Professional Documents
Culture Documents
Introduction To Molecular
Uploaded by
clairole quilantang0 ratings0% found this document useful (0 votes)
46 views1 pagehlj
Original Title
Introduction to Molecular
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documenthlj
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
46 views1 pageIntroduction To Molecular
Uploaded by
clairole quilantanghlj
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
Quilantang,Clairole Marie L.
Gen. Engg I-C
Introduction to Molecular Geometry
Three-Dimensional Arrangement of Atoms in a Molecule
Molecular geometry or molecular structure is the three-dimensional arrangement of atoms within
a molecule. It is important to be able to predict and understand the molecular structure of a molecule
because many of the properties of a substance are determined by its geometry.
The Valence Shell, Bonding Pairs, and VSEPR Model
The outermost electrons of an atom are its valence electrons. The valence electrons are the electrons
that are most often involved in forming bonds and making molecules.
Pairs of electrons are shared between atoms in a molecule and hold the atoms together. These pairs
are called "bonding pairs".
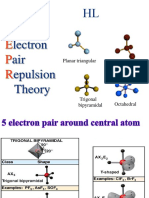
One way to predict the way electrons within atoms will repel each other is to apply the VSEPR
(valence-shell electron-pair repulsion) model. VSEPR can be used to determine a molecule's general
geometry.
Predicting Molecular Geometry
Here is a chart that describes the usual geometry for molecules based on their bonding behavior. To
use this key, first draw out the Lewis structure for a molecule. Count how many electron pairs are
present, including both bonding pairs and lone pairs. Treat both double and triple bonds as if they
were single electron pairs. A is used to represent the central atom. B indicates atoms surrounding A. E
indicates the number of lone electron pairs. Bond angles are predicted in the following order:
lone pair versus lone pair repulsion > lone pair versus bonding pair repulsion > bonding pair versus
bonding pair repulsion
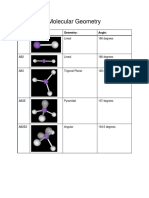
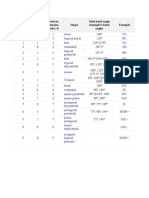
Molecular Geometry
Geometry Type # of Electron Pairs Ideal Bond Angle Examples
linear AB2 2 180 BeCl2
trigonal planar AB3 3 120 BF3
tetrahedral AB4 4 109.5 CH4
trigonal bipyramidal AB5 5 90, 120 PCl5
octohedral AB6 6 90 SF6
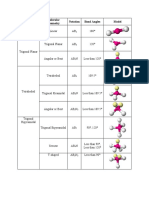
bent AB2E 3 120 (119) SO2
trigonal pyramidal AB3E 4 109.5 (107.5) NH3
bent AB2E2 4 109.5 (104.5) H2O
seesaw AB4E 5 180,120 (173.1,101.6) SF4
T-shape AB3E2 5 90,180 (87.5,<180) ClF3
linear AB2E3 5 180 XeF2
square pyramidal AB5E 6 90 (84.8) BrF5
square planar AB4E2 6 90 XeF4
You might also like
- Analytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryFrom EverandAnalytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryNo ratings yet
- General Chemistry 1 Qt. 2 Week 4Document12 pagesGeneral Chemistry 1 Qt. 2 Week 4Nina Reca OmisolNo ratings yet
- VSEPR TableDocument1 pageVSEPR TableAudrey HizonNo ratings yet
- Molecular Geometry Unit 02Document26 pagesMolecular Geometry Unit 02Iqra BaigNo ratings yet
- Geometry of Molecules ChartDocument6 pagesGeometry of Molecules ChartShamsiNo ratings yet
- LAS Physical-Science Week2Document11 pagesLAS Physical-Science Week2Shekaina Faith Cuizon LozadaNo ratings yet
- Electron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageDocument2 pagesElectron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageBianca GuillermoNo ratings yet
- Bondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eDocument3 pagesBondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eaadhyaNo ratings yet
- MoleDocument2 pagesMoleapi-233333580No ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryIsraClarkeNo ratings yet
- Vsepr-HlDocument25 pagesVsepr-HlRyan BoukaaNo ratings yet
- Worktable For Shape and PolarityDocument2 pagesWorktable For Shape and PolarityDestinee LegendsNo ratings yet
- Molecular GeometryDocument4 pagesMolecular Geometryapi-449127308No ratings yet
- UntitledDocument53 pagesUntitledchandrakanth maheshNo ratings yet
- List of Molecular GeometryDocument1 pageList of Molecular GeometryEsmeNo ratings yet
- List of Molecular GeometryDocument1 pageList of Molecular GeometryEsmeNo ratings yet
- CHM01 CO3 LESSON2 Molecular-ShapesDocument14 pagesCHM01 CO3 LESSON2 Molecular-ShapesErica Mamauag0% (1)
- CHM01 CO3 LESSON2 Molecular-ShapesDocument14 pagesCHM01 CO3 LESSON2 Molecular-ShapesErica MamauagNo ratings yet
- VSEPRDocument1 pageVSEPRĐan KhanhNo ratings yet
- Molecular ShapeDocument1 pageMolecular ShapeNUR DEENA KHALID KM-PensyarahNo ratings yet
- Chemistry-Molecular GeometryDocument2 pagesChemistry-Molecular GeometryBubbles Bubbles100% (1)
- Lewis StructureDocument1 pageLewis Structureits aryamNo ratings yet
- Che2060 Vsepr Geometry Ws KeyDocument5 pagesChe2060 Vsepr Geometry Ws Keyqvcws4h5spNo ratings yet
- Hybridisation and Bond AngleDocument13 pagesHybridisation and Bond Angleskye sueNo ratings yet
- CHM 201 2019-2020 Note1Document38 pagesCHM 201 2019-2020 Note1Adams TemitopeNo ratings yet
- VSEPR GeometriesDocument1 pageVSEPR GeometriesJason JacksonNo ratings yet
- Formula 1Document3 pagesFormula 1ahmad90616No ratings yet
- 3VSEPR Theory 41-48Document8 pages3VSEPR Theory 41-48Raj KishoreNo ratings yet
- Chapter 5: Chemical BondingDocument36 pagesChapter 5: Chemical BondingCt Sophie PheaNo ratings yet
- Vsepr Table PDFDocument1 pageVsepr Table PDFlucasNo ratings yet
- Shapes of Molecules & Ions: Name . . FormDocument2 pagesShapes of Molecules & Ions: Name . . FormjnfjngsdjNo ratings yet
- Bond Angles and VSEPR TheoryDocument3 pagesBond Angles and VSEPR TheoryTaqeeb AbbasNo ratings yet
- 3-6 Molecular Geometry SlidesDocument8 pages3-6 Molecular Geometry Slidesapi-240915238No ratings yet
- Molecular Shapes: Course Outcome 3Document13 pagesMolecular Shapes: Course Outcome 3tin canNo ratings yet
- Nota VSEPR PDFDocument1 pageNota VSEPR PDFMarlene GazconNo ratings yet
- Electron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleDocument2 pagesElectron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleRichamille Ann RicaforteNo ratings yet
- 7 Molecular Geometry AnsDocument6 pages7 Molecular Geometry AnsmamdudurNo ratings yet
- Molecular Geometry: Thinking of Molecules in 3-DimensionsDocument13 pagesMolecular Geometry: Thinking of Molecules in 3-DimensionsFilza AhmadNo ratings yet
- Chemical Bonding Double Prahaar - V1.3-ARCHIDocument60 pagesChemical Bonding Double Prahaar - V1.3-ARCHINeha esaralNo ratings yet
- 3.7 Geometry and Dipole MomentDocument9 pages3.7 Geometry and Dipole Momentelbadry mohamedNo ratings yet
- Scan Jul 22, 2021Document9 pagesScan Jul 22, 2021Nishant MishraNo ratings yet
- VSEPR and ShapesDocument22 pagesVSEPR and ShapesAS & A - Level ChemistryNo ratings yet
- Shape HelpDocument2 pagesShape HelpDerpityNo ratings yet
- Shapes of Molecules: VSEPR TheoryDocument17 pagesShapes of Molecules: VSEPR TheoryANASNo ratings yet
- Shapes of Molecules: VSEPR TheoryDocument17 pagesShapes of Molecules: VSEPR TheoryMervin TorresNo ratings yet
- Shapes of Molecules: VSEPR TheoryDocument17 pagesShapes of Molecules: VSEPR TheoryMervin TorresNo ratings yet
- MolGeom and IMFsDocument21 pagesMolGeom and IMFsJune Dela CruzNo ratings yet
- Class: Xi Inorganic Chemistry DPP. NO.-8Document2 pagesClass: Xi Inorganic Chemistry DPP. NO.-8Radhika MohataNo ratings yet
- Chemical BondingDocument28 pagesChemical BondingPrince DigvijayNo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryDean Joyce Alboroto100% (1)
- Class: Xi Inorganic Chemistry DPP. NO.-9Document2 pagesClass: Xi Inorganic Chemistry DPP. NO.-9Radhika MohataNo ratings yet
- Chemistry WorksheetDocument5 pagesChemistry WorksheetGiezel MadurarNo ratings yet
- Molecular Geometry VseprDocument25 pagesMolecular Geometry Vseprhidayati helmiNo ratings yet
- Anti Butane ConformationsDocument16 pagesAnti Butane ConformationsKONo ratings yet
- Vsepr Theory: Valence Shell Electron Pair RepulsionDocument31 pagesVsepr Theory: Valence Shell Electron Pair Repulsionsoniaali123No ratings yet
- 3 1 3 As Shapes of Molecules ChemsheetsDocument27 pages3 1 3 As Shapes of Molecules ChemsheetsAkshar PatelNo ratings yet
- VSEPR and Molecular Geometries (Summery)Document2 pagesVSEPR and Molecular Geometries (Summery)MihadNo ratings yet
- Math10 Q2 Circles2-1Document13 pagesMath10 Q2 Circles2-1SYLVIE CALUBAQUIBNo ratings yet
- Shapes of MoleculesDocument25 pagesShapes of MoleculesAsaph AharoniNo ratings yet
- Molecular Geometry and Bonding TheoriesDocument85 pagesMolecular Geometry and Bonding TheoriesAnnisa RahmiNo ratings yet
- SummarytujDocument2 pagesSummarytujclairole quilantangNo ratings yet
- Nature of ReligionDocument3 pagesNature of Religionclairole quilantangNo ratings yet
- Article III of The 1987 Constitution - Bill of RightsDocument11 pagesArticle III of The 1987 Constitution - Bill of RightslchieSNo ratings yet
- PoemshifDocument1 pagePoemshifclairole quilantangNo ratings yet
- GFFFRSDDocument2 pagesGFFFRSDclairole quilantangNo ratings yet
- Activity No. 4 Title: Description of Mechanism OBJECTIVE: To Be Able To Write A Description of A DynamoDocument2 pagesActivity No. 4 Title: Description of Mechanism OBJECTIVE: To Be Able To Write A Description of A Dynamoclairole quilantangNo ratings yet
- Song LyricshkkDocument8 pagesSong Lyricshkkclairole quilantangNo ratings yet
- Formal LettersghjjDocument1 pageFormal Lettersghjjclairole quilantangNo ratings yet
- CellllDocument1 pageCellllclairole quilantangNo ratings yet
- Multiple Choice Questions in Engineering Mathematics by Venancio I. Besavilla, Jr. Vol1Document122 pagesMultiple Choice Questions in Engineering Mathematics by Venancio I. Besavilla, Jr. Vol1Lugabaluga100% (1)
- A Project Proposal of Online Enrollment in WmsuDocument8 pagesA Project Proposal of Online Enrollment in Wmsuclairole quilantang33% (3)
- Filipino CollegeIDocument1 pageFilipino CollegeIclairole quilantangNo ratings yet
- (Fire and Spill) : Contingency / Emergency Response PlanDocument1 page(Fire and Spill) : Contingency / Emergency Response PlanClairole Marie QuilantangNo ratings yet
- I Believe in PinkDocument1 pageI Believe in Pinkclairole quilantangNo ratings yet
- Water ResourcesDocument16 pagesWater Resourcesclairole quilantangNo ratings yet
- History 101Document5 pagesHistory 101clairole quilantangNo ratings yet
- Critical AnalysisDocument1 pageCritical Analysisclairole quilantangNo ratings yet
- Chem 2Document5 pagesChem 2clairole quilantangNo ratings yet
- Philippine Revolution: Phase 1 (1896-1898)Document3 pagesPhilippine Revolution: Phase 1 (1896-1898)clairole quilantang100% (1)
- Fast Facts of Mount Apo in PhilippinesDocument1 pageFast Facts of Mount Apo in Philippinesclairole quilantangNo ratings yet
- History 101Document5 pagesHistory 101clairole quilantangNo ratings yet
- Determine The 4th Quantum Number of An Electron Spinning Clockwise in An Orbital Along Oriented XDocument1 pageDetermine The 4th Quantum Number of An Electron Spinning Clockwise in An Orbital Along Oriented Xclairole quilantangNo ratings yet
- ChemDocument1 pageChemclairole quilantangNo ratings yet
- Autobiography (Chem)Document2 pagesAutobiography (Chem)clairole quilantangNo ratings yet
- WMSUDocument1 pageWMSUclairole quilantangNo ratings yet
- Assignment in Philippine LiteratureDocument9 pagesAssignment in Philippine LiteratureClairole Marie QuilantangNo ratings yet
- Philippine Revolution: Phase 1 (1896-1898)Document3 pagesPhilippine Revolution: Phase 1 (1896-1898)clairole quilantang100% (1)
- BoseDocument9 pagesBoseclairole quilantangNo ratings yet
- ALGEBRA (Midterm)Document2 pagesALGEBRA (Midterm)Clairole Marie QuilantangNo ratings yet
- 2nd Semster - Lec01 - Angle & Directions PDFDocument13 pages2nd Semster - Lec01 - Angle & Directions PDFDakheel malekoNo ratings yet
- PWTLecture8 (SedimentationII)Document55 pagesPWTLecture8 (SedimentationII)JojoNo ratings yet
- Wsu Astronomy Lab: Resolving Power of The Human EyeDocument2 pagesWsu Astronomy Lab: Resolving Power of The Human EyeramuNo ratings yet
- Heat 4e Chap04 LectureDocument39 pagesHeat 4e Chap04 Lectureehdfhdhdfhdh100% (1)
- Effect of Surface Area On The Rate of ReactionDocument9 pagesEffect of Surface Area On The Rate of Reactionhas65No ratings yet
- OsidationDocument49 pagesOsidationSadhasivam VeluNo ratings yet
- Applications of Differential Equations in WeatherDocument18 pagesApplications of Differential Equations in WeatherFatimaRaufNo ratings yet
- Filtration Membranes in Cell CultureDocument12 pagesFiltration Membranes in Cell CulturemailreemaNo ratings yet
- Tamano Collapse LimitDocument4 pagesTamano Collapse LimitzhiqianxuNo ratings yet
- Synthetics Lubricant Basestock Brochure - ExxonMobilDocument13 pagesSynthetics Lubricant Basestock Brochure - ExxonMobilRafael Nakazato RecioNo ratings yet
- Titration ConceptDocument10 pagesTitration Conceptbasant kumar singh0% (1)
- IB Math SL IA TopicsDocument3 pagesIB Math SL IA TopicsSimran ZaveriNo ratings yet
- Scheerer Bearing Oil IndustryDocument62 pagesScheerer Bearing Oil IndustrycristinelbNo ratings yet
- III: Polarization: NumericalsDocument2 pagesIII: Polarization: NumericalsNishit PopatNo ratings yet
- Forming RollingDocument3 pagesForming RollingAvinash JhaNo ratings yet
- Bt0213 - Cell Biology Practical ManualDocument31 pagesBt0213 - Cell Biology Practical ManualVenus Divinagracia0% (2)
- Engineering Mechanics: Statics of Deformable BodiesDocument22 pagesEngineering Mechanics: Statics of Deformable BodiesRydelle GuevarraNo ratings yet
- Development of An Apparatus For Biaxial Testing Using Cruciform SpecimensDocument7 pagesDevelopment of An Apparatus For Biaxial Testing Using Cruciform SpecimensmrbeegzNo ratings yet
- National Institute of Technology, Rourkela: Department of Electrical EngineeringDocument11 pagesNational Institute of Technology, Rourkela: Department of Electrical Engineeringvineeth kumarNo ratings yet
- Steel Pile API (LRFD) - Primary DesignDocument2 pagesSteel Pile API (LRFD) - Primary Designbhavdip_shahNo ratings yet
- Exercise4 801Document3 pagesExercise4 801Tehreem FatimaNo ratings yet
- Background Document To EN 1991-1-7 PDFDocument62 pagesBackground Document To EN 1991-1-7 PDFPat GonzalezNo ratings yet
- On The Mechanisms of Pressure Generation in Vented ExplosionsDocument14 pagesOn The Mechanisms of Pressure Generation in Vented Explosionsigor VladimirovichNo ratings yet
- Nick Land Templexity Disordered Loops Through Shanghai Time PDFDocument33 pagesNick Land Templexity Disordered Loops Through Shanghai Time PDFLouly Seif100% (1)
- Engg100 Project 08 HDocument13 pagesEngg100 Project 08 HSean Kurian George100% (1)
- Gas Laws Notes KEY 2015-16 PDFDocument16 pagesGas Laws Notes KEY 2015-16 PDFpankajNo ratings yet
- Euromold Surge Arresters Up To 36kV Interface C 400PB-XSADocument2 pagesEuromold Surge Arresters Up To 36kV Interface C 400PB-XSARaul MañonNo ratings yet
- 3.design and Weight Optimization of Lift Base PlateDocument40 pages3.design and Weight Optimization of Lift Base Plateumesh KudalkarNo ratings yet
- Static Stability of Floating Structures PDFDocument84 pagesStatic Stability of Floating Structures PDFparamarthasom1974No ratings yet
- Walter Isaacson - Einstein, His Life and Universe (2007)Document2 pagesWalter Isaacson - Einstein, His Life and Universe (2007)M.A SamdaniNo ratings yet
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Process Plant Equipment: Operation, Control, and ReliabilityFrom EverandProcess Plant Equipment: Operation, Control, and ReliabilityRating: 5 out of 5 stars5/5 (1)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Nuclear Energy in the 21st Century: World Nuclear University PressFrom EverandNuclear Energy in the 21st Century: World Nuclear University PressRating: 4.5 out of 5 stars4.5/5 (3)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Guidelines for Chemical Process Quantitative Risk AnalysisFrom EverandGuidelines for Chemical Process Quantitative Risk AnalysisRating: 5 out of 5 stars5/5 (1)
- Process Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersFrom EverandProcess Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersNo ratings yet
- Physical and Chemical Equilibrium for Chemical EngineersFrom EverandPhysical and Chemical Equilibrium for Chemical EngineersRating: 5 out of 5 stars5/5 (1)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Tribology: Friction and Wear of Engineering MaterialsFrom EverandTribology: Friction and Wear of Engineering MaterialsRating: 5 out of 5 stars5/5 (1)
- Phase Equilibria in Chemical EngineeringFrom EverandPhase Equilibria in Chemical EngineeringRating: 4 out of 5 stars4/5 (11)