Professional Documents
Culture Documents
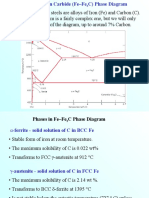
Fe-C Phase Diagram
Fe-C Phase Diagram
Uploaded by
mffritzCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fe-C Phase Diagram
Fe-C Phase Diagram
Uploaded by
mffritzCopyright:
Available Formats
1
Fe-C Phase Diagram
2
At room temperature it exists as ferrite,
or iron.
Upon heating pure Iron experiences two
changes in crystal structure.
When we heat it to 912C it experiences a
polymorphic transformation to austenite,
or iron
At 1394C austenite reverts back to a BCC
phase called ferrite.
1394C
Ferrite
BCC
912C
iron
Austenite
FCC
25C
Ferrite
BCC
1538C
Melts
Pure Iron
2
3
Fe-Fe
3
C Phase Diagram
Only part of the phase
diagram is shown.
The left axis is pure
iron.
On the right the phase
diagram only extends
to 6.70 wt%C
At this concentration
the intermediate
compound iron
carbide, or cementite
(Fe
3
C) is formed
4
Austenite
+ Fe
3
C
Development of Microstructures in Iron-Carbon
Alloys
(Pearlite) 3C Fe +
Various microstructures can be
produced in steel alloys
depending on
carbon content
heat treatment
Equilibrium (slow) cooling from
the region through the eutectoid
composition of 0.76 wt% C
% and Fe
3
C in eutectoid pearlite
3
5
Formation of Pearlite
Micrograph of eutectoid
steel, showing pearlite
microstructure.
Schematic representation of
the formation of pearlite
from austenite
0.76 wt% C
0.022 wt%C
6.7 wt%C
6
Hypo-eutectoid Composition (wt% C <0.76)
+
Upon cooling enter a two-phase
region
Composition 0.002 and 0.76 wt% C
Below 727C the remaining austenite
transforms to pearlite
C Fe3 +
4
Hypo-eutectoid Composition (0.38 wt% C)
White regions:
Proeutectoid
Ferrite
Dark regions:
Pearlite
Close-spaced
layers
Unresolved at this
magnification
Pearlite
wider-spaced
layers
91 m
8
Computing the relative amounts of
proeutectoid and pearlite
Similar to previous lecture.
Use the Lever Rule in
conjunction with a tie line
For hypoeutectic composition
C'
0,
fractions of pro-eutectic ,
and pearlite are:
(total) and Fe
3
C
5
9
Computing the relative amounts of
proeutectoid and pearlite
For hypereutectic composition
C'
1
(total) and Fe
3
C
10
Hyper-eutectoid Composition (wt% C >0.76)
Upon cooling enter a two-phase
region
Composition between 0.76 and 2.14
wt% C
The pro-eutectoid cementite phase has
begun to form along the grain
boundaries
C Fe3 +
6
11
Hyper-eutectoid Composition (1.40 wt% C)
White Pro-
eutectoid cementite
network
Pearlite colonies
30 m
12
Mechanical Properties of Steels
The mechanical properties of steel are largely
dictated by the phase transformations they undergo
upon cooling
If we heat steel to the single phase austenite
region and vary the cooling rate we can control the
microstructure.
Understanding phase transformations of metallic
alloys
7
13
Example: Railway Rails
Eutectoid composition of 0.76 wt%C
100% Pearlite structure
Pearlite is a natural composite
The strength of Pearlite is dictated by its
interlaminar spacing, S (m)
1
4 . 46 140
+ = S y
S
Increased cooling rates
14
Pearlite
Coarse Pearlite Fine Pearlite
8
15
The rate of transformation
of the austenite to pearlite
is dependent on
temperature
This temperature
dependence can be plotted
as % transformation vs.
log. time
Isothermal Transformation Diagrams
Data was collected for
each curve, after rapid
cooling of 100% austenite
to the temperature
indicated
temperature was then
keep constant
throughout the course of
the reaction
16
More convenient to
represent time-
temperature dependence
isothermal
transformation diagram
generated from the %
transformation-versus-
log. times measurements
Note the eutectoid
temperature (727C)
Many tests conducted to
these construct curves.
Isothermal Transformation Diagrams
9
17
Isothermal Transformation Diagrams
18
Spheroidite
Hold steel at high temperature
Large drop in strength occurs
But ductility is greatly
increased
30 m
10
19
Formation of Martensite
Non-Equilibrium Cooling
Carbon must diffuse (move)
in order to form pearlite
Diffusion takes time +
temperature
If the sample is quenched
(plunged into water)
A supersaturated and
unstable structure is formed.
20
Characteristics of Martensite Transformation
Occurs by a non-diffusional process
Transformation occurs extremely fast, i.e. at the
speed of sound
Transformation occurs at temperatures well below
eutectoid temperature
Martensite is very hard (i.e. high
y
) but is
completely brittle
Not useful as an engineering material
Must be tempered
11
21
Martensite
Quench
Needle shaped
White areas austenite
quenched too fast
Tempered
564C
small particles cementite
matrix is ferrite
24.6 m 2 m
Effect of Carbon Content on Hardness
Brinell Hardness 0 - 320 Brinell Hardness 0 - 750
12
23
Effect of Carbon Content on Yield and
Tensile Strength
You might also like
- Microstructure of Metal MaterialsDocument70 pagesMicrostructure of Metal MaterialsEddyWang100% (14)
- ASTM E407-07 Standard Practice For Microetching Metals and AlloysDocument22 pagesASTM E407-07 Standard Practice For Microetching Metals and AlloysRifqiMahendraPutra100% (3)
- The Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 21 to EN 39From EverandThe Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 21 to EN 39Rating: 5 out of 5 stars5/5 (1)
- Fe C Phase DiagramDocument12 pagesFe C Phase DiagramDwi Cahyo NugrohoNo ratings yet
- Cast Iron Phase DiagramDocument97 pagesCast Iron Phase DiagramBhargav Suvagiya100% (1)
- 1 - Heat TreatmentDocument61 pages1 - Heat TreatmentMohamed Karim MohamedNo ratings yet
- TTT and CCT DiagramDocument24 pagesTTT and CCT DiagramArun Raj A C100% (2)
- TTT N AvramiDocument40 pagesTTT N AvramiAmirul AminNo ratings yet
- 03 - Iron - Iron CarbideDocument35 pages03 - Iron - Iron CarbidebotobotoakbarNo ratings yet
- TTT Phase DiagramDocument9 pagesTTT Phase Diagramhari krishnaNo ratings yet
- Publication 4 11889 199Document9 pagesPublication 4 11889 199Mulia AridhoNo ratings yet
- TTT CurvesDocument4 pagesTTT Curvesmanas310jntuhNo ratings yet
- FeC and TTT DiagramsDocument12 pagesFeC and TTT DiagramsMohamed El-WakilNo ratings yet
- EN1101 - MJE - Part 10 - Steel Phase Diagrams - LCDocument13 pagesEN1101 - MJE - Part 10 - Steel Phase Diagrams - LCnwankwo chubyNo ratings yet
- TTT DiagramDocument19 pagesTTT DiagramShah SaudNo ratings yet
- TTTDocument7 pagesTTTMuhammad Hery Al-GhiffaryNo ratings yet
- Review MetalDocument38 pagesReview MetalnisannisaNo ratings yet
- Fe-C Phase Transformations and Hardening of Steel, ContinuedDocument21 pagesFe-C Phase Transformations and Hardening of Steel, ContinuedchenshicatherineNo ratings yet
- The Iron-Iron Carbide Equilibrium DiagramDocument15 pagesThe Iron-Iron Carbide Equilibrium DiagramjhangeerNo ratings yet
- 1 - Heat TreatmentDocument61 pages1 - Heat TreatmentMohamed El SayadNo ratings yet
- Eng Mat Chapter 4Document126 pagesEng Mat Chapter 4VC Chua Yee LeongNo ratings yet
- Lecture Chapter 4 Phase Transformation and Metal AlloysDocument79 pagesLecture Chapter 4 Phase Transformation and Metal AlloysWinni TanNo ratings yet
- Engineering Material-II: Iron Carbide Phase DiagramDocument16 pagesEngineering Material-II: Iron Carbide Phase DiagramAla ZiNo ratings yet
- TTT 11Document55 pagesTTT 11MrDOTNo ratings yet
- Iron - Carbon Phase DiagramDocument33 pagesIron - Carbon Phase Diagramvishnu anand100% (2)
- TTT NotesDocument41 pagesTTT NotesshivanigopiNo ratings yet
- TTTDocument13 pagesTTTharitha gorijalaNo ratings yet
- Materials of Construction and Selection: Faculty of Chemical Engineering Universiti Teknologi MaraDocument80 pagesMaterials of Construction and Selection: Faculty of Chemical Engineering Universiti Teknologi MaraAisyah Addia AzizanNo ratings yet
- EMM LectureDocument38 pagesEMM Lecturelatendra kumar srivastavNo ratings yet
- Heat Treatment Part 1Document32 pagesHeat Treatment Part 1Naman DaveNo ratings yet
- Grey Cast IronDocument97 pagesGrey Cast IronSiddharth GuptaNo ratings yet
- Phase Transformations: Ferrite - BCCDocument65 pagesPhase Transformations: Ferrite - BCCAhsan JunaidNo ratings yet
- Heat TreatmentDocument56 pagesHeat TreatmentAakarsh RastogiNo ratings yet
- 4Document63 pages4ARJUN PESARU 19BME0161No ratings yet
- Heat Treatment - Critical Temperatures - Transformation On Heating / CoolingDocument23 pagesHeat Treatment - Critical Temperatures - Transformation On Heating / Coolingsamurai7_77No ratings yet
- Ch-8 Compatibility ModeDocument58 pagesCh-8 Compatibility Modedreamgurl9011No ratings yet
- Presentation On Heat TreatmentDocument43 pagesPresentation On Heat Treatmentgosaye desalegnNo ratings yet
- Professor Joe Greene Csu, ChicoDocument20 pagesProfessor Joe Greene Csu, ChicotripsachinNo ratings yet
- Iron-Carbon DiagramDocument3 pagesIron-Carbon DiagramnaniNo ratings yet
- 06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramDocument42 pages06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramTalaat Ahmed Mohamed El-Benawy100% (2)
- 3 Fe-Fe3C Phase DiagramDocument33 pages3 Fe-Fe3C Phase DiagramRajat Mishra100% (1)
- FALLSEM2019-20 MEE1005 ETH VL2019201001078 Reference Material I 29-Aug-2019 Fe-Fe3C Phase DiagramDocument33 pagesFALLSEM2019-20 MEE1005 ETH VL2019201001078 Reference Material I 29-Aug-2019 Fe-Fe3C Phase DiagramFazal KhanNo ratings yet
- Lec 7 Fe C DiagramDocument45 pagesLec 7 Fe C DiagramAdnan MehmoodNo ratings yet
- Heat Treatment Lecture NotesDocument48 pagesHeat Treatment Lecture Notes22210021 TANWADE RUTURAJ RAVINDRANo ratings yet
- Heat Treatment of SteelsDocument41 pagesHeat Treatment of Steelsyaswanth1992No ratings yet
- Heat Treatment of Steel PDFDocument8 pagesHeat Treatment of Steel PDFkaviatchennai100% (2)
- Time Temperature Transformation (TTT) or IsothermalDocument26 pagesTime Temperature Transformation (TTT) or IsothermalAfredo TrilasetyaNo ratings yet
- IRON - CARBON DiagramDocument15 pagesIRON - CARBON Diagramgadde39100% (2)
- Iron Carbon Diagram 6Document15 pagesIron Carbon Diagram 6Harris DarNo ratings yet
- Heat TreatmentDocument34 pagesHeat Treatmentrahul72No ratings yet
- TTT DiagramDocument26 pagesTTT Diagrammeckup123No ratings yet
- Carbon Steels: ME 215/205:materials Science LaboratoryDocument9 pagesCarbon Steels: ME 215/205:materials Science Laboratoryfaizan997No ratings yet
- Chapter 5 ContDocument39 pagesChapter 5 ContShishajimooNo ratings yet
- 8.heat TreatmentDocument7 pages8.heat Treatmentrohan_n_desai100% (2)
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo ratings yet
- Welding Inspection, Steels Multi - Choice Question Paper (MFY-007)Document7 pagesWelding Inspection, Steels Multi - Choice Question Paper (MFY-007)Moses_JakkalaNo ratings yet
- Inverse Heat ConductionDocument23 pagesInverse Heat ConductionhsemargNo ratings yet
- Hardenability of Steel PDFDocument59 pagesHardenability of Steel PDFMOHAC KILICASLANNo ratings yet
- Ni HardMaterialDataandApplications 11017 PDFDocument28 pagesNi HardMaterialDataandApplications 11017 PDFhesamalaNo ratings yet
- Valvulas 41000 MasoneilanDocument35 pagesValvulas 41000 MasoneilanGabriel VelardeNo ratings yet
- Material Metallurgy - MCQDocument45 pagesMaterial Metallurgy - MCQSushant GhurupNo ratings yet
- Welding of Ferritic CreepDocument3 pagesWelding of Ferritic CreepMuhammed SulfeekNo ratings yet
- Microstructure of Rapidly Solidified Cu-Al-Ni Shape Memory Alloy RibbonsDocument10 pagesMicrostructure of Rapidly Solidified Cu-Al-Ni Shape Memory Alloy RibbonsMarité MalachevskyNo ratings yet
- Eff Ect of Heat Treatment Parameters On Material Properties of AISI 4140 SteelDocument3 pagesEff Ect of Heat Treatment Parameters On Material Properties of AISI 4140 SteelMatheus Paes PeçanhaNo ratings yet
- The Kinetics of Isothermal Martensitic Transformation of Zirconia Containing A Small Amount of YttriaDocument7 pagesThe Kinetics of Isothermal Martensitic Transformation of Zirconia Containing A Small Amount of YttriaShameekaNo ratings yet
- Lista MateriałówDocument7 pagesLista MateriałówVanessa KowalskaNo ratings yet
- Forming Limit Diagram of Advanced High Strength Steels (AHSS) BasedDocument7 pagesForming Limit Diagram of Advanced High Strength Steels (AHSS) BasedAnup AwateNo ratings yet
- International Journal of Plasticity: Rolf Mahnken, Andreas Schneidt, Thomas AntretterDocument22 pagesInternational Journal of Plasticity: Rolf Mahnken, Andreas Schneidt, Thomas AntretterKhouloud GharbiNo ratings yet
- Critical Review of Hot Stamping Technology For Automotive SteelsDocument13 pagesCritical Review of Hot Stamping Technology For Automotive SteelsFernando BarreraNo ratings yet
- Sub Zero Treatment FinalDocument20 pagesSub Zero Treatment FinalcristisoimosanNo ratings yet
- Material Science and Engineering Ch. 11 SolDocument56 pagesMaterial Science and Engineering Ch. 11 SolPatrick Gibson100% (1)
- Kaljenje I Drugo Po Boji Teorija MalaDocument7 pagesKaljenje I Drugo Po Boji Teorija MalaAnonymous dmhNTyNo ratings yet
- AISI 4340 HSLA Under Quenched and Tempered Conditions PDFDocument9 pagesAISI 4340 HSLA Under Quenched and Tempered Conditions PDFMs3a ProduksiNo ratings yet
- Etchant Composition Conc. Conditions Comments Kalling's No. 1Document1 pageEtchant Composition Conc. Conditions Comments Kalling's No. 1Nick LaveryNo ratings yet
- What Is A Jominy End Quench Test?Document18 pagesWhat Is A Jominy End Quench Test?faqhrulNo ratings yet
- Heat TreatmentDocument30 pagesHeat TreatmentDr-Bharath Vedashantha MurthyNo ratings yet
- The Jominy End Quench TestDocument4 pagesThe Jominy End Quench TestFaiz AmeeriNo ratings yet
- Metallurgical Characterization of M-Wire Nickel-Titanium Shape Memory Alloy Used For Endodontic Rotary Instruments During Low-CDocument3 pagesMetallurgical Characterization of M-Wire Nickel-Titanium Shape Memory Alloy Used For Endodontic Rotary Instruments During Low-C4umNo ratings yet
- Cle-Line CLF Catalog Rev1016Document127 pagesCle-Line CLF Catalog Rev1016Gilberto GarciaNo ratings yet
- The Morphology, Crystallography, and Chemistry of Phases in As-Cast Nickel-Aluminum BronzeDocument9 pagesThe Morphology, Crystallography, and Chemistry of Phases in As-Cast Nickel-Aluminum BronzeKay WhiteNo ratings yet
- Schaffler Diagram For High MN SteelsDocument4 pagesSchaffler Diagram For High MN SteelsEliel OrtizNo ratings yet
- A 1033 - 04 Qtewmzm - PDFDocument14 pagesA 1033 - 04 Qtewmzm - PDFTiến Lượng NguyễnNo ratings yet
- A Study of Austenite Precipitate Growth in Duplex Stainless Steel PDFDocument31 pagesA Study of Austenite Precipitate Growth in Duplex Stainless Steel PDFHarold Armando Muñoz100% (2)