Professional Documents
Culture Documents
InternalAuditSOP 012413
InternalAuditSOP 012413
Uploaded by
noormuddassirkhanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
InternalAuditSOP 012413
InternalAuditSOP 012413
Uploaded by
noormuddassirkhanCopyright:
Available Formats
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 1 of 29
Approved by:
Technical Director
______________________________ (Name)
______________________________ (Signature)
_______________ (Initials)
_________________ (Date)
Quality Assurance Officer
______________________________ (Name)
______________________________ (Signature)
_______________ (Initials)
_________________ (Date)
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 2 of 29
Revision History
Rev
Date
Description of Change
9/5/07
Name
Initial Release
01/13/13
Name
Updated all sections. Reformatted
Appendix A.
Distribution List / Location
This SOP is to be distributed to those individuals involved in the internal audit process of
the lab.
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 3 of 29
Annual Review (The review is to be documented if the document has not been revised in
the past 12 months)
_______________________
Signature
____________________
Title
_______
Date
______________________
Signature
____________________
Title
_______
Date
___________________
Signature
____________________
Title
_______
Date
______________________
Signature
____________________
Title
_______
Date
___________________
Signature
____________________
Title
_______
Date
Training Record
The following laboratory staff have read and agree to follow the latest version of the SOP.
_____________________
______________________ _______
_______
Signature
Name
Initials
Date
_____________________
Signature
______________________
Name
_______
Initials
_______
Date
_____________________
Signature
______________________
Name
_______
Initials
_______
Date
_____________________
Signature
______________________
Name
_______
Initials
_______
Date
_____________________
Signature
______________________
Name
_______
Initials
_______
Date
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 4 of 29
Table of Contents
1. Purpose...........................................................................................................................5
2. Scope...............................................................................................................................5
3. Responsibilities...............................................................................................................5
4. Procedure........................................................................................................................6
5. Related Documentation and References......................................................................8
6. Definitions.......................................................................................................................8
APPENDIX A.....................................................................................................................9
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 5 of 29
1. Purpose
To ensure that the procedures in the quality manual, related to quality systems, and the
labs method manual, related to testing activities, are being followed.
To determine the effectiveness of the labs procedures in controlling the quality of data
reported
To identify, correct, and implement any changes needed in any of the quality system
and testing activities procedures found to be deficient
To ensure all deficiencies in the labs quality system and testing activities are
documented though its corrective action process
2. Scope
The internal audit SOP and associated checklist is used to audit, on an annual basis, the
labs quality system, policies and procedures, work instructions, analytical records, and
reports. In addition, the lab audits its testing activities (each method-technology) on an
annual basis.
3. Responsibilities
Quality Assurance (QA) Manager or QA Officer (QAO):
is knowledgeable and trained in quality system requirements, including internal
audits
initiates all internal audits and ensures they are conducted in an efficient and timely
manner
delegates responsible, trained staff, if applicable, to carry out specific audits of
testing activities
notifies laboratory management , including the technical director, of any
deficiencies (findings) in the quality system or testing activities
documents and monitors corrective actions
documents and tracks staff who have completed auditor training
Auditor:
has completed auditor training
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 6 of 29
has sufficient experience in performing audits
performs audits in an efficient and timely manner
reports all findings to the QAO
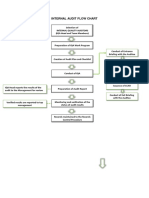
4. Procedure
4.1 The Audit Team
The Quality Assurance Manager or QA Officer (QAO) selects trained staff, if applicable, to
perform the audits defined in this procedure. If trained staff is limited, the QAO and/or
technical director may perform the audits. If trained staff is not limited, the QAO will
designate one of the trained staff to serve as the lead auditor. When ever possible, auditors
are selected from a function not directly involved in the audit.
4.2 Training
Auditors are trained in auditing techniques. Training consists of reading and
understanding this procedure and reference material related to internal auditing, and where
possible, shadowing a trained auditor or completing a formal, external training course. The
auditors are also provided with the applicable auditing guidelines and checklists. Both
quality system and method-specific checklists are to be provided to the auditor.
Evidence of the training includes a signature that the auditor has read and understands this
procedure (see page 3). It may also include documentation of any external seminars or
course work related to quality system auditing. All training records are to be kept for a
minimum of five years.
4.3 Audit Plan
The entire quality system, including testing activities, is audited on an annual basis. The
maximum interval between audits is twelve months. The frequency may be adjusted for
new procedures or deficiencies that resulted from complaints.
The QAO creates the audit schedule. The audit schedule defines the following:
timeframe of the audit
scope of audit
elements and/ or areas to be audited
4.4 Performing the Audit
The QAO notifies the supervisors of the areas to be audited at least a month in advance.
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 7 of 29
The QAO briefs the auditors on the audit procedures and the areas to be audited. The
auditors are to prepare prior to the audit by familiarizing themselves with the audit
procedures.
During the audit, auditors use applicable checklists. They record all findings on the
checklists. The findings are discussed with the staff responsible for performing the function
that was found to be deficient.
4.5 Deficiency Report and Corrective Action Response
A deficiency report is generated by the auditor for each legitimate finding. The QAO
makes the final decision as to whether the finding is legitimate if it can not be resolved
between the auditor and the staff audited. The QAO presents the final report to the
audited staff, as well as, laboratory management, including the technical director.
The audited staff responds to the deficiency report in a manner prescribed by the labs
corrective action procedures, which are included in the Quality Manual. The corrective
action must be completed within 90 days of the date of the finding.
When deficiencies cast doubt on the correctness or validity of the calibration or test results
reported, the lab needs to immediately notify its clients of the situation. A record of the
client notification must be maintained.
4.6 Closing an Audit
Audit findings are closed upon completion of an effective corrective action for each of the
findings. All documents related to the audit, including checklists, deficiency reports,
corrective action responses, are maintained by the QAO.
4.7 Review and Evaluation
The QAO verifies successful implementation of the corrective action by observing
objective evidence supplied by the audited staff as part of the corrective action process.
Follow-up is performed by the QAO, or designated staff, as part of the next scheduled
audit to verify the effectiveness of the corrective actions that were implemented. IN
addition, the QAO reviews the audit report with laboratory management, including the
technical director, as part of the labs annual management review.
5. Related Documentation and References
Audit Plan
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 8 of 29
Audit Checklists, including method-specific checklists
Deficiency (Audit) Report
Corrective Action Response (CAR)
Corrective Action Procedures as noted in Quality Manual
National Environmental Laboratory Accreditation Conference (NELAC), 2003 NELAC Standard,
Approved June 5, 2003, Effective July 1, 2003, 324 pp (EPA/600/R-04/003).
National Environmental Laboratory Accreditation Conference (NELAC), 2009 NELAC Standard,
Approved August 24, 2009, Effective July 1, 2011.
New York State Department of Health (NYS DOH) Environmental Laboratory Approval Program
(ELAP), method-specific checklists,
http://www.wadsworth.org/labcert/elapcert/appforms.htm
New York State Department of Health (NYS DOH), NYCRR Subpart 55-2, Approval of
Laboratories Performing Environmental Analysis, Sections 55-2.1 through 55-2.12 effective
November 17, 2004, and Section 55-2.13 effective October 6, 2004.
6. Definitions
Audit Finding - A conclusion of importance based on observation(s). An undesirable
deviation or nonconformity.
Corrective Action - Action taken to eliminate the root cause(s) and the symptom(s) of an
existing undesirable deviation or nonconformity to prevent recurrence.
Objective Evidence - Verifiable qualitative or quantitative observations, information,
records, or statements of fact pertaining to the quality of a product or service or to the
existence and implementation of quality system element.
Quality Audit - Systematic and independent examination to determine whether quality
activities and related results comply with planned arrangements and whether these
arrangements are implemented effectively and are suitable to achieve objectives
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 9 of 29
APPENDIX A
TOPIC
Organization & Management
1.) Does the laboratory have a policy to ensure its personnel are
free from any commercial, financial and other undue pressures,
which might adversely affect the quality of the work?
2.) Does the laboratory specify and document the responsibility,
authority, and interrelation of all personnel who manage,
perform or verify work affecting the quality of calibrations and
tests in job descriptions for all positions.
3.) Does the laboratory have documented certifications that
personnel performing all tests for which the laboratory is
accredited have the appropriate educational and/or technical
backgrounds?
4.) Does the laboratory nominate deputies in the case of absence
of the technical director or QA officer?
5.) Does the laboratory have documented policies and
procedures to ensure the protection of clients' confidential
information and proprietary rights?
Quality System
1.) Is the quality documentation available to, understood by, and
implemented by all laboratory personnel?
2.) Does the quality manual and related quality documentation
include the objectives and commitments by top management?
N/A
Controlled Document
Comments
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 10 of 29
3.) Does the quality manual and related quality documentation
include the organization and management structure of the
laboratory, its place in any parent organization, and relevant
organizational charts?
4.) Does the quality manual and related quality documentation
include procedures to ensure that all records required under
NELAC are retained?
5.) Does the quality manual and related quality documentation
include procedures for control and maintenance of
documentation through a document control system which
ensures that all standard operating procedures, manuals, or
documents clearly indicate the time period during which the
procedure or document was in force?
6.) Does the quality manual and related quality documentation
include procedures for achieving traceability of measurements?
7.) Does the quality manual and related quality documentation
include a list of all methods under which the laboratory performs
its accredited testing?
8.) Does the quality manual and related quality documentation
include mechanisms for ensuring that the laboratory reviews all
new work to ensure that it has the appropriate facilities and
resources before commencing such work?
9.) Does the quality manual and related quality documentation
include reference to the calibration and/or verification test
procedures used?
10.) Does the quality manual and related quality documentation
include procedures for handling submitted samples?
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 11 of 29
11.) Does the quality manual and related quality documentation
include reference to the major equipment and reference
measurement standards used as well as the facilities and services
used by the laboratory in conducting tests?
12.) Does the quality manual and related quality documentation
include reference to procedures for calibration, verification and
maintenance of equipment?
13.) Does the quality manual and related quality documentation
include reference to verification practices including interlaboratory comparisons, proficiency testing programs, use of
reference materials, and internal quality control schemes?
14.) Does the quality manual and related quality documentation
include procedures to be followed for feedback and corrective
action for failed quality control samples, or when departures
from documented policies, procedures, or NELAC standards
occur?
15.) Does the quality manual and related quality documentation
include procedures for dealing with complaints?
16.) Does the quality manual and related quality documentation
include processes/procedures for establishing that personnel are
adequately experienced in the duties they are expected to carry
out and/or receive any needed training?
17.) Does the quality manual and related quality documentation
include processes and procedures for educating and training
personnel in their ethical and legal responsibilities including the
potential punishments and penalties for improper, unethical, or
illegal actions?
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 12 of 29
18.) Does the quality manual and related quality documentation
include reference to procedures for reporting analytical results?
19.) Does the quality manual and related quality documentation
include a Table of Contents, and applicable lists of references
and glossaries, and appendices?
20.) Does the QA officer keep the quality manual current?
21.) Does the QA officer arrange for or conduct internal audits
on the entire technical operation annually and notify laboratory
management of deficiencies in the quality system and monitor
corrective action
22.) Where a complaint, or any other circumstance, raises doubt
concerning the laboratory's compliance with the laboratory's
policies or procedures, or with the requirements of this Standard
or otherwise concerning the quality of the laboratory's
calibrations or tests, does the laboratory ensure that those areas
of activity and responsibility involved are promptly audited?
23.) Does the laboratory have a procedure for the annual
management review of the quality system?
24.) Is an annual review of the quality system completed by
management to evaluate its continuing suitability and
effectiveness and make any necessary changes or improvements?
25.) Does the annual review take into account reports from
managerial and supervisory personnel, the outcome of recent
internal audits, assessments by external bodies, the results of
interlaboratory comparisons or proficiency tests, any changes in
the volume and type of work undertaken, feedback from clients,
corrective actions and other relevant factors?
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 13 of 29
26.) Are all audits and review findings and any corrective actions
that arise from them documented?
27.) Does the laboratory management ensure that corrective
actions are discharged within the agreed time frame?
28.) Does the laboratory implement checks to monitor the
quality of laboratory results using:
_a__ Internal quality control procedures (using statistical
techniques whenever possible);
_b__ Participation in PT or other interlaboratory comparisons;
_c__ Reference material and/or in-house quality control using
secondary reference materials;
_d__ Replicate testing;
_e__ Re-testing of retained samples; and/or
_f__ Correlation of results for different parameters of a sample.
29.) Does the laboratory have general procedures to be followed
when there are departures from documented policies,
procedures, and QC have occurred?
30.) Do the procedures to be followed when there is a departure
from documented policies, procedures, and QC include but not
limited to:
a___ Identify the individuals responsible for assessing each QC
data type;
b___ Identify the individuals responsible for initiating and/or
recommending corrective actions;
c___ Define how the analyst should treat the data set if the
associated QC measurements are unacceptable;
d___ Specify how out-of-control situations and subsequent
corrective actions are to be documented; and
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 14 of 29
e___ Specify procedures for management (including the QA
officer) to review corrective action reports.
31.) Is a corrective action log maintained, up-to-date?
32.) If a QC measure is out of control and the data is to be
reported, are data qualifiers reported with samples associated
with failed QC measures?
33.) Are all quality control measures assessed and evaluated on
an on-going basis, and quality control acceptance limits used to
determine the usability of the data?
34.) Does the laboratory have procedures for the development
of acceptance/rejection criteria where no method or regulatory
criteria exist?
35.) Are the quality control protocols specified by the
laboratorys method manual followed?
Training
1.) Are training records available for all technical staff that
include:
a___ Evidence that the employee has read, understands, and is
using the latest version of the labs in-house quality
documentation;
b___ Training courses or workshops on specific equipment,
analytical techniques, or lab procedures;
c___ Training courses in ethical and legal responsibilities
including the potential punishments & penalties for violations.
d___ Evidence that the employee has read; acknowledges, and
understands their personal & legal responsibilities including
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 15 of 29
potential punishments & penalties for violations; and
e___ Documentation certifying that the employee has read,
understands, and agrees to use the latest version of a test
method used; and
2.0 Are initial demonstrations, continuing demonstrations and
method certification documented through the use of the forms in
the latest approved NELAC document in Appendix C?
3.) Does the laboratory use another approach, documented in its
Quality Manual, to demonstrate capability for analytes for which
spiking is not an option and for which quality control samples
are not readily available?
4.) Does the laboratory retain all associated supporting data
necessary to reproduce the analytical results summarized in the
IDC certification statement?
5.) Is a copy of the initial demonstration of Capability Certificate
(IDC) in the personnel records for each employee performing a
test method?
6.) Do the training records of each of the technical staff include
documentation of continuing proficiency by at least one of the
following:
___ Acceptable performance of a blind sample;
___ Another demonstration of capability;
___ Successful analysis of a blind performance sample on a
similar test method using the same technology; a
___ Analysis of at least 4 consecutive lab control samples with
acceptable levels of precision and accuracy; or
___ If one of the above can be performed, the analysis of
authentic samples that have been analyzed by another trained
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 16 of 29
analyst with statistically indistinguishable results.
7.) Does the laboratory complete a new demonstration of
capability whenever there is a significant change in instrument
type, personnel, or test method?
8.) Has the laboratory management developed a proactive
program for the detection of improper, unethical, or illegal
actions?
Equipment
1.) Are maintenance procedures documented?
2.) Is each item of equipment including reference materials
labeled, marked or otherwise identified to indicate its calibration
status, when appropriate?
3.) Are maintenance records available?
Support Equipment Calibration and Traceability
1.) Does the laboratory have an established program for the
calibration and verification of its measuring and test equipment
including balances, thermometers and control standards?
2.) Are measurements made by the labs traceable to national
standards of measurement where available?
3.) Does the laboratory maintain records of all certificates that
indicate traceability to national standards of measurement and/or
statements of compliance with an identified metrological
specification?
4.) Is all support equipment calibrated annually, using NIST
traceable references when available, over the entire range in
which the equipment is used?
5.) Are the results of support equipment calibration within the
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 17 of 29
specifications required of the application for which it is used?
6.) Is support equipment removed from service until repaired or
is a deviation curve prepared and all measurements corrected for
the deviation when the calibration is not within acceptance
limits?
7.) Does the laboratory maintain records of established
correction factors to correct measurements?
8.) Are all raw data records retained to document equipment
performance?
9.) Prior to use on each working day, are balances, ovens,
refrigerators, freezers, incubators and water baths checked with
NIST traceable references (where possible) in the expected use
range?
10.) Are mechanical volumetric devices checked for accuracy on
a quarterly basis?
11.) Demonstration of sterilization for biological tests provided
by use of a continuous temperature recording or with the
frequent use of spore strips?
SOPS and Test Methods
1.) Does the laboratory have SOPs for all test methods?
2.) Are all instructions, standards, manuals and reference data
relevant to the work of the laboratory maintained up-to-date and
readily available to the staff?
3.) Are copies of SOPs assessable to all personnel?
4.) Does each SOP clearly indicate:
a___ Effective date of the SOP
b___ Revision number
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 18 of 29
c___ Signature(s) of approving authority
5.) Does the laboratory have an in-house method manual for
each accredited analyte or test method that clearly describes the
labs method?
6.) In cases where modifications are made to published methods
or where the reference test method is ambiguous or provides
insufficient detail, are any modifications, changes, or
clarifications clearly indicated?
7.) Are the practices specified by the laboratorys method
manual followed by all analysts?
8.) Are all essential quality control measures incorporated in the
labs method manual?
9.) Are all quality control measures assessed and evaluated on an
on-going basis?
10.) Does the laboratory have procedures for developing
acceptance/rejection criteria for each test method?
11.) Do the SOPs or the test method SOP reference the details
of the initial calibration procedures, including calculations
integrations, and acceptance criteria associated statistics?
12.) Is the criteria for the acceptance of an initial calibration
established (correlation coefficient or relative percent
difference)?
13.) Are the details of the continuing instrument calibration
procedure, calculations, and associated statistics included or
referenced in the test method SOP?
14.) Does the laboratory establish Standard Operating
Procedures to ensure that the reported data is free from
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 19 of 29
transcription and calculation errors?
15.) Does the laboratory establish Standard Operating
Procedures to ensure that all quality control measures are
reviewed, and evaluated before data is reported?
16.) Are calculations and data transfers subject to checks as
established in the laboratorys SOP?
17.) Do documented procedures exist for the purchase,
reception and storage of consumable materials used for the
technical operations of the laboratory?
18.) Does the laboratory retain records for all standards,
including manufacturer/vendor, the manufacturers Certificate of
Analysis or purity (if supplied), date of receipt, recommended
storage conditions, and an expiration date after which the
material shall not be used unless verified by the laboratory?
19.) Are original reagent containers labeled with the expiration
date?
20.) Are detailed records maintained on reagent and standard
preparation?
21.) Do the records of reagent and standard preparation indicate
traceability to purchased stocks or neat compounds, and include
the date of preparation and preparer's initials?
22.) Are containers of prepared reagents and standards uniquely
identified and include an expiration date and can it be linked to
the documentation of its preparation?
Sample Handling
1.) Does the laboratory have a documented system for uniquely
identifying the items to be tested, to ensure that there can be no
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 20 of 29
confusion regarding the identity of such items at any time?
2.) Does the laboratory assign a unique identification (ID) code
to each sample container received in the laboratory?
3.) Is the laboratory ID code placed on the sample container as a
durable label?
4.) Is the laboratory ID code entered into the laboratory records
(see 5.11.3.d) and does the link that associate the sample with
related laboratory activities such as sample preparation or
calibration?
5.) Does the laboratory have a written sample acceptance policy
that clearly outlines the circumstances under which samples will
be accepted?
6.) Is data from any sample which does not meet the policy
criteria flagged in an unambiguous manner clearly defining the
nature and substance of the variation?
7.) Is the sample acceptance policy made available to sample
collecting personnel and does it include at a minimum all the
policy criteria?
8.) Upon receipt, is the condition of the sample, including any
abnormalities or departures from standard condition as
prescribed in the relevant test method, recorded?
9.) Are all items specified in sample acceptance policy criteria
checked?
10.) Are all samples, which require thermal preservation,
considered acceptable if the arrival temperature is either within
+/-2C of the required temperature or in the method specified
range?
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 21 of 29
11.) For samples with a specified temperature of 4C, are
samples maintained within a temperature of just above freezing
to 6C?
12.) In cases where samples are hand delivered to the laboratory
immediately after collection and do not meet the temperature
criteria considered acceptable, is there evidence that the chilling
process has begun such as arrival on ice?
13.) Does the laboratory have procedures for checking chemical
preservation using readily available techniques, such as pH, free
chlorine or temperature, prior to or during sample preparation
or analysis?
14.) Does the laboratory implement procedures for checking
chemical preservation using readily available techniques, such as
pH, free chlorine or temperature, prior to or during sample
preparation or analysis?
15.) Are the results of all checks recorded?
16.) If the sample does not meet the sample receipt acceptance
criteria does the laboratory do any of the following:
___ Retain correspondence and/or records of conversations
concerning the final disposition of rejected
___ Fully document any decision to proceed with the analysis of
samples not meeting acceptance criteria
___ Is the condition of these samples, at a minimum, noted on
the chain of custody or transmittal form and laboratory receipt
documents?
___ Is the analysis data of these samples appropriately
"qualified" on the final report?
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 22 of 29
17.) Does the laboratory utilize a permanent, sequential log,
such as a logbook or electronic record, to document receipt of
all sample containers?
18.) Is the following information recorded in the laboratory
chronological log?
a___ Client/Project Name
b___ Date and time of laboratory receipt of sample
c___ Unique laboratory ID code (see 5.11.1)
d___ Signature or initials of the person making the entries
19.) Are samples stored away from all standards, reagents, food
and other potentially contaminating sources in such a manner as
to prevent cross contamination?
20.) Are samples, sample fractions, extracts, leachates or other
sample preparation fractions stored according to the conditions
specified by preservation protocols or according to the test
method?
21.) Does the laboratory have standard operating procedures for
the disposal of samples, digestates, leachates and extracts or
other sample preparation products?
Records
1.) Does the laboratory retain on record all original
observations, calculations and derived data, calibration records
and a copy of the test report for five years?
2.) Does the record keeping system allow historical
reconstruction of all laboratory activities that produced the
resultant sample analytical data?
3.) Is the history of the sample readily understood through the
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 23 of 29
documentation including inter-laboratory transfers of samples
and/or extracts?
4.) Do the records include the identity of personnel involved in
sampling, preparation, calibration or testing?
5.) Are all documentation entries signed or initialed by
responsible staff with the reason for the signature or initial
clearly indicated in the records? (Ex. sampled by, prepared
by, reviewed by)
6.) Are all generated data, except those that are generated by
automated data collection systems, recorded directly, promptly
and legibly in permanent ink?
7.) Are entries in records not obliterated by methods such as
erasures, overwritten files or markings?
8.) Are all corrections to record-keeping errors made by one line
marked through the error and the individual making the
correction signing (or initialing) and dating the correction?
9.) Do records that are stored or generated by computers or
personal computers (PCS) have hard copy or write-protected
backup copies?
10.) Does the laboratory have a record management system for
control of laboratory notebooks; instrument logbooks; standards
logbooks; and records for data reduction, validation storage and
reporting?
11.) Is access to archived information documented with an
access log?
12.) Is archived information protected against fire, theft, loss,
environmental deterioration, and vermin and, in the case of
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 24 of 29
electronic records, electronic or magnetic sources?
Reports
1.) Does the test report contain all information necessary for the
interpretation of the test results and all information required by
the method used?
2.) Does the facility management ensure that the appropriate
report items are in the report to the regulatory authority if the
report is prepared by another individual within the organization.
3.) Where the certificate or report contains results of tests
performed by sub-contractors, are these results clearly identified
by subcontractor name or applicable accreditation number?
4.) Does the laboratory certify that the test results meet all
requirements of NELAC or provide reasons and/or justification
if they do not?
Chemistry Quality Control
(To be used with the method checklist)
1.) Is a method blank performed 1 per batch, per matrix type per
sample extraction or preparation method?
2.) Is the analysis stopped, corrected and the problem eliminated
if the blank contamination is greater than 1/10th of the measured
sample contamination or 1/10th of the regulatory limit; or are
the results reported with appropriate data qualifying codes?
3.) Is an LCS (a sample matrix free of analytes of interest spiked
with a verified known amount of analyte) analyzed at a
minimum of 1 per batch of 20 or less samples per matrix, per
sample extraction or preparation method except for analytes for
which spiking solutions are not available?
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 25 of 29
4.) Is a matrix spike (sample prepared by adding a known mass
of target analyte to a specific amount of matrix sample)
performed at a frequency of 1 in 20 samples per matrix, per
sample extraction or preparation method?
5.) Is a matrix spike duplicate (MSD) or laboratory duplicate
performed at a frequency of 1 in 20 samples per matrix, per
sample extraction or preparation method?
6.) Is the initial instrument calibration used directly for
quantitation?
7.) Is the continuing instrument calibration verification used to
confirm the continued validity of the initial calibration?
8.) Are all initial calibrations verified with a standard obtained
from a second source?
9.) If the results of samples are not bracketed by the initial
calibration, are the results reported as having less certainty
(defined qualifiers, flags, or explanation in the case narrative)?
10.) Is the lowest calibration standard of the initial calibration
above the detection limit?
11.) When an initial calibration is not performed on the day of
analysis, does the laboratory verify the validity of the initial
calibration prior to the analysis of samples by analyzing a
continuing instrument calibration verification sample?
12.) Is continuing instrument calibration verification repeated at
the beginning and end of each analytical batch? (If an internal
standard is used, only one continuing calibration verification
must be analyzed per analytical batch)
13.) Are the concentrations of the continuing calibration
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 26 of 29
standard varied within the established calibration range?
14.) Are sufficient raw data records retained to permit
reconstruction of the initial calibration including:
a___ Calibration date
b___ Test method
c___ Instrument
d___ Analysis date
e___ Each analyte name
f___ Concentration
g___ Response
h___ Calibration curve or response factor
15.) Does the laboratory use detection limits that are determined
by the protocol in the mandated test method or applicable
regulation?
16.) Is the quality of water sources monitored and documented
to meet method specified requirements?
Quality Control for Bacteriology
(To be used with the method checklist)
1.) Are temperatures of incubators and water baths recorded
twice daily (morning & afternoon) as required by the methods as
indicated below:
a. ____
b. ____
Total Coliform bacteria incubation at 35.0 +/- 0.5
degrees Celsius (SM9221B, SM9221D,
SM9222B & EPA-600/8-78-017)
Fecal Coliform bacteria incubation at 44.5 +/- 0.2
degrees Celsius (SM9221E, SM9222D, & EPA600/8-78-017)
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 27 of 29
c. ____
Total Coliform & Escherichia coli (E. coli)
incubation at 35.0 +/- 0.5 degrees Celsius
(SM9223 + UV; Colilert, Idexx-18, & Colisure)
d. ____
E. coli incubation at 44.5 +/- 0.2 degrees Celsius
(EC with MUG or Nutrient Agar with MUG)
2.) Is the following support equipment associated with
microbiological testing checked with NIST traceable materials
(where possible)
a. ____ pH meter
b. ____ Balance(s)
c. ____ Conductivity meter
d. ____ Refrigerator(s) for sample storage and/or media
storage
e. ____ Incubators
f. ____ Water baths
3.) Is a minimum of one uninoculated control prepared and
analyzed?
4.) When the same equipment is used to prepare multiple
samples does the laboratory prepare at least one blank at the
beginning, one at the end, with additional blanks inserted after
every 10 samples?
5.) Is a known negative culture analyzed with each set of
samples.
6.) Is each lot of media tested on a monthly basis with at least
one pure culture of a known positive reaction (positive control)?
(Not required if the laboratory has at least one known positive
result of the appropriate organism during the month).
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 28 of 29
7.) Is the positive control test tested with a sample test batch?
8.) Are at least 5% of the suspected positive samples analyzed in
duplicate?
9.) In laboratories with more than one analyst performs the
testing does each analyst make parallel analyses on at least one
positive sample per month?
10.) Are the calculations, data reduction and statistical
interpretations specified by each method followed?
11.) Where the method specifies colony counts, such as
membrane filter or colony counting, is the ability of individual
analysts to count colonies verified at least once per month, by
having two or more analysts count colonies from the same
plate?
12.) In order to demonstrate traceability and selectivity, does the
laboratory use reference cultures of microorganisms obtained
from a recognized national collection or an organization
recognized by the assessor body?
13.) Are the graduations of the temperature measuring devices
appropriate for the required accuracy of measurement?
14.) Are records maintained on all laboratory reagent water
monitoring activities as below when dilution water and/or media
are prepared in house:
a_ Residual Chlorine < 1.0 mg/L.
b_ Conductivity < 2.0 umho/cm at 25 degrees Celsius
c_ Heterotrophic Plate Count < 1000 cfu per mL.
d_ Bacteriological ratio 0.8 3.0.
e_ Cd, Cr, Cu, Ni, Pb, Zn each < 0.05 mg/L, collectively
Controlled Document
Laboratory Name and contact
information
Title: Internal Audit Standard Operating Procedure and Simplified
Quality System Checklist
Doc. No. 2
Rev. No. 2
Date: 01/24/13
Page: 29 of 29
< 0.1 mg/L.
f_ Records maintained for the past five years?
Controlled Document
You might also like
- 3.9 SOP Internal Audit v1Document11 pages3.9 SOP Internal Audit v1Pramod AthiyarathuNo ratings yet
- Monastery of The Seven Rays - Year 1Document193 pagesMonastery of The Seven Rays - Year 1Lykathea Harmony Pax100% (5)
- Internal Audit SOPDocument4 pagesInternal Audit SOPIftikhar Khan100% (1)
- 'Account StatementDocument11 pages'Account StatementSikander Qazi100% (2)
- All About AvanadeDocument16 pagesAll About AvanadeSunil PatidarNo ratings yet
- Internal Quality Audit ProcedureDocument3 pagesInternal Quality Audit ProcedureAcholonu Emeka Jp100% (2)
- Sample Management Review ChecklistDocument4 pagesSample Management Review Checklistpoetoet100% (1)
- QMS 010 Classification Definition and Approval Matrix of GMP Documents SampleDocument5 pagesQMS 010 Classification Definition and Approval Matrix of GMP Documents SampleRosella Planta100% (1)
- Internal Audit SOPDocument2 pagesInternal Audit SOPNazmun100% (3)
- Q2-760-01-Control of Monitoring and Measuring DevicesDocument5 pagesQ2-760-01-Control of Monitoring and Measuring DevicesAlineNo ratings yet
- Stock VerificationDocument11 pagesStock Verificationrockyrr100% (1)
- QMS 065 SampleDocument6 pagesQMS 065 SamplebaluchakpNo ratings yet
- Internal Audit ISO 9001-2008 Checklist 1-20-12Document43 pagesInternal Audit ISO 9001-2008 Checklist 1-20-12TravisNo ratings yet
- VMware vSAN Network Design PDFDocument169 pagesVMware vSAN Network Design PDFFederico MiliaccaNo ratings yet
- Star Magazine - 18 November 2013Document74 pagesStar Magazine - 18 November 2013LouieLongNeckNo ratings yet
- AM-QMS-05-Internal Quality Audit Procedure - Ver 1Document6 pagesAM-QMS-05-Internal Quality Audit Procedure - Ver 1Deepan TravellerNo ratings yet
- InternalAuditSOP 012413Document8 pagesInternalAuditSOP 012413zubair90No ratings yet
- QSP 02 - Record Control ProcedureDocument5 pagesQSP 02 - Record Control ProcedureVivek V100% (1)
- ESCL-SOP-018, Inspection and Test Procedure For Egba Split-ClampsDocument6 pagesESCL-SOP-018, Inspection and Test Procedure For Egba Split-ClampsadiqualityconsultNo ratings yet
- Competency SOPDocument10 pagesCompetency SOPD Tech Dental Technologies100% (1)
- QP-004 Management Review ProcessDocument3 pagesQP-004 Management Review Processesraa asemNo ratings yet
- ISO 13485 PurchasingDocument5 pagesISO 13485 PurchasingSubhashNo ratings yet
- Example of An Internal Audit SOPDocument3 pagesExample of An Internal Audit SOPVeronica Sebald100% (1)
- Quality Assurance (QA) Management Procedures: By: Pharma Tips - Views: 14415 - Date: 06-May-2012Document6 pagesQuality Assurance (QA) Management Procedures: By: Pharma Tips - Views: 14415 - Date: 06-May-2012SrinivasNo ratings yet
- Sop (Internal Audit)Document6 pagesSop (Internal Audit)Arijit Pattanayak100% (2)
- Minutes of Management Review Meeting OkDocument3 pagesMinutes of Management Review Meeting Okdidar100% (4)
- Internal Audit ProcedureDocument1 pageInternal Audit ProcedureXi MoNo ratings yet
- Form IA-002A (Audit Plan) (07-13-2012)Document2 pagesForm IA-002A (Audit Plan) (07-13-2012)granburyjohnstevens100% (1)
- Procedure For Preventive ActionDocument6 pagesProcedure For Preventive ActionHamzah Abbass Sibai100% (1)
- Sample SOP of Internal Audit of A ProcessDocument10 pagesSample SOP of Internal Audit of A Processsarvjeet_kaushalNo ratings yet
- Document Control Effectiveness in ISO 15189 Accredited LaboratoriesDocument12 pagesDocument Control Effectiveness in ISO 15189 Accredited LaboratoriesinventionjournalsNo ratings yet
- Internal Audit Planning and Scheduling Sample FormatDocument3 pagesInternal Audit Planning and Scheduling Sample Formatsameh100% (2)
- Sop PurchasingDocument5 pagesSop PurchasingSteven TanNo ratings yet
- Internal Audit ProceduresDocument15 pagesInternal Audit ProceduresTait G MafuraNo ratings yet
- Iso22000 - Internal Audit ChecklistDocument2 pagesIso22000 - Internal Audit ChecklistGidion Jeffri PoerbaNo ratings yet
- Auditee Feedback Form: Internal Audit DepartmentDocument2 pagesAuditee Feedback Form: Internal Audit DepartmentNiomi GolraiNo ratings yet
- SOP-03 (Employee Performance Monitoring)Document6 pagesSOP-03 (Employee Performance Monitoring)FarhanNo ratings yet
- QSP 8.3.1 Nonconforming Product - SampleDocument3 pagesQSP 8.3.1 Nonconforming Product - SampleGladys Calvo100% (2)
- Objectives and Goals of Auditing Vendors and Production DepartmentDocument15 pagesObjectives and Goals of Auditing Vendors and Production DepartmentFfwms SpainNo ratings yet
- QP-72-03 Customer CommunicationDocument3 pagesQP-72-03 Customer CommunicationSamsudin AhmadNo ratings yet
- G Corrective Action Section 7Document3 pagesG Corrective Action Section 7Ngonidzashe Zvarevashe100% (1)
- ISODocument11 pagesISOaiswaryacdas9853No ratings yet
- How Can ISO 13485 Clause 7.4, Purchasing, Enhance ProcurementDocument3 pagesHow Can ISO 13485 Clause 7.4, Purchasing, Enhance ProcurementPavan MujawdiyaNo ratings yet
- SOPSP05 VendorSelection BSDocument3 pagesSOPSP05 VendorSelection BStroubledsoul100% (1)
- Quality Management System Master18 February 2014Document27 pagesQuality Management System Master18 February 2014shani5573No ratings yet
- QSP-001633 - Rev 04 - Luminus Testing Laboratory Quality Manual207Document34 pagesQSP-001633 - Rev 04 - Luminus Testing Laboratory Quality Manual207SureshNo ratings yet
- New Supplier Survey FormDocument14 pagesNew Supplier Survey Formsutharitessh100% (1)
- Internal Audit NC ReportDocument1 pageInternal Audit NC Reportmorshed_mahamud7055No ratings yet
- Internal Audit Flow ChartDocument1 pageInternal Audit Flow ChartstevierayoNo ratings yet
- Evaluation Reporting of ResultsDocument16 pagesEvaluation Reporting of ResultspurnachandrashekarNo ratings yet
- Procedure On Document ManagementDocument13 pagesProcedure On Document Managementndayiragije JMVNo ratings yet
- Internal Quality Audit Plan Dilg Region 10Document8 pagesInternal Quality Audit Plan Dilg Region 10Cess AyomaNo ratings yet
- Process Audit ChecklistDocument15 pagesProcess Audit ChecklistAbi ParillaNo ratings yet
- Control of Monitoring and Measuring EquipmentDocument3 pagesControl of Monitoring and Measuring EquipmentLinda Setya WatiNo ratings yet
- FDA Volume II - Audits Ora-Lab.4.14Document7 pagesFDA Volume II - Audits Ora-Lab.4.14nilayNo ratings yet
- SOP-4.3-2-0 Creation, Revision and Approval of DocumentsDocument4 pagesSOP-4.3-2-0 Creation, Revision and Approval of DocumentsclairealbertiniNo ratings yet
- QMS Internal External AuditDocument5 pagesQMS Internal External AuditNesanNo ratings yet
- Quality AuditsDocument15 pagesQuality AuditsPrachi PandeyNo ratings yet
- SOP-03 Management Review MeetingsDocument3 pagesSOP-03 Management Review Meetingstrivesh100% (1)
- Purchasing PolicyDocument9 pagesPurchasing PolicyAyman AliNo ratings yet
- E 178 Iso17025 ChecklistDocument19 pagesE 178 Iso17025 ChecklistShweta Rawal VijNo ratings yet
- Auditing Gas Analysis LaboratoriesDocument14 pagesAuditing Gas Analysis LaboratorieshentadwyNo ratings yet
- NQA Aerospace Workshop Webinar 02.10.2020Document53 pagesNQA Aerospace Workshop Webinar 02.10.2020Rony LesbtNo ratings yet
- Form JO Foundation ChecklistDocument1 pageForm JO Foundation ChecklistRony LesbtNo ratings yet
- NQA Webinar - Cyber Security Risk and Assurance in The Supply Chain (WMCRC & Tuned To RISK) 21.04.21Document36 pagesNQA Webinar - Cyber Security Risk and Assurance in The Supply Chain (WMCRC & Tuned To RISK) 21.04.21Rony LesbtNo ratings yet
- NQA Webinar PAS 2060 Carbon Neutrality 30-04-2021Document38 pagesNQA Webinar PAS 2060 Carbon Neutrality 30-04-2021Rony LesbtNo ratings yet
- TBM FormDocument1 pageTBM FormRony LesbtNo ratings yet
- Hand Out Shop Inspection - ISBL - OSBL 210527Document3 pagesHand Out Shop Inspection - ISBL - OSBL 210527Rony LesbtNo ratings yet
- Summary RFI of FDT UG PipeDocument2 pagesSummary RFI of FDT UG PipeRony LesbtNo ratings yet
- Cause Analysis For Spun Pile Crack and BrokenDocument11 pagesCause Analysis For Spun Pile Crack and BrokenRony LesbtNo ratings yet
- 35 - HS-78005 - Isolation ProcedureDocument3 pages35 - HS-78005 - Isolation ProcedureRony LesbtNo ratings yet
- DCC-F9 - Format Job DescriptionDocument1 pageDCC-F9 - Format Job DescriptionRony LesbtNo ratings yet
- TgBin TTB B0 HS 78045 Handling SRCC Documentation and DistributionDocument15 pagesTgBin TTB B0 HS 78045 Handling SRCC Documentation and DistributionRony LesbtNo ratings yet
- Course Assessment Form Date .. . Course Title: . Facilitator: Location: .. 1Document1 pageCourse Assessment Form Date .. . Course Title: . Facilitator: Location: .. 1Rony LesbtNo ratings yet
- Name Target # of Abnormality Finding (# of Mudawalk/month) Week 1 Week 2 Week 3Document3 pagesName Target # of Abnormality Finding (# of Mudawalk/month) Week 1 Week 2 Week 3Rony LesbtNo ratings yet
- Register of Operation On ProcessDocument18 pagesRegister of Operation On ProcessRony LesbtNo ratings yet
- Sop Billing ProcessDocument9 pagesSop Billing ProcessRony Lesbt0% (1)
- P-OkiPL1-100-15-PRO-0002 HSE Induction ProcedureDocument14 pagesP-OkiPL1-100-15-PRO-0002 HSE Induction ProcedureRony Lesbt100% (2)
- P OkiPL1 100 15 PRO 0003 Emergency Preparedness and Emergency ResponseDocument13 pagesP OkiPL1 100 15 PRO 0003 Emergency Preparedness and Emergency ResponseRony LesbtNo ratings yet
- Inter Job Handed OverDocument1 pageInter Job Handed OverRony LesbtNo ratings yet
- WI - Interdiscipline Handed OverDocument5 pagesWI - Interdiscipline Handed OverRony LesbtNo ratings yet
- P-OkiPL1-100-15-PRO-0001 Legal and Other Requirements ProcedureDocument11 pagesP-OkiPL1-100-15-PRO-0001 Legal and Other Requirements ProcedureRony LesbtNo ratings yet
- UT Training Schedule Angkatan Ke-11Document1 pageUT Training Schedule Angkatan Ke-11Rony LesbtNo ratings yet
- Jobmigas 19 August 2013Document2 pagesJobmigas 19 August 2013Rony LesbtNo ratings yet
- Training Traveling Targeting: Welding Inspector - BNSP (Saturday and Sunday, 19.00Document2 pagesTraining Traveling Targeting: Welding Inspector - BNSP (Saturday and Sunday, 19.00Rony LesbtNo ratings yet
- IEE STD C37.104-2002 IEEE Guide For Automatic Reclosing PDFDocument55 pagesIEE STD C37.104-2002 IEEE Guide For Automatic Reclosing PDFJohnatan HernándezNo ratings yet
- Ackermannand Chen 2013 Developing Academic Collocation List AuthorsmanuscriptDocument31 pagesAckermannand Chen 2013 Developing Academic Collocation List AuthorsmanuscriptaridNo ratings yet
- DPP 05Document4 pagesDPP 05urmomNo ratings yet
- End of Term 2 Test A: Grammar VocabularyDocument3 pagesEnd of Term 2 Test A: Grammar VocabularyAngela Lobato MañanesNo ratings yet
- Chemical and Mechanical DesignDocument460 pagesChemical and Mechanical DesignNuriman K-monNo ratings yet
- Effects of Operating Parameters On Nitrogen Oxides Emissions For A Natural GasDocument9 pagesEffects of Operating Parameters On Nitrogen Oxides Emissions For A Natural GasMudhafar MudhafarNo ratings yet
- Introduction To PEG (Parsing Expression Grammar) in PythonDocument71 pagesIntroduction To PEG (Parsing Expression Grammar) in Pythonrwanda0% (1)
- Applied Mechanics I - Fall 2013 PDFDocument4 pagesApplied Mechanics I - Fall 2013 PDFRajeshGupta100% (1)
- Question BankDocument13 pagesQuestion BankFirozNo ratings yet
- Kuji in KanjiDocument25 pagesKuji in Kanjidcamu37100% (3)
- Request For TransferDocument3 pagesRequest For TransferDiomedes ColarNo ratings yet
- 20120229000242SMA3013 - Chapter 1Document105 pages20120229000242SMA3013 - Chapter 1Nurul Hana BalqisNo ratings yet
- Textbook PMDocument46 pagesTextbook PMLai QuocNo ratings yet
- Quays: Healy Power Quarries LTDDocument48 pagesQuays: Healy Power Quarries LTDmwmccarthyNo ratings yet
- Analisis Sootblower Terhadap Head TransferDocument5 pagesAnalisis Sootblower Terhadap Head TransferRDSetyawanNo ratings yet
- Product Info WISI-GT-31-W V3.0 enDocument2 pagesProduct Info WISI-GT-31-W V3.0 enDiego MattaNo ratings yet
- Agency Enrollment FormDocument1 pageAgency Enrollment FormRaymond TenchavezNo ratings yet
- Weld Overlay Cladding1Document3 pagesWeld Overlay Cladding1Yetkin ErdoğanNo ratings yet
- Indian Contract ActDocument18 pagesIndian Contract ActShubhi MittalNo ratings yet
- Kissing FishDocument11 pagesKissing FishkohlerfernandaNo ratings yet
- Razr Iron Scorpions MC Series Book 1 Motorcycle Club Mafia Romance Crossover Universe Book 3 Harley Diamond All ChapterDocument66 pagesRazr Iron Scorpions MC Series Book 1 Motorcycle Club Mafia Romance Crossover Universe Book 3 Harley Diamond All Chapterphillip.mesias887100% (5)
- Study Guide Unit 2.2 - Dna Replication & Protein SynthesisDocument35 pagesStudy Guide Unit 2.2 - Dna Replication & Protein SynthesisGraceNo ratings yet
- GRP WRKDocument5 pagesGRP WRKrheaNo ratings yet
- Uploading 8 23Document12 pagesUploading 8 23Shannara21No ratings yet
- WeldingDocument23 pagesWeldingSuneel Kumar MeenaNo ratings yet