Professional Documents
Culture Documents
Worktable For Shape and Polarity
Uploaded by
Destinee Legends0 ratings0% found this document useful (0 votes)
28 views2 pagesOriginal Title
Worktable for Shape and Polarity
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
28 views2 pagesWorktable For Shape and Polarity
Uploaded by
Destinee LegendsCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
Name: _Maria Isabelle V.
Villacorte__________________________________ Lecture Section _U_______________
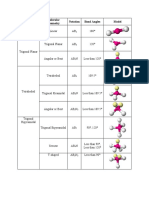
Table 3. Shape and geometry of real molecules
Molecule Lewis structure Number of Number of Molecular shape Bond angle around the central Has polar bonds? Polarity of molecule
bonding lone pairs atom (polar or non-polar)
electron Real (Actual) Model Yes No
pairs (Predicted)
XeF2 2 3 linear 180° 180° / Non-polar
ClF3 3 2 t-shaped 87.5° 90° / Polar
NH3 3 1 trigonal 107.8° 109.5° / Polar
pyramidal
SF4 4 1 seesaw 101.6°, 87.8° 120°, 90° / Polar
XeF4 4 2 square 90° 90° / Non-polar
planar
PCl5 5 0 trigonal 120°, 90° 120°, 90° / Non-polar
bipyramidal
SF6 6 0 octahedral 90° 90° / Non-polar
Geometry and Polarity
Geometry and Polarity
You might also like
- VSEPR TableDocument1 pageVSEPR TableAudrey HizonNo ratings yet
- Bondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eDocument3 pagesBondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eaadhyaNo ratings yet
- LAS Physical-Science Week2Document11 pagesLAS Physical-Science Week2Shekaina Faith Cuizon LozadaNo ratings yet
- Electron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageDocument2 pagesElectron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageBianca GuillermoNo ratings yet
- General Chemistry 1 Qt. 2 Week 4Document12 pagesGeneral Chemistry 1 Qt. 2 Week 4Nina Reca OmisolNo ratings yet
- Introduction To MolecularDocument1 pageIntroduction To Molecularclairole quilantangNo ratings yet
- Molecular ShapeDocument1 pageMolecular ShapeNUR DEENA KHALID KM-PensyarahNo ratings yet
- Molecular Geometry Unit 02Document26 pagesMolecular Geometry Unit 02Iqra BaigNo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryIsraClarkeNo ratings yet
- Geometry of Molecules ChartDocument6 pagesGeometry of Molecules ChartShamsiNo ratings yet
- VSEPR GeometriesDocument1 pageVSEPR GeometriesJason JacksonNo ratings yet
- Lewis StructureDocument1 pageLewis Structureits aryamNo ratings yet
- Vsepr-HlDocument25 pagesVsepr-HlRyan BoukaaNo ratings yet
- ACTIVITY SHEET Geometry of Simple CompoundsDocument4 pagesACTIVITY SHEET Geometry of Simple CompoundsUy, Jhavelaine Cassandra F.No ratings yet
- Electron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleDocument2 pagesElectron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleRichamille Ann RicaforteNo ratings yet
- Properties of 2D Shapes - AnswersDocument1 pageProperties of 2D Shapes - Answerscloud scapeNo ratings yet
- Vsepr Table PDFDocument1 pageVsepr Table PDFlucasNo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryDean Joyce Alboroto100% (1)
- Vsepr ChartDocument1 pageVsepr ChartpankajNo ratings yet
- VSEPRDocument1 pageVSEPRĐan KhanhNo ratings yet
- Co-Efficients and Compass Adjustment ShortDocument2 pagesCo-Efficients and Compass Adjustment Shortehsan nahidNo ratings yet
- Chemistry-Molecular GeometryDocument2 pagesChemistry-Molecular GeometryBubbles Bubbles100% (1)
- Prac 7.2Document3 pagesPrac 7.2Aref DahabrahNo ratings yet
- Convert Your Output To PDF File FormatDocument7 pagesConvert Your Output To PDF File FormatBasti MalonzoNo ratings yet
- Edge Performance of High Power EyepiecesDocument4 pagesEdge Performance of High Power EyepiecesMaria TeresaNo ratings yet
- Rasco - MV - Medium Velocity Directional Spray Nozzle - B.106-Aug21Document39 pagesRasco - MV - Medium Velocity Directional Spray Nozzle - B.106-Aug21Ary SetiawanNo ratings yet
- Fourth Sector AntennaDocument8 pagesFourth Sector AntennaSalaf HayatNo ratings yet
- Geometry Teacher - S Skills Practice Chapter 4 PDFDocument34 pagesGeometry Teacher - S Skills Practice Chapter 4 PDFJamie BaczewskiNo ratings yet
- 3 1 3 As Shapes of Molecules ChemsheetsDocument27 pages3 1 3 As Shapes of Molecules ChemsheetsAkshar PatelNo ratings yet
- Chapter 3 Polygons2Document26 pagesChapter 3 Polygons2Jonard G. TrajanoNo ratings yet
- Unit Circle in Depth HandoutDocument6 pagesUnit Circle in Depth HandoutFayzaNo ratings yet
- AnglesDocument24 pagesAnglespabas9873No ratings yet
- Che2060 Vsepr Geometry Ws KeyDocument5 pagesChe2060 Vsepr Geometry Ws Keyqvcws4h5spNo ratings yet
- New Century Math Yr 9 - Chapter04 Investigation GeometryDocument28 pagesNew Century Math Yr 9 - Chapter04 Investigation GeometryPung Kang QinNo ratings yet
- Shapes of Molecules & Ions: Name . . FormDocument2 pagesShapes of Molecules & Ions: Name . . FormjnfjngsdjNo ratings yet
- 1 2 PDFDocument2 pages1 2 PDF祁伟No ratings yet
- 1. Angle Measure (Degrees and Radians) : Ab=Oa =Ob α= AB rDocument6 pages1. Angle Measure (Degrees and Radians) : Ab=Oa =Ob α= AB rdahlai dahlia oktaviani gintingNo ratings yet
- Molecular ModelDocument1 pageMolecular ModelPedro SuyuNo ratings yet
- STM 001 Reviewer 1Document10 pagesSTM 001 Reviewer 1Armhay Loraine DuavezNo ratings yet
- Chapter 5: Chemical BondingDocument36 pagesChapter 5: Chemical BondingCt Sophie PheaNo ratings yet
- Angle Measure and Unit CircleDocument16 pagesAngle Measure and Unit CircleSnow BollNo ratings yet
- Polygons and QuadrilateralsDocument4 pagesPolygons and QuadrilateralsDaniel DowdingNo ratings yet
- BETE - TF-metric SPIRAL FULL CONEDocument2 pagesBETE - TF-metric SPIRAL FULL CONEOnie Hammamz Oyl100% (1)
- Ángulos 4Document2 pagesÁngulos 4Ana GuardianiNo ratings yet
- Molecular Orbitals PDFDocument6 pagesMolecular Orbitals PDFGeraldNo ratings yet
- TrigonometryDocument9 pagesTrigonometryJustin Rain AlsagonNo ratings yet
- Trigonometric FunctionsDocument48 pagesTrigonometric Functionsegam0004No ratings yet
- Virtual VSEPR Lab ActivityDocument6 pagesVirtual VSEPR Lab ActivityDharm PatelNo ratings yet
- PRECAL Final Module 1 4Document41 pagesPRECAL Final Module 1 4Glen MillarNo ratings yet
- Learning Unit Introduction To Coordinates-Polar Coordinates ExerciseDocument4 pagesLearning Unit Introduction To Coordinates-Polar Coordinates ExerciseShun Long CHEUNGNo ratings yet
- Steric No. Form Shape Angle HybridizationDocument1 pageSteric No. Form Shape Angle Hybridizationmica_tsukadaNo ratings yet
- Trigonometry: Section 4.1 Radian and Degree MeasureDocument4 pagesTrigonometry: Section 4.1 Radian and Degree Measuresarasmile2009No ratings yet
- Ficha Tecnica Boquillas AspersionDocument1 pageFicha Tecnica Boquillas AspersionCesar Muñoz OssesNo ratings yet
- Graphing and Describing 90° and 270° Rotations About The Origin (0, 0)Document13 pagesGraphing and Describing 90° and 270° Rotations About The Origin (0, 0)Paul Hamilton11nNo ratings yet
- Alpha Rv24afDocument4 pagesAlpha Rv24afatsukiNo ratings yet
- Electrical Connector, CN1Document2 pagesElectrical Connector, CN1Ricardo Jorge Horta PequenoNo ratings yet
- MT20, MT20L Manual PDFDocument26 pagesMT20, MT20L Manual PDFKiranNo ratings yet
- Lab Report: Physical Experiment IDocument7 pagesLab Report: Physical Experiment IRaymond QuNo ratings yet
- Theorems On Triangle Inequalities (Exterior Angle Inequality Theorem) - Grade 8Document8 pagesTheorems On Triangle Inequalities (Exterior Angle Inequality Theorem) - Grade 8Ceejey Cardenas GuevarraNo ratings yet
- Introduction To Agricultural Extension EducationDocument25 pagesIntroduction To Agricultural Extension EducationDestinee LegendsNo ratings yet
- RevisedDocument18 pagesRevisedDestinee LegendsNo ratings yet
- HK11 Jumpers KneeDocument7 pagesHK11 Jumpers KneeDestinee LegendsNo ratings yet
- Hains 1992Document6 pagesHains 1992Destinee LegendsNo ratings yet
- List of Reg Advisers 2019 2020Document9 pagesList of Reg Advisers 2019 2020Destinee LegendsNo ratings yet
- CHP 3 PPT BaktDocument40 pagesCHP 3 PPT BaktaroemyNo ratings yet
- Ions and Surfaces: by Mark MichalovicDocument3 pagesIons and Surfaces: by Mark MichalovicDestinee LegendsNo ratings yet
- Egg PhysiologyDocument9 pagesEgg PhysiologyDestinee LegendsNo ratings yet
- Digestive System and Bioenergetics NotesDocument9 pagesDigestive System and Bioenergetics NotesDestinee LegendsNo ratings yet
- HUM 3 E1 - Villacorte - Critique - Heneral LunaDocument9 pagesHUM 3 E1 - Villacorte - Critique - Heneral LunaDestinee LegendsNo ratings yet