Professional Documents
Culture Documents
Organic Chemistry
Organic Chemistry
Uploaded by
raone159632Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organic Chemistry
Organic Chemistry
Uploaded by
raone159632Copyright:
Available Formats
Mollie Burgoon
5.12 exam #3
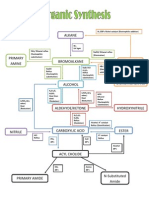
Alkene synthesis
Dehydrohalogenation
strong base
Dehalogenation of a vicinal dibromide
NaI, acetone
Reactions of alkenes
Electrophilic addition of HX
mark
alkyl halide
HX and polar solvent
Free radical addition of HBr
antimark
alkyl halide
HBr, ROOH, heat
Electrophilic H20 addition
mark
alcohol
H+, H20
Oxymercuration/demercuration
mark
alcohol
Hg(OAc)2/H20, NaBH4
Hydroboration/oxidation
antimark
alcohol
BH3/THF, H202/KOH
Hydrogenation
alkane
H2 and Pt/C
Halogenation
vicinal dihalide
X2
Hydrohalogenation
mark (OH)
halohydrin (alcohol moiety)
X2, H20
Syn dihydroxylation
dialcohol
OsO4 or KmnO4, H202
Epoxidation/hydrolysis
anti dialcohol
MCPBA, H2/H2O
Ozonolysis
cleaved double bond
O3, (CH3)2S
Cationic polymerization
BF3 or ROOR
Mollie Burgoon
If you want
alcohol
alkyl halide
vicinal dihalide
halohydrin
alkane
alkene
and you have
alkene
alkene
alkene
alkene
alkene
alkene
alkene
alkene
alkene
alkene
alkyl halide
vicinal dibromide
alcohol
reagents
H+, H20
Hg(OAc)2/H20, NaBH4
BH3/THF, H202/KOH
OsO4, H202
MCPBA, H2/H2O
HX, polar solvent
HBR, ROOH,
X2
X2, H20
H2 and Pt/C
strong base
NaI, acetone
strong acid (H2SO4)
Carbene reactions
acts like a radical
prone to forming 3-membered rings
I2CH2 + Zn or Cu
Expoxidation of alkenes
3-membered ring
uses MCPBA (metachloroperbenzoic acid)
Reactions of alkynes
Lindlars catalyst
Pd + BaSO4 + quinine
turn alkyne into alkene
Acetylide ion
deprotonate --H to get -: only way to make a C-C bond
NaNH2
Look at synthesis of alkynes!!
5.12 exam #3
+/+
+
-
+
+
hydration
oxymercuration
hydroboration
syn dihydroxylation
epoxidation/hydrolysis
electrophilic HX addition
free radical addition
halogenation
halohydrogenation
hydrogenation
dehydrohalogenation
dehalogenation
dehydration of alcohol
You might also like
- Edexcel A Level (A2) Chemistry Organic ChemistryDocument3 pagesEdexcel A Level (A2) Chemistry Organic ChemistryAvrinoxNo ratings yet
- Organic ReagentsDocument15 pagesOrganic ReagentsApoorv Tandon100% (2)
- 16 Hydroxyl compound-24-STDDocument38 pages16 Hydroxyl compound-24-STDManh Doan DucNo ratings yet
- Hydroxyl Compounds: Alcohol & PhenolDocument59 pagesHydroxyl Compounds: Alcohol & PhenolUMMU MARDHIAH ABDUL HALIMNo ratings yet
- UNIT 6 HALO ALKANES & Halo Arenes LatestDocument50 pagesUNIT 6 HALO ALKANES & Halo Arenes Latestsukaina fatimaNo ratings yet
- Alcohols: Chemistry Unit 2 C. Bailey PolackDocument24 pagesAlcohols: Chemistry Unit 2 C. Bailey PolackBritney PattersonNo ratings yet
- Mod 4 Revision Guide 10 Synthetic RoutesDocument2 pagesMod 4 Revision Guide 10 Synthetic RoutesdufraiscNo ratings yet
- 34 ch11Document10 pages34 ch11Mohammed IbrahimNo ratings yet
- Alcohal Phenol PDFDocument10 pagesAlcohal Phenol PDFVedha GowdaNo ratings yet
- Reagent Organic Chemistry HandbookDocument3 pagesReagent Organic Chemistry Handbookdodeda4744No ratings yet
- Haloalkanes and HaloarenesDocument28 pagesHaloalkanes and HaloarenesDevansh TiwaryNo ratings yet
- 11.3B AlcoholsDocument34 pages11.3B AlcoholsЕлнур ИкимбаевNo ratings yet
- CH 17Document18 pagesCH 17MirjanaNo ratings yet
- Carbonyl Compounds Aldehydes and KetonesDocument62 pagesCarbonyl Compounds Aldehydes and KetonesSubhabrata MabhaiNo ratings yet
- Organic ReactionsDocument1 pageOrganic ReactionsFerro FlowNo ratings yet
- 2-28!3!14 Oxidation ReductionDocument11 pages2-28!3!14 Oxidation ReductionNadine Harajli HamzehNo ratings yet
- Organic Compound Reagent/reactant Condition ClassificationDocument2 pagesOrganic Compound Reagent/reactant Condition ClassificationFaridOraha0% (1)
- Alchohols Phenols and EthersDocument5 pagesAlchohols Phenols and EthersPritika Yamini SaiNo ratings yet
- Alcohol Phenol and EtherDocument25 pagesAlcohol Phenol and EtherSmit Domadiya100% (2)
- Chemistry Formula Chapter11 Alcohols, Phenols and EthersDocument26 pagesChemistry Formula Chapter11 Alcohols, Phenols and EthersPramod NairNo ratings yet
- Preparing Halogen DerivativesDocument49 pagesPreparing Halogen DerivativesRenish AryanNo ratings yet
- Aldehydes Ketones Carboxylic AcidsDocument119 pagesAldehydes Ketones Carboxylic AcidsKashvi KhandelwalNo ratings yet
- Organic SynthesisDocument1 pageOrganic Synthesiszozoxo0% (1)
- Word Problems in Organic ChemistryDocument70 pagesWord Problems in Organic ChemistryreghuuchihaNo ratings yet
- Alcohols Phenols and Ethers: Unit 11Document24 pagesAlcohols Phenols and Ethers: Unit 11Meenal JoshiNo ratings yet
- Organic Reactions Summary SheetDocument2 pagesOrganic Reactions Summary Sheetthacheee64% (11)
- Lecture. 13,14,15 PDFDocument45 pagesLecture. 13,14,15 PDFsarah100% (1)
- Notes - Alkyl Halides and Aryl HalidesDocument34 pagesNotes - Alkyl Halides and Aryl HalidesDivya MehtaNo ratings yet
- Alcohols Phenols & EtherDocument10 pagesAlcohols Phenols & EtherVipin AroraNo ratings yet
- Reactions of Alcohols, Phenols, Aldehydes and KetonesDocument44 pagesReactions of Alcohols, Phenols, Aldehydes and KetonesGlen Mangali100% (4)
- Reducing Agents: Hydride Reagents and DihydrogenDocument5 pagesReducing Agents: Hydride Reagents and DihydrogenBhakteeNo ratings yet
- Week 12 Alkohol Dan PhenolDocument62 pagesWeek 12 Alkohol Dan PhenolAgitha FarihaNo ratings yet
- Chemistry Notes For Class 12 Chapter 11 Alcohols, Phenols and EthersDocument25 pagesChemistry Notes For Class 12 Chapter 11 Alcohols, Phenols and Ethershamdy solimanNo ratings yet
- Alcohol and PhenolDocument117 pagesAlcohol and Phenolsulihah12100% (2)
- Alkenes: 1. From Dehydration of AlcoholDocument14 pagesAlkenes: 1. From Dehydration of AlcoholPratik TimalsinaNo ratings yet
- Aromatic Compounds DPP 02Document4 pagesAromatic Compounds DPP 02Rinku Arun JainNo ratings yet
- Reaction List v002Document5 pagesReaction List v002cecil3414No ratings yet
- Organic Reagents PDFDocument4 pagesOrganic Reagents PDFKartikey Jain100% (6)
- Alcohols Phenols Ethers PDFDocument61 pagesAlcohols Phenols Ethers PDFRohan HotaNo ratings yet
- Alcohols 1630993189Document56 pagesAlcohols 1630993189Sahisa MahatNo ratings yet
- UNIT - 11. Alcohols Phenols and Ethers - NotesDocument17 pagesUNIT - 11. Alcohols Phenols and Ethers - NotesAngelina DaisyNo ratings yet
- Organic ReactionsDocument1 pageOrganic ReactionsMasrulIsmailNo ratings yet
- NEO - JEE - 12 - P1 - CHE - E - Haloalkanes and Haloarenes. - S5 - 209Document115 pagesNEO - JEE - 12 - P1 - CHE - E - Haloalkanes and Haloarenes. - S5 - 20916739No ratings yet
- Alcohols, Phenols and EthersDocument11 pagesAlcohols, Phenols and EthersSandeepNo ratings yet
- Chapter 7 - Alkyl HalidesDocument24 pagesChapter 7 - Alkyl HalidesClinton NdhlovuNo ratings yet
- 51 Reactions From PDFDocument5 pages51 Reactions From PDFAbhinandan Sinha33% (3)
- All Organic Reagent PDFDocument6 pagesAll Organic Reagent PDFreaper1371293No ratings yet
- Organic ReactionsDocument4 pagesOrganic ReactionsRobbing_HoodNo ratings yet
- Chapter 7Document45 pagesChapter 7Wai Kwong ChiuNo ratings yet
- Reagents in Organic ChemistryDocument3 pagesReagents in Organic ChemistryParam SoniNo ratings yet
- Aldehydes, Ketones and Carboxylic AcidDocument19 pagesAldehydes, Ketones and Carboxylic AcidPraneel BhattNo ratings yet
- Reaction Summary: ALKENES: Reagent Conditions Products Observations Example/DiagramDocument1 pageReaction Summary: ALKENES: Reagent Conditions Products Observations Example/DiagramVictoria KairooNo ratings yet
- Alcohol Phenol and Ether FinalDocument33 pagesAlcohol Phenol and Ether FinalC.S. KrithikNo ratings yet
- Chem Class12 Chapter 8Document16 pagesChem Class12 Chapter 8rohithardy45No ratings yet
- 12 - Cbs - Aldehydes Ketones Carboxylic AcidsDocument6 pages12 - Cbs - Aldehydes Ketones Carboxylic AcidsShauryaNo ratings yet
- Chemistry Form 6 Sem 3 07Document65 pagesChemistry Form 6 Sem 3 07Ng Swee Loong StevenNo ratings yet
- Pdf-Haloalkanes and HaloarenesDocument159 pagesPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)