Professional Documents
Culture Documents
CHM456 Tutorial 2 MAY 2012 Chapter 4,5 and 6: CL A) B) C)
Uploaded by
wa2345Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHM456 Tutorial 2 MAY 2012 Chapter 4,5 and 6: CL A) B) C)
Uploaded by
wa2345Copyright:
Available Formats
CHM456 TUTORIAL2 MAY2012
CHAPTER4,5AND6
QUESTION 1
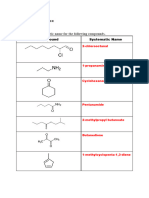
Give the IUPAC names for the following compounds.
Cl
Br
F
a)
b) c)
d) e)
f)
QUESTION 2
Consider the 3 different butane conformations as shown below
a) Name each of the butane conformation (I-III).
b) Arrange the stability of the conformation in increasing order. Explain your answer.
QUESTION 3
Consider the following reaction scheme.
CHM456 TUTORIAL2 MAY2012
CHAPTER4,5AND6
a) Give
i) the reagents of P, Q, R and U.
ii) the structure of the products of S and T.
b) Name the rule which dictates the product of S.
QUESTION 4
Classify the mechanism of each of the following reactions as SN1, SN2, E1 or E2.
QUESTION 5
Propose a mechanism leading to the following products.
CHM456 TUTORIAL2 MAY2012
CHAPTER4,5AND6
QUESTION
a) Using examples, state the differences between E1 and E2 elimination reactions.
b) Explain the formation of approximately 50% inverted and 50% racemic products from an
SN1 reaction.
QUESTION 5
a) Write the mechanisms for the following conversions.
QUESTION 6
Each of the following alcohols, Y and Z, has been subjected to acid catalyzed dehydration
and yields a mixture of two isomeric alkenes. Draw the isomers of the alkenes in each case,
and determine which one is the major product.
QUESTION 7
(S)-3-bromo-3-methylhexane is reacted with a strong nucleophile of thiol (SH) to give W
as the product via SN2 reaction as shown below.
i) Determine whether the starting material of (S)-3-bromo-3-methylhexane is optically
active or not.
ii) Give the structure of the product, W.
iii) Suggest the absolute configuration of W.
iv) If the nucleophile of SH" is changed to a weaker nucleophile of H2O, give the
structure of the new product and its stereochemistry. Explain your answer.
You might also like
- Pillared Metal-Organic Frameworks: Properties and ApplicationsFrom EverandPillared Metal-Organic Frameworks: Properties and ApplicationsNo ratings yet
- 2013 Homework KimorDocument23 pages2013 Homework KimorDiamondNadinaNo ratings yet
- Macromolecular Chemistry—8: Plenary and Main Lectures Presented at the International Symposium on Macromolecules Held in Helsinki, Finland, 2—7 July 1972From EverandMacromolecular Chemistry—8: Plenary and Main Lectures Presented at the International Symposium on Macromolecules Held in Helsinki, Finland, 2—7 July 1972K. SaarelaNo ratings yet
- Kendriya Vidyalaya Sangathan-Bangalore Region IDocument5 pagesKendriya Vidyalaya Sangathan-Bangalore Region IjagpreetNo ratings yet
- Theory and Applications of the Empirical Valence Bond Approach: From Physical Chemistry to Chemical BiologyFrom EverandTheory and Applications of the Empirical Valence Bond Approach: From Physical Chemistry to Chemical BiologyFernanda DuarteNo ratings yet
- CHEM1280 2011 Midterm Exam PDFDocument3 pagesCHEM1280 2011 Midterm Exam PDFLouisNo ratings yet
- Dynamic Covalent Chemistry: Principles, Reactions, and ApplicationsFrom EverandDynamic Covalent Chemistry: Principles, Reactions, and ApplicationsNo ratings yet
- Aldehyde, Ketone & Carboxylic Acid - Xii Group-2-2023Document14 pagesAldehyde, Ketone & Carboxylic Acid - Xii Group-2-2023Shashwat R TripathyNo ratings yet
- For The Following Cyclic Compounds, Determine Whether Each Is Aromatic, Anti-Aromatic or Non-AromaticDocument11 pagesFor The Following Cyclic Compounds, Determine Whether Each Is Aromatic, Anti-Aromatic or Non-AromaticRaja AkmalNo ratings yet
- Assingment Fundamental Organic Chemistry Chm457Document4 pagesAssingment Fundamental Organic Chemistry Chm457Natasha AdreenaNo ratings yet
- Pharm D POC QuestionsDocument16 pagesPharm D POC Questionspradeep36No ratings yet
- 2015 Apr-Biochemistry & Physical ChemistryDocument2 pages2015 Apr-Biochemistry & Physical ChemistryD PrajnaNo ratings yet
- NSS Chemistry Part 11 Chemistry of Carbon CompoundsDocument47 pagesNSS Chemistry Part 11 Chemistry of Carbon CompoundsFelix YueNo ratings yet
- Tutorial 3 NSC 2410 Unit 2 Biomolecules Proteins JULY 2021Document3 pagesTutorial 3 NSC 2410 Unit 2 Biomolecules Proteins JULY 2021Boyd benson kayomboNo ratings yet
- Subjective Question BankDocument2 pagesSubjective Question BankWajahat AliNo ratings yet
- Post Mid Term9th PaperDocument7 pagesPost Mid Term9th PaperJyoti SumanNo ratings yet
- CHE 232 Test 1Document11 pagesCHE 232 Test 1moatlhodiNo ratings yet
- SCGS F.7 AL Chemistry Assignment 2 - HALOALKANESDocument1 pageSCGS F.7 AL Chemistry Assignment 2 - HALOALKANESsachinkurhekarNo ratings yet
- 2nd Sem End July 2015-ModifiedDocument4 pages2nd Sem End July 2015-ModifiedbgroyNo ratings yet
- CBSE 12 Chemistry Question Paper 2010 PDFDocument33 pagesCBSE 12 Chemistry Question Paper 2010 PDFsarvansirNo ratings yet
- Part IAInorg 2008Document12 pagesPart IAInorg 2008Chris LittleNo ratings yet
- Assignment 1-2 MSE21 Ch1227Document18 pagesAssignment 1-2 MSE21 Ch1227mr.abdullah03mseNo ratings yet
- Alkenes and AlkynesDocument2 pagesAlkenes and AlkynesLewis AlfonsoNo ratings yet
- Chemistry 2003 Sample PaperDocument3 pagesChemistry 2003 Sample PaperSachin KukrejaNo ratings yet
- Tutorial 2 PDFDocument3 pagesTutorial 2 PDFAlees RahaizanNo ratings yet
- Chap 15-24Document49 pagesChap 15-24Linh NguyễnNo ratings yet
- Answers 7Document22 pagesAnswers 7SureshkumaryadavNo ratings yet
- Chapter 4 QuestionsDocument2 pagesChapter 4 Questionsdaniday1977100% (1)
- Orgo 1 QuzzesDocument34 pagesOrgo 1 QuzzesDanny NguyenNo ratings yet
- Organic Chem ExDocument15 pagesOrganic Chem Exwan zhouNo ratings yet
- OC Part B QuestionsDocument10 pagesOC Part B QuestionsSunanda 2004No ratings yet
- Iuniversit Ifg Asgo: Degree Examination Level-2Document14 pagesIuniversit Ifg Asgo: Degree Examination Level-2staticfmNo ratings yet
- Chapter 7 QuestionsDocument4 pagesChapter 7 Questionsdaniday19770% (1)
- Mescellaneous Questions From Chapters 1-3Document12 pagesMescellaneous Questions From Chapters 1-3dgetachew513No ratings yet
- Alkanes and AlkenesDocument7 pagesAlkanes and Alkenestminghui3.86No ratings yet
- Chemistry Unit 4 Goodie BagDocument29 pagesChemistry Unit 4 Goodie BagJacob Salkin100% (2)
- Chemistry 3719 Old ExamsDocument300 pagesChemistry 3719 Old ExamsHY-11 Đỗ Quốc TiệpNo ratings yet
- Tutorial 13 ArenesDocument2 pagesTutorial 13 ArenesDomNo ratings yet
- 05 Chemistry UG MODEL PAPERS ANU 2020-21Document8 pages05 Chemistry UG MODEL PAPERS ANU 2020-21Maria Rayappan S.No ratings yet
- CH4103-CH4153 Organic Chemistry 2A - E.O'Reilly M.Zacharska Autumn 2017Document8 pagesCH4103-CH4153 Organic Chemistry 2A - E.O'Reilly M.Zacharska Autumn 2017tadhg.barrett2112No ratings yet
- Exam 3 ReviewDocument12 pagesExam 3 ReviewEvan TryonNo ratings yet
- Sp2001 Final Organic II 200pts (Weighted As 300) : RNH C N H O O O R RDocument25 pagesSp2001 Final Organic II 200pts (Weighted As 300) : RNH C N H O O O R RUmmi Khairani UrfaNo ratings yet
- Che230 Exam 3 Aq15Document7 pagesChe230 Exam 3 Aq15Emily MosherNo ratings yet
- Haloalkanes Q BankDocument7 pagesHaloalkanes Q BankVishnuNo ratings yet
- SCH 2202 Organic Chemistry IiDocument3 pagesSCH 2202 Organic Chemistry Iimichael100% (1)
- Q.P. Code: 564252Document3 pagesQ.P. Code: 564252vinay0717No ratings yet
- Organic PaperDocument2 pagesOrganic PaperBablu RajputNo ratings yet
- CHM 102Document4 pagesCHM 102Fumzy AdelakunNo ratings yet
- Assignment - HydrocarbonsDocument7 pagesAssignment - HydrocarbonsYash KumarNo ratings yet
- Tutorial 3: CHM258 - SEP18-JAN19Document4 pagesTutorial 3: CHM258 - SEP18-JAN19OberonNo ratings yet
- TEST-1 KetoneDocument3 pagesTEST-1 Ketonecarsk403No ratings yet
- Organ Part I A PaperDocument10 pagesOrgan Part I A PaperMinh TieuNo ratings yet
- Exam II - '05Document3 pagesExam II - '05kitthiNo ratings yet
- CL-XII SC Chemistry Pre BoardDocument15 pagesCL-XII SC Chemistry Pre BoardRapelly NagarajuNo ratings yet
- Hydrocarbons JeeDocument9 pagesHydrocarbons JeeDanish AlamNo ratings yet
- Tutorail Sheet 1 NSC 2210Document3 pagesTutorail Sheet 1 NSC 2210Panashe MaluwaNo ratings yet
- Sample Paper Gr11Document3 pagesSample Paper Gr11Enoca AJNo ratings yet
- Organic SynthesisDocument5 pagesOrganic SynthesisGuruKPONo ratings yet
- Inter 2nd Year ChemistryDocument20 pagesInter 2nd Year ChemistryRsp Srinivas50% (4)
- Chemistry SQP XII PDFDocument14 pagesChemistry SQP XII PDFIshikaGuptaNo ratings yet
- Graph of Molar ConductivityDocument1 pageGraph of Molar Conductivitywa2345No ratings yet
- English Sem 6Document2 pagesEnglish Sem 6wa2345No ratings yet
- CHM556 Organic Chemistry Ii Laboratory: The TechniquesDocument3 pagesCHM556 Organic Chemistry Ii Laboratory: The Techniqueswa2345No ratings yet
- EventDocument10 pagesEventwa2345No ratings yet
- K L S E: Uala Umpur Tock XchangeDocument1 pageK L S E: Uala Umpur Tock Xchangewa2345No ratings yet
- Tutorial Chapter 2Document3 pagesTutorial Chapter 2wa2345No ratings yet
- Tutorial Chapter 3Document21 pagesTutorial Chapter 3wa2345No ratings yet
- Example of Appointed LetterDocument1 pageExample of Appointed Letterwa2345No ratings yet
- K L S E: Uala Umpur Tock XchangeDocument1 pageK L S E: Uala Umpur Tock Xchangewa2345No ratings yet
- K L S E: Uala Umpur Tock XchangeDocument1 pageK L S E: Uala Umpur Tock Xchangewa2345No ratings yet
- Tutorial Chapter 2Document3 pagesTutorial Chapter 2wa2345No ratings yet
- PDLC StepsDocument1 pagePDLC Stepswa2345No ratings yet
- Tutorial Chapter 2Document3 pagesTutorial Chapter 2wa2345No ratings yet
- PDLC StepsDocument1 pagePDLC Stepswa2345No ratings yet
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Pocket Guide to Flanges, Fittings, and Piping DataFrom EverandPocket Guide to Flanges, Fittings, and Piping DataRating: 3.5 out of 5 stars3.5/5 (22)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Transformer: The Deep Chemistry of Life and DeathFrom EverandTransformer: The Deep Chemistry of Life and DeathRating: 4.5 out of 5 stars4.5/5 (13)