Professional Documents
Culture Documents
Tabel Perhitungan HCL KONDUKTO
Uploaded by
Febrina Putri RCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tabel Perhitungan HCL KONDUKTO
Uploaded by
Febrina Putri RCopyright:
Available Formats
volu
me
HCl
volu
me
NaOH
N HCl
N
NaOH
mol
HCl
mol
NaOH
mol
berlebih
v
total
M Larutan
L H+
L Cl-
L HCl
teori
L HCl
prakte
k
%
kesalah
an
10
0.2
0.002
200
0.001
0.3498
0.0763
59,877
0.2
0.645
3.171
0.2
2.921
0.545
2.735
10
0.2
2.483
0.616
2.235
10
0.2
2.050
0.691
1.713
10
0.2
1.621
0.815
21.082
10
0.2
1.196
0.96
31.528
10
0.2
0.682258
653
0.602316
702
0.523162
395
0.444784
059
0.367170
479
0.290310
36
0.214192
87
0.138807
249
3.363
10
3.127838
492
2.761341
838
2.398456
17
2.039127
968
1.683305
811
1.330937
929
0.981974
651
0.636366
654
3.583
0.2
0.0089417
91
0.0078940
59
0.0068566
5
0.0058294
11
0.0048121
95
0.0038048
54
0.0028072
46
0.0018192
3
0.864
0.001797
3
0.001594
6
0.001391
9
0.001189
2
0.000986
5
0.000783
8
0.000581
1
0.000378
4
201
10
0.00020
27
0.00040
54
0.00060
81
0.00081
08
0.00101
35
0.00121
62
0.00141
89
0.00162
16
0.426
1
3.810
1.062
0.00
2
0.00
2
0.00
2
0.00
2
0.00
2
0.00
2
0.00
2
0.00
2
0.00
2
10
0.202
7
0.202
7
0.202
7
0.202
7
0.202
7
0.202
7
0.202
7
0.202
7
0.202
7
0.775
1.125
45.857
202
203
204
205
206
207
208
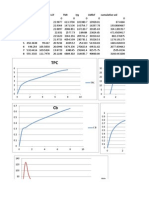
Mol HCl = V.HCl x N. HCl
v.total = (200 ml HCl + air) + v. NaOH/1000
Mol NaOH= V.NaOH x N.NaOH
M.Larutan = mol berlebih/v.total
Mol berlebih = mol HCl - mol NaOH
LH+ =349,8 S. cm2.mol-1 x M.Larutan /1000cm3. L-1
LCl-=76,3 S. cm2.mol-1 x M.Larutan /1000cm3. L-1
LHCl = LH+ + LCl-
You might also like
- Kurva Absorbansi Terhadap PH Kurva Konsentrasi Terhadap PH Kurva 2Ph Terhadap LogdDocument3 pagesKurva Absorbansi Terhadap PH Kurva Konsentrasi Terhadap PH Kurva 2Ph Terhadap LogdNovira ChandisaNo ratings yet
- Multicomponent Equilibrium Flash Calculation: TotalsDocument2 pagesMulticomponent Equilibrium Flash Calculation: Totalsprateek_bhoirNo ratings yet
- V (ML) HCLR (G-Mole) C (G-Mole/Ml) X Time (Min.) V (L) N (G-Mole) D (N) /DT K (Ml/G-Mole-Min.) FTCDocument1 pageV (ML) HCLR (G-Mole) C (G-Mole/Ml) X Time (Min.) V (L) N (G-Mole) D (N) /DT K (Ml/G-Mole-Min.) FTCKrishnamoorthy VijayalakshmiNo ratings yet
- Book 1Document216 pagesBook 1Quan ThànhNo ratings yet
- Bismillah NM Fix2Document280 pagesBismillah NM Fix2taufikrachmansusantoNo ratings yet
- Primary Groups Used in The Stefanis Panayiotou Group Contribution Method (Continued)Document1 pagePrimary Groups Used in The Stefanis Panayiotou Group Contribution Method (Continued)palanaruvaNo ratings yet
- Kurva Hcl-Naoh: Hantaran JenisDocument3 pagesKurva Hcl-Naoh: Hantaran JenisNovira ChandisaNo ratings yet
- Contoh Perhitungan MargulesDocument25 pagesContoh Perhitungan MargulesBangkit GotamaNo ratings yet
- Group A - Exp 7 - RTD - CSTRDocument8 pagesGroup A - Exp 7 - RTD - CSTRAkhilesh ChauhanNo ratings yet
- Lampiran B C + GrafikDocument2 pagesLampiran B C + GrafikrikaariskaaaNo ratings yet
- IsoelecDocument1 pageIsoelecroshanraja1983No ratings yet
- Problem 5.4-8Document3 pagesProblem 5.4-8Nguyễn AnNo ratings yet
- C:/Users/carre/Desktop/Simulacion Produccion de Caprolactama, HSC MainDocument22 pagesC:/Users/carre/Desktop/Simulacion Produccion de Caprolactama, HSC MainCamila Florencia ScarlatoNo ratings yet
- C:/Users/carre/Desktop/Simulacion Produccion de Caprolactama, HSC MainDocument22 pagesC:/Users/carre/Desktop/Simulacion Produccion de Caprolactama, HSC MainCamila Florencia ScarlatoNo ratings yet
- 00 Perhitungan Alat Besar 1-DeSKTOP-NJGKKH8Document359 pages00 Perhitungan Alat Besar 1-DeSKTOP-NJGKKH8Muhammad FakhrizalNo ratings yet
- Benzene Plant WorksheetDocument6 pagesBenzene Plant WorksheetMuhammad sherazNo ratings yet
- X-Ray Diffraction: Lab ReportDocument16 pagesX-Ray Diffraction: Lab ReportFikan Mubarok RohimsyahNo ratings yet
- Chem300Quiz 2ChEA LibutlibutDocument15 pagesChem300Quiz 2ChEA LibutlibutEredson LibutlibutNo ratings yet
- New Termwork4.1Document12 pagesNew Termwork4.1Ankit ModiNo ratings yet
- Appendix10 GPSA Physical PropertiesDocument9 pagesAppendix10 GPSA Physical PropertiesJuan Vargas SerranoNo ratings yet
- Tugas 3 - Laily Fitri Pelawi - 1806154116Document8 pagesTugas 3 - Laily Fitri Pelawi - 1806154116LailyNo ratings yet
- Experiment Adsorbtie MB Pe Carbune Activ Granular: Conc Abs 0 0 2 0.363 4 0.745 6 1.024 8 1.395 10 1.622Document3 pagesExperiment Adsorbtie MB Pe Carbune Activ Granular: Conc Abs 0 0 2 0.363 4 0.745 6 1.024 8 1.395 10 1.622OanaMaiorNo ratings yet
- BiofarDocument24 pagesBiofarNovita Cahya PuspitasariNo ratings yet
- CopperDocument3 pagesCopperEkta TejwaniNo ratings yet
- Result PL FransiscoDocument21 pagesResult PL FransiscoRBDaniel Tumpal Sinurat 089No ratings yet
- Conductivity of Solutions - Chem 101 Lab - 1Document8 pagesConductivity of Solutions - Chem 101 Lab - 1Erin Twomey50% (2)
- Tablas de Antoine VERDADERASDocument13 pagesTablas de Antoine VERDADERASmarych1990100% (1)
- Anasthasia Putri / 02 Kurva Kesetimbangan Uap-Cair 1641420066 / 3B D4 Chloroform - 1,4 Dioxine (P 100kpa)Document5 pagesAnasthasia Putri / 02 Kurva Kesetimbangan Uap-Cair 1641420066 / 3B D4 Chloroform - 1,4 Dioxine (P 100kpa)Anasthasia PutriNo ratings yet
- Datos Experimentales Cloroformo +2-Propanol A 72kpaDocument8 pagesDatos Experimentales Cloroformo +2-Propanol A 72kpamaria paulaNo ratings yet
- Trifasico 3.0 Version CerrarDocument22 pagesTrifasico 3.0 Version CerrarCamila Florencia ScarlatoNo ratings yet
- Copia de Calculos Practica 2-LQF1Document24 pagesCopia de Calculos Practica 2-LQF1Fran Morales GalanNo ratings yet
- AntoineDocument23 pagesAntoineHector Silva GutiérrezNo ratings yet
- NM MantappisanDocument224 pagesNM MantappisanRisky Andi0% (1)
- Antoine Coefficients For Vapor PressureDocument32 pagesAntoine Coefficients For Vapor PressureJesicaidat9No ratings yet
- N XN Yn 0.1 (Tn-Yn) SOL. ANALITICA ERRORDocument9 pagesN XN Yn 0.1 (Tn-Yn) SOL. ANALITICA ERRORRamiro HuacalloNo ratings yet
- Correlação S MicrogeoDocument80 pagesCorrelação S MicrogeoPaulino TaveiraNo ratings yet
- Coef Therm Exp Liq OrgDocument200 pagesCoef Therm Exp Liq OrgLeidy ValenciaNo ratings yet
- N1 2256 Tr/min (40HZ) : PR Opt (W) Popt (Pa)Document3 pagesN1 2256 Tr/min (40HZ) : PR Opt (W) Popt (Pa)Khene Mohamed LamineNo ratings yet
- Constantes AntoineDocument24 pagesConstantes Antoinecamiluda100% (2)
- Reaction Kinetics: Rate ConstantsDocument7 pagesReaction Kinetics: Rate Constantsprateek_bhoirNo ratings yet
- Reaction Kinetics: Rate ConstantsDocument7 pagesReaction Kinetics: Rate ConstantsEdgar Velastegui GonzálezNo ratings yet
- T Awp Perimeter LCF Tmi Iyy Lmilcf Cumulative Vol 0 0.5 1 242.3197 1.5 280.4816 2 3 352.7904 4 5 450.3638 6 7 532.4416 109.5824 8 555.3315 111.7978Document15 pagesT Awp Perimeter LCF Tmi Iyy Lmilcf Cumulative Vol 0 0.5 1 242.3197 1.5 280.4816 2 3 352.7904 4 5 450.3638 6 7 532.4416 109.5824 8 555.3315 111.7978roy thomasNo ratings yet
- Hydrogen Density (KG/M) at Different Temperatures (C) and Pressures (Mpa)Document1 pageHydrogen Density (KG/M) at Different Temperatures (C) and Pressures (Mpa)AdityaAnugerahPutraNo ratings yet
- Log X/M Log X/MDocument4 pagesLog X/M Log X/MNurlaeli NaelulmunaNo ratings yet
- Antoine Coefficient TableDocument14 pagesAntoine Coefficient TableDeva AfrgNo ratings yet
- HeterogenityDocument2 pagesHeterogenityAkashShuklaNo ratings yet
- WB AGA 8 Working-5Document303 pagesWB AGA 8 Working-5Waqar BhattiNo ratings yet
- Neraca FDocument37 pagesNeraca FRahmat AgustrionoNo ratings yet
- Reprezentarea Reprezentarea Ferecventei RelDocument6 pagesReprezentarea Reprezentarea Ferecventei RelPeter SzocsNo ratings yet
- Results SimpleDocument1 pageResults SimplePARTHA SARATHYNo ratings yet
- United States Census Figures Back to 1630From EverandUnited States Census Figures Back to 1630No ratings yet
- Progress in Inorganic ChemistryFrom EverandProgress in Inorganic ChemistryKenneth D. KarlinNo ratings yet
- Interpretation of MS-MS Mass Spectra of Drugs and PesticidesFrom EverandInterpretation of MS-MS Mass Spectra of Drugs and PesticidesNo ratings yet
- Fluids, Colloids and Soft Materials: An Introduction to Soft Matter PhysicsFrom EverandFluids, Colloids and Soft Materials: An Introduction to Soft Matter PhysicsAlberto Fernandez-NievesNo ratings yet