Professional Documents
Culture Documents
Stabilization of 1-Adamantyl Cation
Uploaded by
DeepMukherjee0 ratings0% found this document useful (0 votes)
16 views4 pagesProject
Original Title
docpppp(2)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentProject
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
16 views4 pagesStabilization of 1-Adamantyl Cation
Uploaded by
DeepMukherjeeProject
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 4
Part-III
Conclusion and Summary
(By Arijit Das,Int PHD)
Summary
In this experiment kinetic isotopic effect was studied on different deuterium
substituted adamantyl cation and from the data obtained the reasons for
stabilization of 1-adamantyl -cation was explained.
The following three factors stabilizes the 1-adamantyl cation:
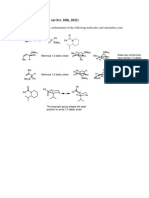
1. Carbon-Hydrogen Hyperconjugation
Carbon hydrogen hyperconjugation in 1-adamantyl cation has very little
effect in stabilizing the cation which evident from the valence bond
description of hyperconjugation (Bredts Rule).But this occurs in superacid
condition and the physical evidence I,e X-ray structure is shown below.
2. Homohyperconjugation
As carbon hydrogen hyperconjugation plays very little effect for the
stabilization that means other effects are also there,one of them is
homohyperconjugation.
The positive charge is distributed over the four carbon atom and stabilize
the cation.
3. Carbon-Carbon Hyperconjugation
This effect also stabilzes the cation.The evidence is if we substitute the
gamma position by methyl,amine,cyano groups stabilization energy
changes.
SCOPE:
1. Adamantyl cation can be used as a probe for understanding long range
substitution effects.(Appeared in Vol.1, No9, pp.6671, 2011,Resonance,J
Chandrasekhar,)
2. The adamantyl group is large, inert, and hydrophobic and can be
incorporated suitably to make fat-soluble drugs. (Appeared in Vol.1, No9, pp.66
71, 2011,Resonance, J Chandrasekhar)
Further Application;
These all kind of hyperconjugative effect plays a significant role.
All the effects seem valid and explained theoretically and experimentally.
The actual mechanism involving these effects needs further attention which is the
major effect for the stabilization of these systems is presently address
You might also like
- SAR of benzodiazepines structure-activity relationshipsDocument8 pagesSAR of benzodiazepines structure-activity relationshipsSomnath MondalNo ratings yet
- Classical MechanicsDocument297 pagesClassical MechanicsOwais Khalid100% (1)
- Alkyne zipper reaction: isomerizes internal to terminal alkynesDocument28 pagesAlkyne zipper reaction: isomerizes internal to terminal alkynesRajeswari RajiNo ratings yet
- Atomistic Simulation of Tensile Tests On Iron and FerriteDocument20 pagesAtomistic Simulation of Tensile Tests On Iron and FerriteSupun RanganaNo ratings yet
- Cinetica BriquetagemDocument7 pagesCinetica BriquetagemJUAN CANELLAS BOSCH NETONo ratings yet
- Organic 2022Document134 pagesOrganic 2022xapodi8776No ratings yet
- Olefin Polymerization With Ziegler-Natta Catalyst - Chemistry LibreTextsDocument3 pagesOlefin Polymerization With Ziegler-Natta Catalyst - Chemistry LibreTextsasad100% (1)
- IzomerizareDocument22 pagesIzomerizareTogan CristianNo ratings yet
- PROBLEM - SET - 3 - AnswerDocument5 pagesPROBLEM - SET - 3 - AnswerDylan BobNo ratings yet
- Resonance Interactions in Acyclic Systems: IupacDocument8 pagesResonance Interactions in Acyclic Systems: IupacAmOo ChurailNo ratings yet
- (Grupo 3) Photocatalyzed Direct Α-Alkylation of Esters Using Styrenes Adv Synth Catal - 2023 - WangDocument6 pages(Grupo 3) Photocatalyzed Direct Α-Alkylation of Esters Using Styrenes Adv Synth Catal - 2023 - Wangjuan.ceballos.anayaNo ratings yet
- Stability of Metal ComplexesDocument29 pagesStability of Metal Complexesjyothi sai sriNo ratings yet
- Vrouw, Mar 2011Document4 pagesVrouw, Mar 2011emediageNo ratings yet
- Elimination Reactions: 1. The E2 ReactionDocument8 pagesElimination Reactions: 1. The E2 ReactionNurul HidayahNo ratings yet
- 1515565547CHE P1 M25 EtextDocument17 pages1515565547CHE P1 M25 Etextafrozshams1869No ratings yet
- Ammonia Adsorption On Keggin-Type Heteropolyacid Catalysts Explored by DensityDocument7 pagesAmmonia Adsorption On Keggin-Type Heteropolyacid Catalysts Explored by DensityChau MaiNo ratings yet
- CH 3 Test ReviewDocument4 pagesCH 3 Test RevieweherrerahghsNo ratings yet
- ORGANIC 2019Document24 pagesORGANIC 2019xapodi8776No ratings yet
- Reaction IntermediatesDocument5 pagesReaction Intermediatescybercp100% (1)
- Problem Set 2Document2 pagesProblem Set 2Dylan BobNo ratings yet
- FMO Approach Explains Sigmatropic ReactionsDocument18 pagesFMO Approach Explains Sigmatropic ReactionsPrajwal AcharyaNo ratings yet
- Reaction Mechanism TutorialDocument12 pagesReaction Mechanism TutorialSarah CipawNo ratings yet
- A Collection of Questions in Organic Chemistry and Their Detailed SolutionsDocument69 pagesA Collection of Questions in Organic Chemistry and Their Detailed SolutionsShambo BasuNo ratings yet
- Have You Played This Game?: 9/10/2020 Dr. Erum Akbar 1Document10 pagesHave You Played This Game?: 9/10/2020 Dr. Erum Akbar 1Rabia FiazNo ratings yet
- Sem-4 - Active Methylene CompoundsDocument16 pagesSem-4 - Active Methylene CompoundshazemNo ratings yet
- Effect On Chemical PropertiesDocument6 pagesEffect On Chemical PropertiesSandeep KumarNo ratings yet
- Base Catalyzed RearrangementsDocument7 pagesBase Catalyzed RearrangementsAlvaroEnriqueQuinterosNo ratings yet
- Rotamer Barriers in Saturated and Unsaturated CompoundsDocument11 pagesRotamer Barriers in Saturated and Unsaturated CompoundsRojo JohnNo ratings yet
- Hydrocarbon Absorption in Tetrachloroaluminate Catalysts Effects 1998 CatalyDocument12 pagesHydrocarbon Absorption in Tetrachloroaluminate Catalysts Effects 1998 CatalyBianca BugdanoviczNo ratings yet
- Lecture 2Document19 pagesLecture 2RajeshNo ratings yet
- Types of Isomerisms Chain IsomerismDocument5 pagesTypes of Isomerisms Chain IsomerismRahul DubeyNo ratings yet
- The of Formation and Dissociation Actin-Myosin: RatesDocument8 pagesThe of Formation and Dissociation Actin-Myosin: RatesyicinenNo ratings yet
- PDF - Chemical-Arithmatic StoichiometryDocument25 pagesPDF - Chemical-Arithmatic StoichiometrySushrut GhimireNo ratings yet
- 1456899116CHE_P1_M3_e-Text (1)Document14 pages1456899116CHE_P1_M3_e-Text (1)akhiluiopNo ratings yet
- Organic Chemistry 2021Document26 pagesOrganic Chemistry 2021xapodi8776No ratings yet
- Determination of Serum Proteins by Biuret ReactionDocument16 pagesDetermination of Serum Proteins by Biuret ReactionCatalina López MendozaNo ratings yet
- Chemical Engineering Science: Danlu Tong, Geoffrey C. Maitland, Martin J.P. Trusler, Paul S. FennellDocument14 pagesChemical Engineering Science: Danlu Tong, Geoffrey C. Maitland, Martin J.P. Trusler, Paul S. Fennellmppatilmayur1679No ratings yet
- Chemguide Energy ProfilesDocument8 pagesChemguide Energy ProfilesEriani WulandariNo ratings yet
- Polyelectrolyte Conformation Controlled by A Trivalent-Rich Ion JacketDocument6 pagesPolyelectrolyte Conformation Controlled by A Trivalent-Rich Ion JacketLaura Daniela HenaoNo ratings yet
- ChenCatChem silaneDocument7 pagesChenCatChem silaneHimadri SahaNo ratings yet
- CHEM F111: General Chemistry: PilaniDocument16 pagesCHEM F111: General Chemistry: Pilanicukdbjsisns shsusbsbvzNo ratings yet
- Mammen Jchemphys2015Document6 pagesMammen Jchemphys2015Luca BrunoNo ratings yet
- Tetrahedro N Letters: Brandi M. Hudson, Jason G. Harrison, Dean J. TantilloDocument4 pagesTetrahedro N Letters: Brandi M. Hudson, Jason G. Harrison, Dean J. TantilloDavi HenriqueNo ratings yet
- Polymerisation and KineticsDocument20 pagesPolymerisation and KineticsMaulik KotadiyaNo ratings yet
- Electropolymerization of Pyrrole and Electrochemical Study of Polypyrrole: 1. Evidence For Structural Diversity of PolypyrroleDocument16 pagesElectropolymerization of Pyrrole and Electrochemical Study of Polypyrrole: 1. Evidence For Structural Diversity of PolypyrroleEmmanuel Avalos HuarteNo ratings yet
- Chapter IDocument4 pagesChapter Iم.احمد سالمNo ratings yet
- Sigmatropic Rearrangement - Anushka SunamDocument29 pagesSigmatropic Rearrangement - Anushka SunamAnushka SunamNo ratings yet
- This Report Has Been Delimited and Cleared For Purl'C Release Under Dod Directive No Restrictions Are Imposed Upon Its Use and DisclosureDocument24 pagesThis Report Has Been Delimited and Cleared For Purl'C Release Under Dod Directive No Restrictions Are Imposed Upon Its Use and DisclosureroylesterlaraNo ratings yet
- $R5XNVLLDocument10 pages$R5XNVLLvivekdhandNo ratings yet
- Conformational, Steric, and Stereo-Electronic Effects Anomeric EffectDocument8 pagesConformational, Steric, and Stereo-Electronic Effects Anomeric Effectyayooo2004No ratings yet
- Effect of Ezolith On Isomerization c4Document7 pagesEffect of Ezolith On Isomerization c4zahiraNo ratings yet
- NMR Analysis Identifies Photochemical Dimerization ProductDocument3 pagesNMR Analysis Identifies Photochemical Dimerization ProductEkin Dwi ArifNo ratings yet
- J Molcata 2009 03 027Document5 pagesJ Molcata 2009 03 027Henry ArceoNo ratings yet
- Organic Chemistry AnswersDocument18 pagesOrganic Chemistry AnswersKavi100% (1)
- Conjugated SystemsDocument32 pagesConjugated SystemsprocesspipingdesignNo ratings yet
- Conformational Isomerism by MuneebDocument11 pagesConformational Isomerism by MuneebMuneeb Ur RehmanNo ratings yet
- Thermodynamic Versus Kinetic Reaction ControlDocument15 pagesThermodynamic Versus Kinetic Reaction ControlUdasi Raqs Kerti HaiNo ratings yet
- Properties of C-C Bonds in N-Alkanes: Relevance To Cracking MechanismsDocument11 pagesProperties of C-C Bonds in N-Alkanes: Relevance To Cracking MechanismsMoses M. HammanNo ratings yet
- Kinetics of Selective Catalytic Reduction of NO by NH On Fe-Mo /ZSM-5 CatalystDocument4 pagesKinetics of Selective Catalytic Reduction of NO by NH On Fe-Mo /ZSM-5 CatalystRISHA RAJUNo ratings yet
- Stereoelectronic Effects: A Bridge Between Structure and ReactivityFrom EverandStereoelectronic Effects: A Bridge Between Structure and ReactivityNo ratings yet
- Ride Details Fare Details: Thanks For Travelling With Us, Deep MukherjeeDocument3 pagesRide Details Fare Details: Thanks For Travelling With Us, Deep MukherjeeDeepMukherjeeNo ratings yet
- Red DragonDocument2 pagesRed DragonZega AgustianNo ratings yet
- Register Avast Antivirus Product KeysDocument1 pageRegister Avast Antivirus Product KeysximadiosNo ratings yet
- 14 Scat Theo Eps Converted ToDocument1 page14 Scat Theo Eps Converted ToDeepMukherjeeNo ratings yet
- C:/NPPDF 32 Log/DebuglogDocument7 pagesC:/NPPDF 32 Log/DebuglogDeepMukherjeeNo ratings yet
- Assignment 3Document3 pagesAssignment 3DeepMukherjeeNo ratings yet
- 2005 RD 1 Questions tcm18-190744Document12 pages2005 RD 1 Questions tcm18-190744DeepMukherjeeNo ratings yet
- Mess Menu h1 July-August2014Document1 pageMess Menu h1 July-August2014DeepMukherjeeNo ratings yet
- Assignment2 (1) cd214Document1 pageAssignment2 (1) cd214DeepMukherjeeNo ratings yet
- IRCTC e-ticket cancellation slipDocument1 pageIRCTC e-ticket cancellation slipDeepMukherjeeNo ratings yet
- GolapBagan by Moti NondiDocument55 pagesGolapBagan by Moti NondiDeepMukherjeeNo ratings yet
- A Physics BooklistDocument15 pagesA Physics BooklistDeepMukherjeeNo ratings yet