Professional Documents
Culture Documents
Alkyne Metathesis & Enyne Metathesis
Uploaded by
polypeptideCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alkyne Metathesis & Enyne Metathesis
Uploaded by
polypeptideCopyright:
Available Formats
-
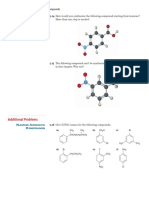
alkyne metathesis cleaves the C-C triple bonds in two alkynes to form two new alkynes; it is used to make conjugated polymers with interesting electronic properties and to synthesize macrocycles with E or Z alkenes after reduction ; it is catalyzed by metal alkylidenes
the intermediate of alkyne metathesis involves a metallacyclobutadiene complex
alkyne cross metathesis can be used to synthesize unsymmetrical alkynes
ring closing alkyne metathesis + reduction can be used to synthesize macrocyclic natural products containing cis olefins
enyne metathesis generates dienes from alkynes and alkenes by splitting the alkene in half and adding them across the alkyne ; high heterofunction tolerance when used with Grubbs catalyst
enyne metathesis can be used with olefin metathesis to form bicyclic products
the mechanism of enyne metathesis involves the formation of an alkylidene complex and the subsequent formation of a metallocyclobutene complex
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Exp 6 Williamson Ether SynthesisDocument5 pagesExp 6 Williamson Ether SynthesisSherry0% (1)
- Glass Care Safe Handling PDFDocument16 pagesGlass Care Safe Handling PDFAdinda SaraswatiNo ratings yet
- Glass Care Safe Handling PDFDocument16 pagesGlass Care Safe Handling PDFAdinda SaraswatiNo ratings yet
- Chemical Equilibrium (Final Review)Document20 pagesChemical Equilibrium (Final Review)polypeptideNo ratings yet
- NMR Yield CalculationDocument4 pagesNMR Yield CalculationpolypeptideNo ratings yet
- Chem 14A Discussion Section: Coordination ChemistryDocument3 pagesChem 14A Discussion Section: Coordination ChemistrypolypeptideNo ratings yet
- Units of Measurement: Introduction To ChemistryDocument4 pagesUnits of Measurement: Introduction To ChemistrypolypeptideNo ratings yet
- Mechanism of Microbial InfectionsDocument122 pagesMechanism of Microbial InfectionspolypeptideNo ratings yet
- Lecture of Zoonoses at NTU 2015Document159 pagesLecture of Zoonoses at NTU 2015polypeptideNo ratings yet
- Homework 3Document13 pagesHomework 3polypeptideNo ratings yet
- Chapter 28 LCDocument5 pagesChapter 28 LCpolypeptideNo ratings yet
- Midterm RecitationDocument12 pagesMidterm RecitationpolypeptideNo ratings yet
- Homework 3Document13 pagesHomework 3polypeptideNo ratings yet
- Chemistry GRE SampleDocument0 pagesChemistry GRE Sampleyoostan100% (2)
- Viral Diseases MechanismsDocument105 pagesViral Diseases MechanismspolypeptideNo ratings yet
- Animal ConditioningDocument27 pagesAnimal ConditioningpolypeptideNo ratings yet
- General Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentDocument2 pagesGeneral Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentpolypeptideNo ratings yet
- Aic I HW7Document2 pagesAic I HW7polypeptideNo ratings yet
- Chapter1s StatMechDocument22 pagesChapter1s StatMechpolypeptideNo ratings yet
- Chapter24 Materials ChemDocument25 pagesChapter24 Materials ChempolypeptideNo ratings yet
- Chapter 7 Intro To Optical InstrumentsDocument10 pagesChapter 7 Intro To Optical InstrumentspolypeptideNo ratings yet
- pKa Table AnalysisDocument6 pagespKa Table AnalysisAlessandro MoreniNo ratings yet
- Alkyne Metathesis & Enyne MetathesisDocument3 pagesAlkyne Metathesis & Enyne MetathesispolypeptideNo ratings yet
- Functional Groups in Organic ChemistryDocument1 pageFunctional Groups in Organic ChemistrygznmemberNo ratings yet
- E26 QuestionsDocument5 pagesE26 QuestionspolypeptideNo ratings yet
- Protective GroupDocument26 pagesProtective Groupchhan4No ratings yet
- FLUXIONALITYDocument7 pagesFLUXIONALITYpolypeptideNo ratings yet
- Literature Report 2Document12 pagesLiterature Report 2polypeptideNo ratings yet
- C H ActivationDocument55 pagesC H Activationpolypeptide100% (1)
- Est-Ce QueDocument6 pagesEst-Ce QuepolypeptideNo ratings yet
- Test Taking StrategiesDocument193 pagesTest Taking StrategiesAmy Kennedy100% (1)
- ADISI Aldehid Dan KetonDocument2 pagesADISI Aldehid Dan Ketondarkbreaker3244No ratings yet
- ALKYL HALIDESNomenclature and PreparationDocument20 pagesALKYL HALIDESNomenclature and Preparationsatourigojo594No ratings yet
- Total Synthesis of the Sesquiterpene EremophiloneDocument4 pagesTotal Synthesis of the Sesquiterpene EremophilonePrabodh SatyalNo ratings yet
- Synthesis of Esters Lab ReportDocument6 pagesSynthesis of Esters Lab ReportTrisha Alibin PreNo ratings yet
- Lecture Outline: Organic ChemistryDocument30 pagesLecture Outline: Organic Chemistrysungyeon heoNo ratings yet
- Characterization of Lignin Extracted CompoundDocument13 pagesCharacterization of Lignin Extracted CompoundJitender SinghNo ratings yet
- Bischler-Napieralski Reaction - Wikipedia, The Free EncyclopediaDocument4 pagesBischler-Napieralski Reaction - Wikipedia, The Free EncyclopediaEvs GoudNo ratings yet
- PD Pincer ComplexesDocument4 pagesPD Pincer ComplexesBenjamín Marc Ridgway de SassouNo ratings yet
- CHEM F311 Lecture 38 39 1,5-Dicarbonyl CompoundsDocument9 pagesCHEM F311 Lecture 38 39 1,5-Dicarbonyl Compoundsliving luxuriousNo ratings yet
- TIME TABLE 04-Dec To 11-Dec - For WebsiteDocument13 pagesTIME TABLE 04-Dec To 11-Dec - For WebsiteAnsuman GuptaNo ratings yet
- Liebermann-Burchard and Salkowski Tests for CholesterolDocument2 pagesLiebermann-Burchard and Salkowski Tests for Cholesteroljsbren50% (6)
- Alcohols - Organic Chemistry (1) - 3Document12 pagesAlcohols - Organic Chemistry (1) - 3Defaults rulezNo ratings yet
- Chemistry SSC-II Exam Answer SheetDocument6 pagesChemistry SSC-II Exam Answer SheetNawazNo ratings yet
- Organic Chemistry Questions and Answers - Volume I: July 2020Document166 pagesOrganic Chemistry Questions and Answers - Volume I: July 2020Simona ButanNo ratings yet
- Cyclo Alkanes CompltDocument8 pagesCyclo Alkanes CompltKaneez AsmaNo ratings yet
- Chem. Rev. 2009, 109, 5069-5119Document51 pagesChem. Rev. 2009, 109, 5069-5119dhuglNo ratings yet
- Fat Facts PDFDocument6 pagesFat Facts PDFEko Ariyanto Wong PlembangNo ratings yet
- Nomenclature, Bonding, and Isomers FlashcardsDocument106 pagesNomenclature, Bonding, and Isomers FlashcardsLejNo ratings yet
- P.G. Taylor, J.M.F. Gagan Alkenes and AromaticsDocument183 pagesP.G. Taylor, J.M.F. Gagan Alkenes and AromaticsCeleste PonceNo ratings yet
- Sodium Ethoxide: General DiscussionDocument1 pageSodium Ethoxide: General DiscussionDevendra ShuklaNo ratings yet
- SCH 4U0 Alkanes, Alkenes, Alkynes and Cyclic Hydrocarbons WorksheetDocument3 pagesSCH 4U0 Alkanes, Alkenes, Alkynes and Cyclic Hydrocarbons WorksheetMo NassifNo ratings yet
- Previous Years AIPMTNEET &AIIMS QuestionsDocument11 pagesPrevious Years AIPMTNEET &AIIMS QuestionsSohag DeNo ratings yet
- Sni, Nighbouring GP Participation & E1cbDocument19 pagesSni, Nighbouring GP Participation & E1cbsaheedvkNo ratings yet
- Controlled Radical Polymerization GuideDocument52 pagesControlled Radical Polymerization GuideBeryl MawaridNo ratings yet
- Neighbouring Group Participation; SN1 vs SN2 MechanismsDocument3 pagesNeighbouring Group Participation; SN1 vs SN2 MechanismsIshaan ChaturvediNo ratings yet
- NCERT Solutions For Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic AcidsDocument17 pagesNCERT Solutions For Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acidssinghrathoreganga814No ratings yet
- 10.haloalkanes and Haloarenes KCET PYQsDocument1 page10.haloalkanes and Haloarenes KCET PYQsPunith kumar100% (2)
- Combinepdf-7 CompressedDocument21 pagesCombinepdf-7 Compressedaichiii.bearNo ratings yet
- RSC Advances: ReviewDocument14 pagesRSC Advances: ReviewLuana Alves MachadoNo ratings yet