0% found this document useful (0 votes)
644 views3 pagesReport Lab
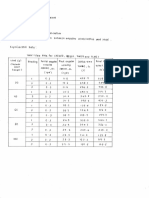
This document describes an experiment on thermodynamics investigating the compression and depression of an ideal gas at constant volume. The experiment aims to apply concepts of the ideal gas law relating temperature, volume, and pressure. Students will collect data on temperature, pressure, and volume over time while compressing and depressing a gas using a TEPGC unit and connected tanks. Safety precautions must be followed, and the experiment is to be conducted under supervision. Results tables show the recorded measurements for temperature, pressure in two tanks of different volumes over intervals of time.
Uploaded by
Amirul AriffCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
644 views3 pagesReport Lab
This document describes an experiment on thermodynamics investigating the compression and depression of an ideal gas at constant volume. The experiment aims to apply concepts of the ideal gas law relating temperature, volume, and pressure. Students will collect data on temperature, pressure, and volume over time while compressing and depressing a gas using a TEPGC unit and connected tanks. Safety precautions must be followed, and the experiment is to be conducted under supervision. Results tables show the recorded measurements for temperature, pressure in two tanks of different volumes over intervals of time.
Uploaded by
Amirul AriffCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd