Professional Documents
Culture Documents
Nomenclature
Uploaded by
crystalgal9462Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nomenclature
Uploaded by
crystalgal9462Copyright:
Available Formats
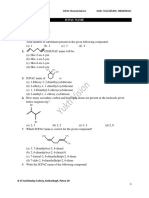
NOMENCLATURE 1) Give the IUPAC name for each of the following compounds:
2) Draw a structural formula for each of the following compounds (either dash or bond-line formula): a) 4-t-butylheptane b) 2-methylhexan-2-ol c) 3-ethyl-5,5,7-trimethylnonane d) (1-methylethyl)cyclohexane e) 3-ethylcyclopentanol f) 4-methylpent-3-en-1-ol g) pent-2-yn-1-ol h) 3-methylcyclopentene i) 3-bromo-2-methylhex-2-ene j) 4-methylpent-2-yne k) 1-isopentylcyclohexene
3) Explain why each of the following names is incorrect. a) 3-propylhexane b) 4-methylhexane c) 2-methylhexan-6-ol d) 3,5,6,7-tetramethylnonane e) 2,2-dimethyl-3-ethylpentane f) 6-isopentyldecane
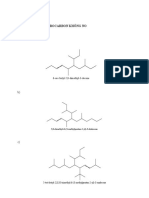
4) Give the IUPAC name for each of the following:
5) Draw a structural formula for compounds you will encounter in the laboratory sessions. a) toluene b) acetone c) benzoic acid d) diethyl ether 6) More practice problem: Draw a structural formula for each of the following compounds (either dash or bond-line formula): a) ethyl isopropyl ether g) trans-1-methoxybut-1-ene e) ethyl 4-aminobenzoate f) 1,4-dimethoxybenzene g) cyclohexanone
b) trans-1-bromobutene c) 5-ethylheptan-3-one d) cis-pent-2-enoic acid e) ethyl butanoate f) 2-chloroethyl benzoate
h) 2,2-dimethylcyclopentanone i) 4-ethyl-5,5,7-trimethyloctanal j) 2,3-dimethylhexanoic acid k) methyl trans-hex-3-enoate l) n-butylmethylamine
You might also like
- Chem Test 1Document5 pagesChem Test 1dishaali110No ratings yet
- Studyon Alkane NameDocument3 pagesStudyon Alkane NamekjjkimkmkNo ratings yet
- Chem 141 Problem-Set 6 Friday 27th September 2013Document3 pagesChem 141 Problem-Set 6 Friday 27th September 2013Riley BenoitNo ratings yet
- Exercise - Nomenclature of Organic Compounds FinalDocument10 pagesExercise - Nomenclature of Organic Compounds FinalVina YuNo ratings yet
- Nomenclature _ DPP 02 (Of Lec 04) __ (Arjuna JEE 2023)Document2 pagesNomenclature _ DPP 02 (Of Lec 04) __ (Arjuna JEE 2023)jeemainsmaterial97No ratings yet
- Nomenclature - Prob SetDocument7 pagesNomenclature - Prob SetGenny MarantanNo ratings yet
- Problem Set 2: Eloc, Arriane Mae J. Gaganting, Ephraim Joash A. Gregorio, Dona-Mae EDocument5 pagesProblem Set 2: Eloc, Arriane Mae J. Gaganting, Ephraim Joash A. Gregorio, Dona-Mae EEPHRAIM JOASH ABEJO GAGANTINGNo ratings yet
- 13 DPP 2 New Batch A-N +ans For StudentsDocument32 pages13 DPP 2 New Batch A-N +ans For StudentskljNo ratings yet
- Testbank4 Testbank4 Nomenklatur Konform+ Svarnomenklatur Konform+ SvarDocument12 pagesTestbank4 Testbank4 Nomenklatur Konform+ Svarnomenklatur Konform+ SvarScott LarmerNo ratings yet
- Organic Chemistry I Worksheet on Alkanes and CycloalkanesDocument6 pagesOrganic Chemistry I Worksheet on Alkanes and Cycloalkanesneneng rohayatiNo ratings yet
- Tutorial Problems, 2013/2014: Che 153 Organic Chemistry For EngineersDocument21 pagesTutorial Problems, 2013/2014: Che 153 Organic Chemistry For EngineersIng Rashid BawahNo ratings yet
- Homework Assignments Chapter-7: AlkenesDocument8 pagesHomework Assignments Chapter-7: AlkenesandrewNo ratings yet
- Practice Problems for Organic Chemistry Chapter 21Document14 pagesPractice Problems for Organic Chemistry Chapter 21Malak SamehNo ratings yet
- Bnbi104 Tutorial1Document1 pageBnbi104 Tutorial1Ashria Sonali PrakashNo ratings yet
- Chapter 3 2Document41 pagesChapter 3 2jerrica thomasNo ratings yet
- PROBSET-Drawing-And-Nomenclature Grp10 PT14 Sanchez, Sarmiento, ThettathDocument4 pagesPROBSET-Drawing-And-Nomenclature Grp10 PT14 Sanchez, Sarmiento, ThettathDale Miko SanchezNo ratings yet
- 655dae70717b6700190a1295 - ## - IUPAC Nomenclature DPP-02 (Of Lecture 03)Document3 pages655dae70717b6700190a1295 - ## - IUPAC Nomenclature DPP-02 (Of Lecture 03)anubhab256224No ratings yet
- Latihan Nomenclature AlkaneDocument14 pagesLatihan Nomenclature AlkanekjjkimkmkNo ratings yet
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetDocument4 pages12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetRehnuma BasherNo ratings yet
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetDocument4 pages12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetValerie Duran-ArzagaNo ratings yet
- Work Sheet Alkanes Sbi 09Document5 pagesWork Sheet Alkanes Sbi 09Elfi Susanti VHNo ratings yet
- 2023 - 03 - 25 - Oc Mka 10 Hours Maha One ShotDocument197 pages2023 - 03 - 25 - Oc Mka 10 Hours Maha One ShotgvygtvcNo ratings yet
- Organic Chemistry QuizDocument7 pagesOrganic Chemistry QuizCarlo Jay BasulNo ratings yet
- Naming Alkanes AnsDocument1 pageNaming Alkanes AnskevinamyNo ratings yet
- Q2 2022 OrgchemDocument2 pagesQ2 2022 OrgchemHazeljoyce AlcantaraNo ratings yet
- 11 DPP 02a Iupac of Alkane Emerge 1657625376769Document4 pages11 DPP 02a Iupac of Alkane Emerge 1657625376769Nothing 2No ratings yet
- Chapter 03Document40 pagesChapter 03AC BañaresNo ratings yet
- Problem Set 2 Solution PDFDocument2 pagesProblem Set 2 Solution PDFLouisNo ratings yet
- Tutorial 5Document4 pagesTutorial 5Eqieyn JerrNo ratings yet
- 5 - AlkenesDocument12 pages5 - AlkenesSean Gabriel LacambraNo ratings yet
- Chapter 4 QuestionsDocument2 pagesChapter 4 Questionsdaniday1977100% (1)
- CH 4 StudyDocument11 pagesCH 4 StudyLiz Hans0% (1)
- Organic Chemistry Naming ExaminationDocument6 pagesOrganic Chemistry Naming ExaminationHaa Kksak100% (1)
- Prof. Rakesh Rathi Organic Chemistry, Moles, IUPAC Name TutorialDocument4 pagesProf. Rakesh Rathi Organic Chemistry, Moles, IUPAC Name TutorialPrakash KapadiaNo ratings yet
- DPP NomenclatureDocument7 pagesDPP Nomenclaturegamishtag18No ratings yet
- Bài Tập Danh PhápDocument9 pagesBài Tập Danh PhápTrần Thị Kiều TrangNo ratings yet
- PRACTICE SHEET - 01 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)Document6 pagesPRACTICE SHEET - 01 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)ABD 17No ratings yet
- 655eed7353c28900188cd636 - ## - IUPAC Nomenclature DPP-03 (Of Lecture 04)Document3 pages655eed7353c28900188cd636 - ## - IUPAC Nomenclature DPP-03 (Of Lecture 04)anubhab256224No ratings yet
- IUPAC Naming QuizDocument2 pagesIUPAC Naming QuizErica WuNo ratings yet
- Ejercicios de AlacanosDocument16 pagesEjercicios de AlacanosRicardo Alcántara ReyesNo ratings yet
- Chapter Test Alcohol and Ether Nomenclature 1Document5 pagesChapter Test Alcohol and Ether Nomenclature 1Allyza Mikaella LabradorNo ratings yet
- IUPAC NOMENCLATURE REVISION WORKSHEET-1Document3 pagesIUPAC NOMENCLATURE REVISION WORKSHEET-1AAYUSHNo ratings yet
- Tarea 6 AlcanosDocument13 pagesTarea 6 AlcanosLuis Petrikowski :3No ratings yet
- Document 1Document9 pagesDocument 1Nishi tomarNo ratings yet
- WS SL NamingPracticeDocument1 pageWS SL NamingPracticeGüşta İrem SakızNo ratings yet
- OC02 - Alkenes, Alkynes and Cyclic Hydrocarbons - Worksheet - ANSWERSDocument5 pagesOC02 - Alkenes, Alkynes and Cyclic Hydrocarbons - Worksheet - ANSWERSAlizay Imran80% (5)
- Alkanes, Alkenes, Alkynes and Cyclic Hydrocarbons - Worksheet - ANSWERSDocument5 pagesAlkanes, Alkenes, Alkynes and Cyclic Hydrocarbons - Worksheet - ANSWERSSocdal Abdi100% (1)
- 2016 Worksheet For Medicine StudentsDocument2 pages2016 Worksheet For Medicine StudentsbedadadenbalprosperityNo ratings yet
- OC02 Alkenes Alkynes and Cyclic Hydrocarbons Worksheet ANSWERSDocument4 pagesOC02 Alkenes Alkynes and Cyclic Hydrocarbons Worksheet ANSWERSakshayddsbNo ratings yet
- IUPAC NomanclatureDocument21 pagesIUPAC NomanclatureDARK KILLERNo ratings yet
- Organic Chemistry 8Th Edition Bruice Test Bank Full Chapter PDFDocument36 pagesOrganic Chemistry 8Th Edition Bruice Test Bank Full Chapter PDFtonya.paongo686100% (10)
- Chương 3 Các Hợp Chất Hydrocarbon Không No: 6-sec-butyl-5,8-dimethyl-3-deceneDocument4 pagesChương 3 Các Hợp Chất Hydrocarbon Không No: 6-sec-butyl-5,8-dimethyl-3-decenelong hungNo ratings yet
- Classification and Nomenclature of Organic Compounds NJ 247Document29 pagesClassification and Nomenclature of Organic Compounds NJ 247gauravydv5306rgrNo ratings yet
- Chapter Test Cyclic Hydrocarbon Nomenclature 1Document4 pagesChapter Test Cyclic Hydrocarbon Nomenclature 1Allyza Mikaella LabradorNo ratings yet
- Organic Functional Groups WorksheetDocument4 pagesOrganic Functional Groups WorksheetCorey Becker33% (6)
- Handbook of Proton-NMR Spectra and Data: IndexFrom EverandHandbook of Proton-NMR Spectra and Data: IndexAsahi Research Center Co., Ltd.No ratings yet
- Matrix Metalloproteinase BiologyFrom EverandMatrix Metalloproteinase BiologyIrit SagiNo ratings yet
- Guide to Trivial Names, Trade Names and Synonyms for Substances Used in Analytical Nomenclature: International Union of Pure and Applied Chemistry: Analytical Chemistry DivisionFrom EverandGuide to Trivial Names, Trade Names and Synonyms for Substances Used in Analytical Nomenclature: International Union of Pure and Applied Chemistry: Analytical Chemistry DivisionNo ratings yet
- Fuel Cells, Solar Panels, and Storage Devices: Materials and MethodsFrom EverandFuel Cells, Solar Panels, and Storage Devices: Materials and MethodsNo ratings yet