brunetto et al. (2007), showed the existence of a relationship between the content in methylxanthines and the genotype of the cocoa. These studies were focused on the theobromine/caffeine relationship, allowing its classication into Forastero, Trinitario and Criollo cocoa. In the present study, the correlation between the cocoa extracts analyzed and the theobromine/caffeine relationship was examined. The results obtained are presented in Fig. 3. Most of the farms sampled produce Trinitario
Fig. 3. Relationship between methylxanthine content and cocoa genotype. The X and Y axes represent the theobromine/caffeine concentration ratio and %caffeine in cocoa beans, respectively.
cocoa beans, except bS_2 which is clearly a Forastero cocoa crop, characterized by high theobromine content and low caffeine concentration, whereas the other samples (Trinitario) contain intermediate theobromine/caffeine levels. Even though aCA is a sample classied as Trinitario cocoa, based on the present results it is also clear that this sample is close to Criollo cocoa mainly due to its low theobromine/ caffeine ratio (Fig. 3). The avan-3-ols, ()-epicatechin and (+)-catechin (also known as catechins) were identied and quantied in cocoa extracts of various cocoa-growing areas, and the results are shown in Table 2. Considerable variations in the concentrations of these compounds in cocoa extracts were observed, depending on the geographical origin of the samples. ()-epicatechin dominated among avan-3-ols, with the highest concentrations ranging from 3.787 0.070 to 1.405 0.080 mg/g of defatted cocoa powders, and decreasing in the following order bAR N aL_1 N cCT_1 N cCT_2 N bGM_2 N bTA_2 aVC N bS_2 N aGT N bGM_1 N bS_1 N aL_2 N bVM N cCT_3 N aL_3 N aSH N bTA_1 N aCA. On the other hand, (+)-catechin concentrations were low, ranging between 1.297 0.031 and 0.226 0.006 mg/g of defatted cocoa powders, and decreased in the order of aL_1 N bTA_2 N bVM N bAR N bS_2 N cCT_3 N bS_1 N bGM_1 N aL_2 N aGT N cCT_1 N bGM_2 N aSH N cCT_2 N aCA N aVC N aL_3 N bTA_1. The comparison of avan-3-ol content in the cocoa beans tested with those obtained by other authors shows some differences. Namely, Niemenak et al. (2006), determined the content of catechins by reversed-phase high-performance liquid chromatography (RP-HPLC), using 60% aqueous acetone as the extraction solvent on freshly harvested seeds of different cocoa pods from different clones, and found higher contents of catechin (1.2514.42 mg/g) and epicatechin (14.4343.90 mg/g) than the ones obtained in the present study. Gotti, Furlanetto, Pinzauti, and Cavrini (2006), used 71% aqueous ethanol as extraction solvent and modied cyclodextrin to quantify catechins in cocoa beans by micellar electrokinetic chromatography, and determined 0.06 0.008 mg/g of catechin and 4.10 0.20 mg/g of epicatechin in Venezuelan cocoa beans, which are in agreement with our results. Ortega et al. (2008), prepared two kinds of phenolic extracts of cocoa beans, the phenol cocoa extract (CE), by solid/liquid extraction, and the puried extract (PCE), by solid phase extraction (SPE). Although they did not mention the extraction solvent used, the results are quite similar to those obtained here; they reported 0.335 0.083 and 0.434 0.055 mg/g of catechin for CE and PCE respectively, while for epicatechin the contents were 3.011 0.053 and 4.314 0.371 mg/g for CE and PCE respectively.
Please cite this article as: Carrillo, L.C., et al., Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia, Food Research International (2013), http://dx.doi.org/10.1016/j.foodres.2013.06.019
L.C. Carrillo et al. / Food Research International xxx (2013) xxxxxx
It is known that the higher the altitude, the less dense is the atmosphere through which UV radiation must pass to reach the soil. UV-B (280315 nm) radiation is a minor component of the solar spectrum, yet it has the potential to disproportionately affect metabolic processes in humans, animals, microorganisms and plants (Jansen et al., 2001). Earlier investigations on wild as well as cultivated plants, showed signicant positive correlations between the altitude of the site of growth and the amounts of certain phenolic antioxidants (Alonso-Amelot, Oliveros-Bastidas, & Calcagnopisarelli, 2007). Such variations with the altitude are assumed to be associated with the enzymes involved in the synthesis of phenolics that are produced in greater quantities or show increased activity at low temperature (Chalker-Scott & Scott, 2004). In tropical conditions, temperature decreases with increase in altitude, but solar UV radiation also changes, increasing with increased altitude and proximity to the sea. Quantitatively, it is known that UV-B increases 1418% per 1.000 m in altitude increase. Therefore, with greater UV radiation at higher altitudes, plants are expected to show increased content of polyphenols and better antioxidant properties (Kishore et al., 2010). Our results regarding TPC, avan-3-ol, catechin and epicatechin contents suggest that cocoa plants may experience the opposite effect, since samples such as cCT_1, cCT_2, bAR and aGT, that are in the lowest altitude range are among the samples whose content of these bioactive compounds is the highest (Table 2). These ndings are in agreement with those obtained by Mateus, Marques, Gonalves, Machado, and De Freitas (2001), who investigated the inuence of the altitude of vineyards on the developmental changes of catechins in seeds and skin of two grape varieties. The results showed that vineyard altitude and its related climate are important factors for grape maturity and varietal avan-3-ol composition. At the end of maturity, low altitude (higher temperature and humidity) appears to be advantageous to produce higher concentrations of catechins, low molecular weight avan-3-ol oligomers, and total extractable procyanidins. Regardless of the vegetable matrix, this could also be happening in the cocoa beans used here, since the main polyphenol compounds produced by the plant are avan-3-ol oligomers and their polymers (procyanidins). Nonetheless, further investigations are required to conclude on this subject. Finally, a recent study has proposed the inuence of the environment and the genotype of native Andean potato, on polyphenol content and antioxidant activity (Andre et al., 2009). According to the results obtained, potato plants can adjust their polyphenol content in response to changing environmental conditions, but more important was the prevailing genotype on the effects generated by the environment, concerning variations in polyphenol content. Variation in genotype was not taken into account in the current study; consequently, the differences observed in the results can also be inuenced by this variable. Hence, further research using specic cocoa ecotypes are needed to reach outright conclusions. 3.3. Antioxidant capacity of cocoa extracts Recognition of many health benets provided by polyphenol compounds has encouraged increased scientic interest in determining the antioxidant capacity of various plant-derived products. However, a standardized method for the determination of antioxidant properties of certain foods and beverages has not yet been established. The ORAC assay has become a widely used method to assess antioxidant capacity in biological samples and foods (Prior et al., 2003) and has been broadly applied as a method of choice by academics and the food and supplement industry. The evaluation of antioxidant capacity by the ORAC assay was performed for cocoa extracts of eighteen tested cocoa farms and the results obtained are shown in Table 2. The decreasing order of cocoa bean extracts based on the antioxidant capacity evaluated with the ORAC assay, closely follows the TPC, avan-3-ol and epicatechin contents: bAR N aL_1 N cCT_1 N bVM N bGM_2 N aVC N bS_2 N bS_1 N
aSH N cCT_2 N aCA N aGT N bTA_2 N cCT_3 N aL_3 N aL_2 N bTA_1 N bGM_1. These results are also conrmed by the moderate Pearson correlation coefcients obtained between ORAC and these three variables (r = 0.678, r = 0.685 and r = 0.677 for TPC, avan-3-ol and epicatechin contents, respectively). In spite of this, our results are quite similar to those reported by the ORAC database of the United States Department of Agriculture for cocoa beans and dry cocoa powder (Nutrient Data Laboratory, 2010). 3.4. Principal component analysis (PCA) Multivariate analysis has traditionally been employed to evaluate food quality as well as wines and other products. PCA is a frequently employed statistical analysis method and has been successfully applied to analytical results, both for individual compounds and component combinations (Kallithraka et al., 2001). PCA can compress the data based on similarities and differences, by reducing the number of dimensions without much loss of information, and dene the number of principal components, offering a visual description of group differences or clustering and outliers (Ieri, Innocenti, Andrenelli, Vecchio, & Mulinacci, 2011; Rodriguez-Campos, Escalona-Buenda, Orozco-Avila, Lugo-Cervantes, & Jaramillo-Flores, 2011). To evaluate the possibility of differentiating the samples taking into account their origin, we applied this statistical tool. TPC, avan-3-ol, epicatechin, catechin, caffeine and theobromine contents as well as theobromine/ caffeine ratio, ORAC values, temperature and altitude, were considered as attributes to identify the principal components. Generally, the two rst principal components (PCs) are sufcient to explain the maximum variation in all original data. In our case, the three rst principal components represented 79.60% of the total variability, the total polyphenol, avan-3-ol and epicatechin contents being the dominating features in the PC1 (39.65% of the total variability), while altitude, theobromine/ caffeine ratio and caffeine content are the features with highest weight in the PC2 (25.68% of the total variability). Temperature constituted the most important element in the third PC (14.28% of the total variability). Fig. 4 shows a classical representation of the scores on PC1 and PC2 (65.32% of total variance). Cocoa beans from aL_1 and bAR contain large amounts of polyphenols, avan-3ols and epicatechin and this is why PC1 clearly separates them from the other samples, whereas the opposite occurs for the aCA and bTA_1 beans. Examining a twodimensional score plot in the space dened by PC1 and PC2 shows that the distribution of samples follows a pattern that depends on PC2 (Fig. 4). Thus the rst group comprising 7 cocoa-growing areas, is slightly dominated by features in the PC1, but highly related with altitude and the theobromine/caffeine ratio (PC2). The second group comprises 7
Fig. 4. Principal component analysis: Biplot (score and loading plots are overlaid) of the rst two principal components associated with differentiation of samples according to growing areas (TPC: total polyphenol content, FLV: avan-3-ols, EPI: epicatechin, CAT: catechin, CAF: caffeine, TEO: theobromine, TE/CA: theobromine/caffeine ratio, TEMP: temperature, ALT: altitude, ORAC values). The abbreviations of cocoa-growing areas are explained in Table 1.
Please cite this article as: Carrillo, L.C., et al., Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia, Food Research International (2013), http://dx.doi.org/10.1016/j.foodres.2013.06.019
L.C. Carrillo et al. / Food Research International xxx (2013) xxxxxx
other cocoa-growing areas and is also slightly dominated by features in the rst principal component, but highly linked to caffeine content (PC2). It is important to highlight that samples within the clusters are clearly differentiated by regional origins, as shown in Fig. 1. aVC, aCA, bS_1, bS_2, aL_1, aL_2, aL_3 and aSH were cultivated and harvested in the Colombian Andean mountains, whereas the other samples were from atlands. Chemometric techniques have been commonly used to assure food authenticity and quality (Cruz et al., 2013; Souza et al., 2011) as well as to classify their geographical origin based on chemical composition (Cheng, Qin, Guo, Hu, & Wu, 2013; Luykx, & van-Ruth, S. M., 2008). It is very usual to nd researches applying univariate methods for determining relationships between total or individual phenolics with antioxidant properties of cocoa beans and cocoa by-products. However, this one-dimensional approach fails to guarantee the determination of simultaneous correlations among all results. In order to overcome this problem, the interest in chemometric methods, which are multivariate by nature, has been recognized as a valuable tool in the food science (Granato, Katayama, & Castro, 2010). Specically in cocoa and chocolates eld, by using PCA and hierarchical cluster analysis Niemenak et al. (2006), classied different cocoa clones according to their polyphenol and anthocyanin contents; Miller et al. (2009), established that most measures of avanol chemistry, including epicatechin and the oligomeric and polymeric avan-3-ols (procyanidins), have a predictable relationship with the degree of processing of cocoa byproducts (cocoa powder, baking chocolate, dark chocolate, milk chocolates, and others); and more recently Rodriguez-Campos et al. (2012), used PCA to determine the effect of fermentation times and drying temperature on the composition of volatile compounds in cocoa beans. As far as we are concerned, the current work is one of the rst assessing the potential relationships between the major cocoa components (catechins and methylxanthines) and geographical origin. There are several internal and external factors affecting the quality and/or quantity of bioactive compounds in cocoa plants. These include the genetic (varietal and regional) diversity as well as many environmental variables, i.e. growth conditions such as light intensity, humidity, temperature, the use of fertilizers, wounding, infections or other stress factors (Niemenak et al., 2006). Whether or not the polyphenol, avan-3-ol and epicatechin contents in cocoa samples are important for health benets, our results indicate that caffeine content and the theobromine/caffeine ratio could be used as parameters to establish a cocoa bean classication according to the geographical area of the cocoa-growing regions, mainly depending on the altitude of the crops. Acknowledgment The authors thank the Universidad de Antioquia for logistic and nancial support of the project Sustainability Strategy 2011/2012, and especially to Anne-Lise Haenni (INSTITUT JACQUES MONOD, CNRS Universit Paris-Diderot) for her important contributions in the development of this work. References
Alonso-Amelot, M., Oliveros-Bastidas, A., & Calcagnopisarelli, M. (2007). Phenolics and condensed tannins of high altitude Pteridium arachnoideum in relation to sunlight exposure, elevation, and rain regime. Biochemical Systematics and Ecology, 35, 110. Andre, C. M., Our, M., Hoffmann, L., Hausman, J. F., Rogez, H., Larondelle, Y., et al. (2009). Inuence of environment and genotype on polyphenol compounds and in vitro antioxidant capacity of native Andean potatoes (Solanum tuberosum L.). Journal of Food Composition and Analysis, 22(6), 517524. Baltrusaityte, V., Venskutonis, P. R., & Ceksteryte, V. (2007). Radical scavenging activity of different oral origin honey and beebread phenolic extracts. Food Chemistry, 101(2), 502514. Belscak, A., Komes, D., Horzic, D., Ganic, K. K., & Karlovic, D. (2009). Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Research International, 42(56), 707716.
Brunetto, M. d. R., Gutirrez, L., Delgado, Y., Gallignani, M., Zambrano, A., Gmez, ., Ramos, G., & Romero, C. (2007). Determination of theobromine, theophylline and caffeine in cocoa samples by a high-performance liquid chromatographic method with on-line sample cleanup in a switching-column system. Food Chemistry, 100, 459467. Chalker-Scott, L., & Scott, J. D. (2004). Elevated ultraviolet-B radiation induces cross-protection to cold in leaves of Rhododendron under eld conditions. Photochemistry and Photobiology , 79 (2), 199 204. Cheng, H., Qin, Z. H., Guo, X. F., Hu, X. S., & Wu, J. H. (2013). Geographical origin identication of propolis using GCMS and electronic nose combined with principal component analysis. Food Research International, 51, 813822. Criado, M. N., Morello, J. R., Motilva, M. J., & Romero, M. P. (2004). Effect of growing area on pigment and phenolic fractions of virgin olive oils of the Arbequina variety in Spain. Journal of the American Oil Chemists' Society, 81(7), 633640. Cruz, A. G., Cadena, R. S., Alvaro, M. B. V. B., Sant'Ana, A. S., Oliveira, C. A. F., Faria, J. A. F., et al. (2013). Assessing the use of different chemometric techniques to discriminate low-fat and full-fat yogurts. LWT Food Science and Technology, 50, 210214. Cuatrecasas, J. (1964). Cacao and its allies: A taxonomic revision on the genus Theobroma. Bulletin of the United States National Museum, 35. (pp. 79614). Washington: Smithsonian Institution, 79614. Gotti, R., Furlanetto, S., Pinzauti, S., & Cavrini, V. (2006). Analysis of catechins in Theobroma cacao beans by cyclodextrin-modied micellar electrokinetic chromatography. Journal of Chromatography. A, 1112(12), 345352. Granato, D., Katayama, F. C. U., & Castro, I. A. (2010). Assessing the association between phenolic compounds and the antioxidant activity of Brazilian red wines using chemometrics. LWT Food Science and Technology, 43, 15421549. Ieri, F., Innocenti, M., Andrenelli, L., Vecchio, V., & Mulinacci, N. (2011). Rapid HPLC/DAD/MS method to determine phenolic acids, glycoalkaloids and anthocyanins in pigmented potatoes (Solanum tuberosum L.) and correlations with variety and geographical origin. Food Chemistry, 125, 750759. Jansen, M. A. K., van den Noort, R. E., Tan, M. Y. A., Prinsen, E., Lagrimini, L. M., & Thorneley, R. N. F. (2001). Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiology, 126(3), 10121023. Kallithraka, S., Arvanitoyannis, I. S., Kefalas, P., El-Zajouli, A., Soueros, E., & Psarra, E. (2001). Instrumental and sensory analysis of Greek wines; implementation of principal component analysis (PCA) for classication according to geographical origin. Food Chemistry, 73, 501514. Kishore, G., Ranjan, S., Pandey, A., & Gupta, S. (2010). Inuence of altitudinal variation on the antioxidant potential of tartar buckwheat of Western Himalaya. Food Science and Biotechnology, 19(5), 13551363. Li, Y., Feng, Y., Zhu, S., Luo, C., Ma, J., & Zhong, F. (2012). The effect of alkalization on the bioactive and avor related components in commercial cocoa powder. Journal of Food Composition and Analysis, 25, 1723. Liu, A. H., Li, L., Xu, M., Lin, Y. H., Guo, H. Z., & Guo, D. A. (2006). Simultaneous quantication of six major phenolic acids in the roots of Salvia miltiorrhiza and four related traditional Chinese medicinal preparations by HPLCDAD method. Journal of Pharmaceutical and Biomedical Analysis, 41, 4856. Londoo, J., Montoya, G., Guerrero, K., Aristizabal, L., & Arango, G. (2006). Citrus juice inhibits low density lipoprotein oxidation: Relationship between free radical scavenger activity and electrophoretic mobility. Revista Chilena de Nutricion, 33(3), 8. Lugo, A. E., Brown, S. L., Dodson, R., Smith, T. S., & Shugart, H. H. (1999). The Holdridge life zones of the conterminous United States in relation to ecosystem mapping. Journal of Biogeography, 26, 10251038. Luykx, D. M. A. M., & van-Ruth, S. M. (2008). An overview of analytical methods for determining the geographical origin of food products. Food Chemistry, 107, 897911. Markham, K. R., & Bloor, S. J. (1998). Analysis and identication of avonoids in practice. In C. A. Rice-Evans, & L. Packer (Eds.), Flavonoids in health and disease. New York, Basel, Hong Kong: Marcel Dekker Inc. Mateus, N., Marques, S., Gonalves, A. C., Machado, J. M., & De Freitas, V. (2001). Proanthocyanidin composition of red Vitis vinifera varieties from the Douro Valley during ripening: Inuence of cultivation altitude. American Journal of Enology and Viticulture, 52(2), 115121. Miller, K. B., Hurst, W. J., Flannigan, N., Ou, B., Lee, C. Y., Smith, N., et al. (2009). Survey of commercially available chocolate- and cocoa-containing products in the United States. 2. Comparison of avan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. Journal of Agricultural and Food Chemistry, 57(19), 91699180. Motamayor, J. C., Risterucci, A. M., Lopez, P. A., Ortiz, C. F., Moreno, A., & Lanaud, C. (2002). Cacao domestication I: The origin of the cacao cultivated by the Mayas. Heredity, 89, 380386. N'Goran, J. A. K., Laurent, V., Risterucci, A. M., & Lanaud, C. (1994). Comparative genetic diversity studies of Theobroma cacao L. Using RFLP and RADP markers. Heredity, 73, 589597. Niemenak, N., Rohsius, C., Elwers, S., Ndoumou, D. O., & Lieberei, R. (2006). Comparative study of different cocoa (Theobroma cacao L.) clones in terms of their phenolics and anthocyanins contents. Journal of Food Composition and Analysis, 19(67), 612619. Nutrient Data Laboratory, U. S. D. A. (2010). Database for the Oxygen Radical Absorbance Capacity (ORAC) of selected foods, 2. (pp. 39). Ortega, N., Romero, M. P., Macia, A., Reguant, J., Angles, N., Morello, J. R., et al. (2008). Obtention and characterization of phenolic extracts from different cocoa sources. Journal of Agricultural and Food Chemistry, 56(20), 96219627. Othman, A., Ismail, A., Ghani, N. A., & Adenan, I. (2007). Antioxidant capacity and phenolic content of cocoa beans. Food Chemistry, 100(4), 15231530.
Please cite this article as: Carrillo, L.C., et al., Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia, Food Research International (2013), http://dx.doi.org/10.1016/j.foodres.2013.06.019
L.C. Carrillo et al. / Food Research International xxx (2013) xxxxxx Semiz, G., Heijari, J., Isik, K., & Holopainen, J. K. (2007). Variation in needle terpenoids among Pinus sylvestris L. (Pinaceae) provenances from Turkey. Biochemical Systematics and Ecology, 35(10), 652661. Souza, S. S., Cruz, A. G., Walter, E. H. M., Faria, J. A. F., Celeghini, R. M. S., Ferreira, M. M. C., et al. (2011). Monitoring the authenticity of Brazilian UHT milk: A chemometric approach. Food Chemistry, 124, 692695. Temime, S. B., Campeol, E., Cioni, P. L., Daoud, D., & Zarrouk, M. (2006). Volatile compounds from Chtoui olive oil and variations induced by growing area. Food Chemistry, 99(2), 315325. Toms-Barbern, F., Cienfuegos-Jovellanos, E., Marn, A., Muguerza, B., Gil-Izquierdo, A., Cerd, B., et al. (2007). A new process to develop a cocoa powder with higher avonoid monomer content and enhanced bioavailability in healthy humans. Journal of Agricultural and Food Chemistry, 55, 39263935. Wollgast, J., & Anklam, E. (2000). Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identication and quantication. Food Research International, 33, 423447.
Ou, B., Hampsch-Woodill, M., & Prior, R. L. (2001). Development and validation of an improved oxygen radical absorbance capacity assay using uorescein as the uorescent probe. Journal of Agricultural and Food Chemistry , 49 , 4619 4626. Prior, R. L., Hoang, H., Gu, L., Wu, X., Bacchiocca, M., Howard, L., et al. (2003). Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. Journal of Agricultural and Food Chemistry, 51(11), 32733279. Rodriguez-Campos, J., Escalona-Buenda, H. B., Contreras-Ramos, S. M., Orozco-Avila, I., Jaramillo-Flores, E., & Lugo-Cervantes, E. (2012). Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chemistry, 132, 277288. Rodriguez-Campos, J., Escalona-Buenda, H. B., Orozco-Avila, I., Lugo-Cervantes, E., & Jaramillo-Flores, M. E. (2011). Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Research International, 44, 250258.
Please cite this article as: Carrillo, L.C., et al., Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia, Food Research International (2013), http://dx.doi.org/10.1016/j.foodres.2013.06.019
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- AreaPercent - VOLATILES CACAO1345837297Document2 pagesAreaPercent - VOLATILES CACAO1345837297Laura NogueraNo ratings yet
- Qualvolatilescacao 23512Document109 pagesQualvolatilescacao 23512Laura NogueraNo ratings yet
- Fec2 1Document1 pageFec2 1Laura NogueraNo ratings yet
- Tamaño de GranoDocument7 pagesTamaño de GranoLaura NogueraNo ratings yet
- 1 s2.0 S0021967300005355 MainDocument41 pages1 s2.0 S0021967300005355 MainLaura NogueraNo ratings yet
- Textura y Apariencia Del ChocolateDocument19 pagesTextura y Apariencia Del ChocolateLaura NogueraNo ratings yet
- Croma To GramaDocument1 pageCroma To GramaLaura NogueraNo ratings yet
- TIC: FIBRAEXT8512-2.D/data - MS: AbundanceDocument1 pageTIC: FIBRAEXT8512-2.D/data - MS: AbundanceLaura NogueraNo ratings yet
- Campos 2010Document13 pagesCampos 2010Laura NogueraNo ratings yet
- Differences Between The Content of Phenolic Compounds in Criollo, Forastero and Trinitario Cocoa Seed (Theobroma Cacao L.)Document12 pagesDifferences Between The Content of Phenolic Compounds in Criollo, Forastero and Trinitario Cocoa Seed (Theobroma Cacao L.)Laura NogueraNo ratings yet
- Effect of Temperature and Air Velocity on Drying Rate and Constant of Cocoa BeansDocument7 pagesEffect of Temperature and Air Velocity on Drying Rate and Constant of Cocoa BeansLaura NogueraNo ratings yet
- A Powerful Flavor Impact Constituent of Grapefruit JuiceDocument10 pagesA Powerful Flavor Impact Constituent of Grapefruit JuiceLaura NogueraNo ratings yet
- Food Research International: Luis C. Carrillo, Julián Londoño-Londoño, Andrés GilDocument8 pagesFood Research International: Luis C. Carrillo, Julián Londoño-Londoño, Andrés GilLaura NogueraNo ratings yet
- Free Radical Scavenging by Natural Polyphenols: Atom Versus Electron TransferDocument11 pagesFree Radical Scavenging by Natural Polyphenols: Atom Versus Electron TransferLaura NogueraNo ratings yet
- Jonfiaessen 2007Document5 pagesJonfiaessen 2007Laura NogueraNo ratings yet
- Brunetto 2009Document5 pagesBrunetto 2009Laura NogueraNo ratings yet
- 0 Sensory Evaluation PracticesDocument1 page0 Sensory Evaluation PracticesLaura NogueraNo ratings yet
- ) Stabilization of Enzymes by Intramolecular Cross-Linking Using Bi Functional ReagentsDocument12 pages) Stabilization of Enzymes by Intramolecular Cross-Linking Using Bi Functional ReagentsLaura NogueraNo ratings yet
- Aculey 2010Document8 pagesAculey 2010Laura NogueraNo ratings yet
- Aculey 2010Document8 pagesAculey 2010Laura NogueraNo ratings yet
- Buffers - Principles and PracticeDocument15 pagesBuffers - Principles and PracticeLaura NogueraNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)