Professional Documents
Culture Documents
Phosphorus Cycle
Phosphorus Cycle
Uploaded by
Jorge VillalobosCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Phosphorus Cycle
Phosphorus Cycle
Uploaded by
Jorge VillalobosCopyright:
Available Formats
PHOSPHORUS CYCLE
The phosphorus cycle is the biogeochemical cycle which characterizes the
transport and chemical transformation of phosphorus through the geosphere,
hydrosphere and biosphere. Unlike many other biogeochemical cycles, the
atmosphere does not play a significant role in the movement of phosphorus,
since phosphorus and phosphorus-based compounds are typically solids at the
normal ranges of temperature and pressure found on Earth. Therefore most of
the phosphorus remains within rock, sediments, sand, and the ocean floor, with
a fraction in living biomass. Phosphorus moves among trophic levels in an
ecosystem by plant growth, herbivory and carnivory.
caption
The aquatic phosphorus cycle. (Source EPA)
Occurrence in the Earth crust
Phosphorus in the Earth's crust generally occurs in its maximally oxidized state,
such as inorganic phosphate rocks. Phosphates are liberated from rocks in the
weathering process of the natural environment. The small phosphorus losses in
a terrestrial system caused by leaching through the action of rain are
countered by gains from weathering rocks. In soil, phosphate is absorbed on
clay surfaces and organic matter particles and becomes incorporated.
Role in biota
Plant species dissolve ionized forms of phosphate and take the mineral into
their system. Herbivores obtain phosphorus by consuming plant biomass, and
carnivores by consuming herbivores. Herbivores and carnivores excrete
phosphorus as a waste product in urine and feces. Phosphorus is then released
back into the soil when plants or animal matter decomposes and the cycle
repeats. Phosphorus is an essential nutrient for plants and animals in the form
of ions, including phosphate, PO43- and hydrogen phosphate, HPO42-.
Phosphates are effective fertilizers, but they also cause pollution problems in
lakes streams. Because phosphorus is often the nutrient in limited supply, even
a small increase in availability can cause a significant effect. Over-enrichment
of phosphate can lead to algae blooms. This excess of algae causes increased
consumption by bacteria, which then leads to even higher bacterial
concentrations. In the process the bacteria use up much of the dissolved
oxygen in the water during cellular respiration and thereby cause the death of
fish due to oxygen deprivation. The primary biological importance of
phosphates is as a component of nucleotides, which serve as energy storage
within cells (Adenosine triphosphate [ATP]) or, when linked together, form the
nucleic acids deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
Phosphorus, primarily in the form of hydroxyapatite, Ca5(PO4)3OH, is a key
structural component of animals. Approximately 80% of the phosphorus in
vertebrate animals is in their bones and teeth. This element is also an
important constituent of phospholipids, which are in all biological membranes.
Anthropogenic influence
Human influences in the phosphorus cycle arise chiefly from the introduction of
synthetic fertilizers. Use of fertilizers mainly has significantly altered both the
phosphorus and nitrogen cycles. Vegetation may not be able to utilize all of the
phosphate fertilizer applied; as a consequence, much of the phosphate applied
as fertilizer is lost from the land through water surface runoff. The dissolved
phosphate in surface runoff is eventually precipitated as sediment at the
bottom of the water body. In certain lakes and ponds, this phosphate may be
redissolved and recycled, often as an excessive nutrient. Animal wastes or
manure are also be applied to land as fertilizer, particularly in developing
countries.
If misapplied on frozen ground during the winter, much of the fertilizer may be
lost when ice melts and forms runoff. In certain areas very large or intense feed
lots of animals, may result in excessive surface runoff of phosphate and nitrate
into streams. Other human sources of phosphate are in the out flows from
municipal sewage treatment plants. Without an expensive tertiary treatment,
the phosphate in sewage is not removed during various treatment operations.
Again an extra amount of phosphate enters the water.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5814)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- LAB EXERCISE Blastula and GastrulaDocument8 pagesLAB EXERCISE Blastula and GastrulaJOSHUA MOLO100% (1)
- Recombinant DNA TECHNOLOGYDocument29 pagesRecombinant DNA TECHNOLOGYsannsannNo ratings yet
- Disease and Immunity MicroorganismsDocument8 pagesDisease and Immunity MicroorganismsAmna SaleemNo ratings yet
- Government College of Nursing, Jodhpur: Presentation ONDocument7 pagesGovernment College of Nursing, Jodhpur: Presentation ONpriyanka0% (1)
- Pest ControlDocument22 pagesPest ControlRika Yunita Chandra Harimurti100% (1)
- HH SuiteDocument5 pagesHH Suiteemma698No ratings yet
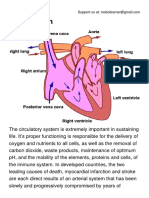
- The Circulatory System PDFDocument4 pagesThe Circulatory System PDFPerry SinNo ratings yet
- Grade 10 Science SyllabusDocument2 pagesGrade 10 Science SyllabusGlebert Cañete Dadol100% (2)
- FORTIGEL CollagenDocument6 pagesFORTIGEL CollagenranasoftNo ratings yet
- Adherence, Anti-Adherence, and Oligosaccharides Preventing Pathogens From Sticking To The Host.Document61 pagesAdherence, Anti-Adherence, and Oligosaccharides Preventing Pathogens From Sticking To The Host.Didier Alexis Rosales MosqueraNo ratings yet
- IntroductionDocument9 pagesIntroductionGray Odyssey M.No ratings yet
- Deocument - 147full Download Book Nutrition and Lifestyle in Neurological Autoimmune Diseases Multiple Sclerosis PDFDocument41 pagesDeocument - 147full Download Book Nutrition and Lifestyle in Neurological Autoimmune Diseases Multiple Sclerosis PDFadolph.wright782100% (20)
- AMI Equipment Services and Solutions, Inc. Biomedical Engineering DepartmentDocument11 pagesAMI Equipment Services and Solutions, Inc. Biomedical Engineering Departmentvandolph siribanNo ratings yet
- Cardiac Stem Cell Therapy: An Overview: AKM M Islam, AAS Majumder, F Doza, MM Rahman, H JesminDocument15 pagesCardiac Stem Cell Therapy: An Overview: AKM M Islam, AAS Majumder, F Doza, MM Rahman, H JesminNavojit ChowdhuryNo ratings yet
- 1 s2.0 S071734581630001X MainDocument7 pages1 s2.0 S071734581630001X MainTAUHID ALAMNo ratings yet
- AHMADDocument7 pagesAHMADSUNDAY MUSANo ratings yet
- 2 Importance of Halophilic and Halotolerant Lactic Acid Bacteria in CheesesDocument9 pages2 Importance of Halophilic and Halotolerant Lactic Acid Bacteria in Cheesesagussalim mattiNo ratings yet
- Luakan National High School-Annex San Ramon Dinalupihan, Bataan First Grading Examination SY: 2018-2019 Science Grade-9Document1 pageLuakan National High School-Annex San Ramon Dinalupihan, Bataan First Grading Examination SY: 2018-2019 Science Grade-9Melanie perez cortezNo ratings yet
- 101-Normal Skin MCQsDocument25 pages101-Normal Skin MCQsHybat ElsheikhNo ratings yet
- WBI01 Jan 2015 MSDocument28 pagesWBI01 Jan 2015 MSTammyKittikoonNo ratings yet
- The Impact of Cell Phone, Laptop Computer, and Microwave Oven Usage On Male FertilityDocument17 pagesThe Impact of Cell Phone, Laptop Computer, and Microwave Oven Usage On Male FertilitymaxsmaxNo ratings yet
- Bio Cell TheoryDocument22 pagesBio Cell TheoryCHLOE BEA DILLANo ratings yet
- Cockroach Lecture 3Document14 pagesCockroach Lecture 3Mohit SharmaNo ratings yet
- M.Sc. BotanyDocument170 pagesM.Sc. BotanyPraveen Kumar100% (1)
- Presentation 2Document27 pagesPresentation 2sameer78680% (1)
- (NATO Conference Series 13 - IV Marine Sciences) Alan Longhurst (Auth.), M. J. R. Fasham (Eds.) - Flows of Energy and Materials in Marine Ecosystems - Theory and Practice-Springer US (1984) PDFDocument721 pages(NATO Conference Series 13 - IV Marine Sciences) Alan Longhurst (Auth.), M. J. R. Fasham (Eds.) - Flows of Energy and Materials in Marine Ecosystems - Theory and Practice-Springer US (1984) PDFEzel Galindo PérezNo ratings yet
- BSC Lecture Notes Semester 1Document7 pagesBSC Lecture Notes Semester 1satasha28No ratings yet
- Biotechnology Statement of PurposeDocument2 pagesBiotechnology Statement of PurposeJames WachiraNo ratings yet
- Breeding StrategiesDocument53 pagesBreeding StrategiesRazelle Manceras100% (1)
- IGCSE 2009 Science Double Award 4SC0 Specification ISSUE 2 March09Document62 pagesIGCSE 2009 Science Double Award 4SC0 Specification ISSUE 2 March09Harry WatkinsonNo ratings yet