Professional Documents
Culture Documents
Lecture 5 Figure 4 - 8 TGA PT Oxides
Uploaded by
ZUL KAMARUDDIN0 ratings0% found this document useful (0 votes)
13 views1 pagesains note
Original Title
Lecture 5 Figure 4_8 TGA Pt Oxides
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentsains note
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
13 views1 pageLecture 5 Figure 4 - 8 TGA PT Oxides
Uploaded by
ZUL KAMARUDDINsains note
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 1
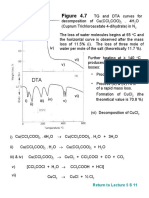
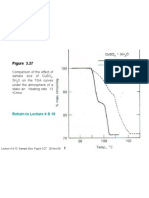
Figure 4.
8 TGA curves for the oxides of the Pt metals
(atmosphere: O2, pressure: 100 Torr, and heating rate: 10 oC/min)
Pt group metals: Pt, Ir and Ru form
solid oxides PtO, IrO2 and RuO2
respectively in atmospheric oxygen,
which are volatile at higher
temperatures.
Most of the metal oxides are stable at
high temperature. They contain the
respective metals at their highest
oxidation numbers.
However, paladium oxide (PdO) tends
to decompose at temperature higher
than 800 oC.
Formation and decomposition of
the metal oxides depend on the
oxygen partial pressure, the
heating rate and the surface area
of the metal powder.
Return to Lecture 5 S 12
Lecture 5 Figure 4_8 TGA Pt oxides
You might also like
- High Temperature Corrosion: Fundamentals and EngineeringFrom EverandHigh Temperature Corrosion: Fundamentals and EngineeringNo ratings yet
- M.C. Mayoral, J.M. Andrés, M.T. Izquierdo, B. Rubio: Article InfoDocument8 pagesM.C. Mayoral, J.M. Andrés, M.T. Izquierdo, B. Rubio: Article InfoLuis Miguel Gómez AcevedoNo ratings yet
- Thermite PDFDocument8 pagesThermite PDFPui KuanNo ratings yet
- Thermite: Chemical ReactionsDocument8 pagesThermite: Chemical ReactionsPui KuanNo ratings yet
- Embrittlement PDFDocument5 pagesEmbrittlement PDFtheerapat patkaewNo ratings yet
- Furnace BrazingDocument26 pagesFurnace BrazingNatKThNo ratings yet
- Decarbonization in HeattreatmentDocument37 pagesDecarbonization in Heattreatmentreza haghjooNo ratings yet
- HQTNDocument38 pagesHQTNAusuNo ratings yet
- Crevice Corrosion of Grade-2 Titanium in Saline Solutions at Different Temperatures and Oxygen ConcentrationsDocument8 pagesCrevice Corrosion of Grade-2 Titanium in Saline Solutions at Different Temperatures and Oxygen ConcentrationsGeovanny JaenzNo ratings yet
- Reduction of Iron Ore Pellets, Sinter and Lumps in Blast FurnaceDocument8 pagesReduction of Iron Ore Pellets, Sinter and Lumps in Blast FurnacePSS PrasadNo ratings yet
- Anor TitaniumDocument16 pagesAnor Titaniumcahya larasatiNo ratings yet
- Thermodynamics of Titanium and Oxygen Dissolved in Liquid Iron Equilibrated With Titanium OxidesDocument10 pagesThermodynamics of Titanium and Oxygen Dissolved in Liquid Iron Equilibrated With Titanium Oxidesarchivossubidos_No ratings yet
- A Mechanism For The Oxidation-Related in Uence On The Thermoelectric Behavior of PalladiumDocument32 pagesA Mechanism For The Oxidation-Related in Uence On The Thermoelectric Behavior of PalladiumGlobal QualityNo ratings yet
- Furnace BrazingDocument26 pagesFurnace BrazingRandallharwellNo ratings yet
- High-Temperature Behavior of Laser ElectrodispersiDocument18 pagesHigh-Temperature Behavior of Laser Electrodispersiateer6727No ratings yet
- Base Metals and Base-Metal Family Groups: Metallurgical ReactionsDocument114 pagesBase Metals and Base-Metal Family Groups: Metallurgical ReactionsYasa CossioNo ratings yet
- High temp corrosion mechanisms under 40 charsDocument20 pagesHigh temp corrosion mechanisms under 40 charssanu patilNo ratings yet
- CarbonDocument2 pagesCarbonnikolaNo ratings yet
- Extraction of Metals NotesDocument6 pagesExtraction of Metals NotesAyush JadiaNo ratings yet
- IronDocument43 pagesIronjosevitorromualdoNo ratings yet
- Aiche-33-01Catalyst Poisoning6Document17 pagesAiche-33-01Catalyst Poisoning6Hsein WangNo ratings yet
- HSC ExamplesDocument14 pagesHSC ExamplesAlejandro López OrtizNo ratings yet
- Chou 2001Document5 pagesChou 200144 print scan copyNo ratings yet
- Catalytic properties of nanomaterialsDocument23 pagesCatalytic properties of nanomaterialsGoutam Giri100% (1)
- CaTi0.9Fe0.1O3 Perovskite Powder for MedicineDocument10 pagesCaTi0.9Fe0.1O3 Perovskite Powder for MedicineLưu Thu HàNo ratings yet
- Presentasion Feb 2013Document15 pagesPresentasion Feb 2013rbcahyonoNo ratings yet
- Makalah Natrium: Material TeknikDocument12 pagesMakalah Natrium: Material TeknikMahfud EffendiNo ratings yet
- Lecture 4: Slag in Steelmaking ContentsDocument10 pagesLecture 4: Slag in Steelmaking ContentsSonu MishraNo ratings yet
- Corrosion Science: Shujun Gao, Bruce Brown, David Young, Marc SingerDocument10 pagesCorrosion Science: Shujun Gao, Bruce Brown, David Young, Marc SingerErick Roldan HermosilloNo ratings yet
- Applied Chemical: Created By: Elfi Nur Rohmah MSU Class March, 2019Document60 pagesApplied Chemical: Created By: Elfi Nur Rohmah MSU Class March, 2019elfiNo ratings yet
- Ohtsuka 2010Document7 pagesOhtsuka 2010Rodrigo Regla MuñozNo ratings yet
- High Temperature Corrosion MechanismsDocument30 pagesHigh Temperature Corrosion Mechanismspkn_pnt9950No ratings yet
- High-Temperature Metal Hydrides As Heat Storage Materials For Solar and Related ApplicationsDocument20 pagesHigh-Temperature Metal Hydrides As Heat Storage Materials For Solar and Related Applicationsaradhya0809No ratings yet
- When Gold Is Not NobleDocument13 pagesWhen Gold Is Not NobleSumit KumarNo ratings yet
- Inorganic Chem 2Document81 pagesInorganic Chem 2et0191zemeneNo ratings yet
- The Effect of Water VaporDocument5 pagesThe Effect of Water VaporAbigail GuerreroNo ratings yet
- Ch-19 Gas Welding, Gas Cutting - Arc WeldingDocument85 pagesCh-19 Gas Welding, Gas Cutting - Arc WeldingdiptyaNo ratings yet
- CorrosionDocument9 pagesCorrosionsterferNo ratings yet
- 2000 Basic Ceramic Consideration For Lost Wax ProcessingDocument17 pages2000 Basic Ceramic Consideration For Lost Wax ProcessingehsanNo ratings yet
- Review of The Green Ammonia ProcessDocument5 pagesReview of The Green Ammonia ProcessBrendan JonesNo ratings yet
- Theory of Oxy-Fuel Gas Cutting: Understanding the Chemical ReactionsDocument1 pageTheory of Oxy-Fuel Gas Cutting: Understanding the Chemical ReactionsManjunath EaswaranNo ratings yet
- metals and non metals class 10Document8 pagesmetals and non metals class 10Gowtham LNo ratings yet
- Seminar On Oxidation Resistant CoatingDocument18 pagesSeminar On Oxidation Resistant CoatingManoj IyengarNo ratings yet
- Kuliah Korosi 2008Document169 pagesKuliah Korosi 2008dwi_atmaja_3No ratings yet
- Purification and Recovery of Rhodium Metal by The Formation of Intermetallic CompoundsDocument3 pagesPurification and Recovery of Rhodium Metal by The Formation of Intermetallic CompoundsAbdulrahman JradiNo ratings yet
- Corrosion and Metal FinishingDocument19 pagesCorrosion and Metal FinishingShlok GuptaNo ratings yet
- The Role of Oxidation in The Wear of Alloys: F. H. StottDocument11 pagesThe Role of Oxidation in The Wear of Alloys: F. H. Stottkannanmech87No ratings yet
- 1986 Hydrolysis of Titanium Alkoxide and Effects of HydrolyticDocument6 pages1986 Hydrolysis of Titanium Alkoxide and Effects of HydrolyticSimon LeluyerNo ratings yet
- Thermochemistry c4Document1 pageThermochemistry c4vonashNo ratings yet
- Hot Corrosion and Performance of Nickel-Based CoatingsDocument7 pagesHot Corrosion and Performance of Nickel-Based CoatingsStanford BrownNo ratings yet
- Sakurai 5MPa RWGSDocument2 pagesSakurai 5MPa RWGSDanielNo ratings yet
- ThoriumDocument4 pagesThoriumZaira SanjuanNo ratings yet
- Catalysis of Gold NanoparticlesDocument14 pagesCatalysis of Gold NanoparticleslarguedasNo ratings yet
- Catalysts 11 01475 v2Document19 pagesCatalysts 11 01475 v2ERIKO DARMAWANNo ratings yet
- Effect of Heat Treatment On Microstructure and Hardness of Eurofer 97Document4 pagesEffect of Heat Treatment On Microstructure and Hardness of Eurofer 97Toramaru UtsunomiyaNo ratings yet
- Properties of MetalsDocument17 pagesProperties of MetalsDavies MasumbaNo ratings yet
- Corti Christopher PaperDocument28 pagesCorti Christopher PaperLuis CoronaNo ratings yet
- Soalan Seni PPT 2018Document5 pagesSoalan Seni PPT 2018ZUL KAMARUDDIN100% (1)
- Representative Clay Bodies TembikarDocument1 pageRepresentative Clay Bodies TembikarZUL KAMARUDDINNo ratings yet
- Alatan TEMBIKARDocument2 pagesAlatan TEMBIKARZUL KAMARUDDINNo ratings yet
- Other Klin TembikarDocument1 pageOther Klin TembikarZUL KAMARUDDINNo ratings yet
- Coiling TembikarDocument2 pagesCoiling TembikarZUL KAMARUDDINNo ratings yet
- ResumeDocument2 pagesResumeZUL KAMARUDDINNo ratings yet
- Soalan Seni PPT 2018Document5 pagesSoalan Seni PPT 2018ZUL KAMARUDDIN100% (1)
- Suhu Commercial Clays After Firing TembikarDocument1 pageSuhu Commercial Clays After Firing TembikarZUL KAMARUDDINNo ratings yet
- ScorpionsDocument3 pagesScorpionsZUL KAMARUDDINNo ratings yet
- Lecture 8 Application DTA & DSC01Document22 pagesLecture 8 Application DTA & DSC01ZUL KAMARUDDINNo ratings yet
- You and IDocument3 pagesYou and IZUL KAMARUDDINNo ratings yet
- Lecture 6 Dta & Dsc01Document22 pagesLecture 6 Dta & Dsc01ZUL KAMARUDDINNo ratings yet
- Bahtera MerdekaDocument1 pageBahtera MerdekaZUL KAMARUDDINNo ratings yet
- RESUME ConfirmDocument3 pagesRESUME ConfirmZUL KAMARUDDINNo ratings yet
- Lecture 7 Application DTA & DSC01Document34 pagesLecture 7 Application DTA & DSC01ZUL KAMARUDDINNo ratings yet
- Lecture 6 Figure 6 - 8 ThermocoupleDocument1 pageLecture 6 Figure 6 - 8 ThermocoupleZUL KAMARUDDINNo ratings yet
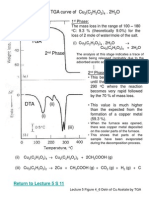
- Lecture 5 Figure 4 - 7 TGA Cu TrichloacetateDocument1 pageLecture 5 Figure 4 - 7 TGA Cu TrichloacetateZUL KAMARUDDINNo ratings yet
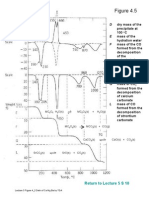
- Lecture 5 Figure 4 - 6 Detn of Cu Acetate by TGADocument1 pageLecture 5 Figure 4 - 6 Detn of Cu Acetate by TGAZUL KAMARUDDINNo ratings yet
- Lecture 4 Sample Holder Figure 3 - 23 To 3 - 26Document4 pagesLecture 4 Sample Holder Figure 3 - 23 To 3 - 26ZUL KAMARUDDINNo ratings yet
- Lecture 6 Table 6.1Document1 pageLecture 6 Table 6.1ZUL KAMARUDDINNo ratings yet
- Lecture 6 Figure 6 - 2 Compare Thermal DTADocument1 pageLecture 6 Figure 6 - 2 Compare Thermal DTAZUL KAMARUDDINNo ratings yet
- Lecture 5 Figure 4 - 9 Decomposition RH OxidesDocument1 pageLecture 5 Figure 4 - 9 Decomposition RH OxidesZUL KAMARUDDINNo ratings yet
- Lecture 5 Figure 4 - 5 Detn of CA MG Ba by TGADocument1 pageLecture 5 Figure 4 - 5 Detn of CA MG Ba by TGAZUL KAMARUDDINNo ratings yet
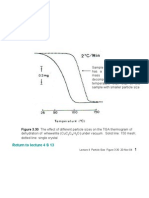
- Lecture 4 Particle Size Figure 3 - 30Document1 pageLecture 4 Particle Size Figure 3 - 30ZUL KAMARUDDINNo ratings yet
- Lecture 4 Heating Rate Figure 3 - 17Document1 pageLecture 4 Heating Rate Figure 3 - 17ZUL KAMARUDDINNo ratings yet
- Lecture 4 Partial Pressure Figure 3 - 19Document1 pageLecture 4 Partial Pressure Figure 3 - 19ZUL KAMARUDDINNo ratings yet
- Lecture 4 Sample Size Figure 3 - 27Document1 pageLecture 4 Sample Size Figure 3 - 27ZUL KAMARUDDINNo ratings yet
- Thermal Analysis SSK 4242Document17 pagesThermal Analysis SSK 4242ZUL KAMARUDDINNo ratings yet
- Lecture 4 Heating Rate Figure 3 - 20Document1 pageLecture 4 Heating Rate Figure 3 - 20ZUL KAMARUDDINNo ratings yet