0% found this document useful (0 votes)
94 views2 pagesMole
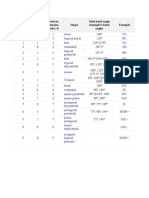
This document describes the molecular geometry of different compounds based on the number of electron pairs, lone pairs, and electron domains. It lists common molecular shapes such as linear, trigonal planar, tetrahedral, trigonal pyramidal, bent, and their corresponding ideal bond angles. Examples of compounds that adopt each shape are also provided along with images of their molecular structures.
Uploaded by
api-233333580Copyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
94 views2 pagesMole
This document describes the molecular geometry of different compounds based on the number of electron pairs, lone pairs, and electron domains. It lists common molecular shapes such as linear, trigonal planar, tetrahedral, trigonal pyramidal, bent, and their corresponding ideal bond angles. Examples of compounds that adopt each shape are also provided along with images of their molecular structures.
Uploaded by
api-233333580Copyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd