Professional Documents
Culture Documents
62 Experiment #5. Titration of An Acid Using A PH Meter
Uploaded by
Samiullah IlyasOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
62 Experiment #5. Titration of An Acid Using A PH Meter
Uploaded by
Samiullah IlyasCopyright:
Available Formats
62
Experiment #5. Titration of an Acid; Using a pH Meter
TITRATION OF AN ACID; USING A pH METER
Introduction
The pH meter is an instrument that measures the pH of a solution and affords a
direct method of obtaining a titration curve.
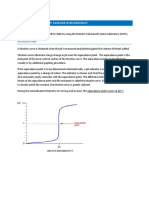
A titration curve is a graph of
measured pH values versus the volume (milliliters) of titrant added. The figure
below is an example of a titration curve, illustrating the numerous data points and
the best smooth curve drawn through the points.
Figure 1. Titration Curve of acid HA
The equivalence point is the point at which an equal amount of acid has been added
to the amount of base present or vice versa. The equivalence point occurs on the
titration curve in the region where there is a relatively large change in pH with a
relatively small change in volume. The steeper the curve in the region of the
equivalence point the more precisely it may be established. Once a titration curve is
constructed and the equivalence point established the experimenter could then
choose an indicator that would give a suitable endpoint (point at which the indicator
changes colour).
63
Experiment #5. Titration of an Acid; Using a pH Meter
Selection of the equivalence point
In this experiment you will graph the measured pH against the volume of standard
NaOH solution added. The best smooth curve should be drawn through these points.
The equivalence point can be established using the steepest tangent to the smooth
curve where the pH changes rapidly. The equivalence point is the mid point between
the two lines intersecting the volume axis.
The method is summarized below:
Equivalence Point Determination
11
10
9
pH
8
7
6
5
4
3
23.5
24
24.5
25
Volume NaOH (mL)
25.5
26
Figure 2: Equivalence Point Determination for acid HA
The equivalence point selected using this method is a more accurate method than
using an indicator in the titration.
A second method maybe used to determine the equivalence point. To use this
method a graph is constructed of
method.
!pH
vsVaverage . The graph below illustrates this
!V
64
Experiment #5. Titration of an Acid; Using a pH Meter
Equivalence Point Determination
50
dpH/dV
40
30
20
10
0
24
24.5
25
25.5
V average
26
26.5
27
Figure 3: Equivalence Point Determination for acid HA using the First
Derivative Method
The volume at the point where the graph reaches the maxima is the equivalence
point of the titration.
A disadvantage of the titration curve method is the time and effort required to make
the measurements and to construct the graph. This disadvantage can be overcome
by using a recording pH meter, which provides a chart record of the pH of a solution
as a function of time.
The pH of a solution is related to the H+ ion concentration by the equation:
(1)
pH = log[H+]
The ionization constant, Ka, for a generic acid HA is:
(2)
HA
H+ + A
Ka =
[H+][A]
[HA]
and the pKaof an acid is simply:
(3)
pKa = log Ka
If we take equation (2)
65
Experiment #5. Titration of an Acid; Using a pH Meter
(2)
Ka =
[H+][A]
[HA]
and now take log of both sides:
[A]
log Ka = log[H+] log
[HA]
from equation (3):
[A]
pKa= -log[H+] log
[HA]
and therefore from (1):
[A]
pKa = pH log
[HA]
But since pKais constant and pH varies:
[A]
pH = pKa + log
[HA]
Therefore when [A] = [HA]
pH = pKa
This is the midway point to the equivalence point. Therefore pKa values can
also be directly read from your titration curves.
Procedure
In preparation for this procedure, read Appendix B for directions on the proper use of
a balance and how to weigh by difference, and Appendix C for directions on the
proper use of a buret in a titration. Before lab have a draft of the procedures for
the preparation and the standardization of ~300mL of ~1M NaOH solution ready
for your instructor to look over.
Obtain from your instructor an unknown acid sample.
instructions on the pH meter before you start.
You will also be given
Hint: A similar procedure was used in the Chemistry 1000 laboratory.
SAFETY NOTE: Sodium hydroxide is a strong base and can cause burns if
it is left on the skin for too long. If your hands feel slippery or soapy at any
point during the lab, wash them well to remove the sodium hydroxide.
66
Experiment #5. Titration of an Acid; Using a pH Meter
Weigh accurately 2.00 - 2.25 g (+
0.0001 g) of your sample into a 250
mL beaker. Dissolve the sample in
50 mL of water. Prepare your buret
for titration (clean, rinse, etc.) and
fill the buret with standardized ~1
M NaOH solution. Obtain a pH
probe from your instructor and set up
your apparatus as shown. Check
with your instructor before starting
to be sure everything is set up
correctly.
Proceed to add standardized ~1 M base at the rate of 1 mL per addition taking the
buret reading and also the pH reading after each addition. Once the pH starts to
change more rapidly the size of the additions should be reduced to 0.5 mL or less.
As the pH changes become larger (this may occur at a pH of about 4 to 5) reduce the
size of the NaOH additions until single drops are being added. Continue until you
are satisfied the equivalence point has been passed or the pH is approximately 11 to
11.5. When you are finished, remove your apparatus, rinse the pH electrode and
return the pH probe to your instructor.
Note that your unknown acid will have more than one equivalence point if it is
polyprotic.
Report
1.
2.
3.
4.
Construct a titration curve graph of pH values versus mL of 1M NaOH solution
added.
!pH
Construct a graph of
vsVaverage . Using this graph determine the
!V
equivalence point for the titration. Illustrate on the titration curve the pKa(s)
of your unknown acid.
Calculate and report the Ka value(s) and the molar mass of your unknown
sample.
Give a balanced, generic reaction equation for your unknown acid.
67
Experiment #5. Titration of an Acid; Using a pH Meter
Titration of an Acid; Using a pH Meter
DATA SHEET:
Partner Credit:
Name: _______________________
Y
(Circle if yes)
Unknown # or letter:
Instructor's signature:
Partner's Name: ________________
_______________
_______________________
Lab Section: _______________
68
Experiment #5. Titration of an Acid; Using a pH Meter
Experimental Procedure (draft only): Please prepare before the start of class.
Instructor's signature:
_______________________
You might also like
- Potentiometric Titration CurvesDocument5 pagesPotentiometric Titration CurvesDavid GrahamNo ratings yet
- Experiment 1 Preparation of Buffer SolutionsDocument16 pagesExperiment 1 Preparation of Buffer Solutionsmohamad ashaziq89% (57)
- Weak Acid Strong Base Titration LabDocument8 pagesWeak Acid Strong Base Titration Labapi-265089380100% (1)
- Discussion On Potentiometric TitrationsDocument16 pagesDiscussion On Potentiometric TitrationsKcirtap Zketh60% (5)
- Titration Lab ReportDocument38 pagesTitration Lab Reportadillaanis100% (4)
- Experimental approaches to Biopharmaceutics and PharmacokineticsFrom EverandExperimental approaches to Biopharmaceutics and PharmacokineticsNo ratings yet
- Determination of Ka of Unknown AcidDocument23 pagesDetermination of Ka of Unknown AcidShasha0% (1)
- Lab Experiment 3 Ka Determination Through PH TitrationDocument4 pagesLab Experiment 3 Ka Determination Through PH TitrationxmusiqaNo ratings yet
- 06 and 07 Standardization of NaOH and Acid Base TitrationDocument16 pages06 and 07 Standardization of NaOH and Acid Base TitrationTyler Hardy80% (5)
- AP Chemistry Investigation 4 - Judy, Paul, AnthonyDocument13 pagesAP Chemistry Investigation 4 - Judy, Paul, AnthonyAnthony HowerNo ratings yet
- Experiment 4 - Potentiometric TitrationDocument11 pagesExperiment 4 - Potentiometric TitrationJoemer Absalon Adorna100% (2)
- Practical Manual of Analytical ChemistryFrom EverandPractical Manual of Analytical ChemistryRating: 4.5 out of 5 stars4.5/5 (3)
- Experiment 11 Results and Discussion Report: Potentiometric Determination of The Purity and Dissociation Constant of Potassium Hydrogen PhthalateDocument4 pagesExperiment 11 Results and Discussion Report: Potentiometric Determination of The Purity and Dissociation Constant of Potassium Hydrogen PhthalateNathalie Dagmang80% (10)
- Acid-Base Titrations Curve Formal LabDocument9 pagesAcid-Base Titrations Curve Formal LabAshley StraubNo ratings yet
- Lab Report Experiment 2aaa - EditDocument17 pagesLab Report Experiment 2aaa - EditAtikah Jembari100% (1)
- Experiment 6 Titration II - Acid Dissociation ConstantDocument8 pagesExperiment 6 Titration II - Acid Dissociation ConstantPanneer SelvamNo ratings yet
- Acetic Acid Dissociation Constant S11Document7 pagesAcetic Acid Dissociation Constant S11Ayesha ShahidNo ratings yet
- 24 Acid-Base TitrationDocument5 pages24 Acid-Base Titrationgardarr11No ratings yet
- CHEM A 24 COMP Half TitrationDocument4 pagesCHEM A 24 COMP Half TitrationSung Hoon ParkNo ratings yet
- Practical 4Document2 pagesPractical 4vimukthi gunasinghaNo ratings yet
- Mixture of Carbonate BicarbonateDocument9 pagesMixture of Carbonate BicarbonateIan Justine SanchezNo ratings yet
- KaDocument5 pagesKaSonu DubeyNo ratings yet
- Lab Report Acid BaseDocument4 pagesLab Report Acid Basexuni34No ratings yet
- Potentiometric Titration Ex17Document10 pagesPotentiometric Titration Ex17Tien HaminhNo ratings yet
- Exp 6 - Acid Base Titration-2Document9 pagesExp 6 - Acid Base Titration-2liquidsnake007No ratings yet
- Potentiometric Titration of A Weak Acid: Chemistry 135 Clark CollegeDocument14 pagesPotentiometric Titration of A Weak Acid: Chemistry 135 Clark CollegeMay LeeNo ratings yet
- Lab Report Experiment 2 Determination of Ka Value of A Weak AcidDocument17 pagesLab Report Experiment 2 Determination of Ka Value of A Weak AcidarisyahariffNo ratings yet
- CWV 35 COMP Phosphoric - Acid PDFDocument4 pagesCWV 35 COMP Phosphoric - Acid PDFNaveen KumarNo ratings yet
- Potentiometric TitrationDocument3 pagesPotentiometric TitrationDaniele Joseph HizonNo ratings yet
- Determination of The Ka Ofa Weak AcidDocument7 pagesDetermination of The Ka Ofa Weak AcidFikrie MuhdNo ratings yet
- Lab 2 Eng Chem LabDocument19 pagesLab 2 Eng Chem LabillyzlNo ratings yet
- Practical 04 - Estimation of PKa by Half Neutralization MethodDocument10 pagesPractical 04 - Estimation of PKa by Half Neutralization Methodsandi fernandoNo ratings yet
- Acid Base TitrationDocument12 pagesAcid Base TitrationMsfaeza HanafiNo ratings yet
- Written Report Expt.8Document5 pagesWritten Report Expt.8Nicole NatanauanNo ratings yet
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDocument5 pagesAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- Quantitative Determination of Potassium Acid Phthalate KHPDocument17 pagesQuantitative Determination of Potassium Acid Phthalate KHPMichelle Cruz AbrilNo ratings yet
- 5: PH Measurement and Its Applications (Experiment) : ObjectivesDocument19 pages5: PH Measurement and Its Applications (Experiment) : ObjectivesNajmi NasirNo ratings yet
- CH142Exp5Titration PDFDocument7 pagesCH142Exp5Titration PDFSako RasheedNo ratings yet
- Sinha TitrationcurvesDocument11 pagesSinha TitrationcurvesRadu StafiNo ratings yet
- PH Titration LabQuest PDFDocument3 pagesPH Titration LabQuest PDFAntónio MatiasNo ratings yet
- Titration Curves of Strong and Weak Acids and BasesDocument3 pagesTitration Curves of Strong and Weak Acids and BasesMatthew Runyon50% (2)
- GA7 Potentio Titr Rev7 99Document9 pagesGA7 Potentio Titr Rev7 99Jerome SadudaquilNo ratings yet
- Sample Lab Report For Experiment 2Document2 pagesSample Lab Report For Experiment 2Ashfaq AhmadNo ratings yet
- LSM1101 Practical 1Document6 pagesLSM1101 Practical 1givena2ndchance100% (1)
- Acid - Base Lab 2016 BegleyDocument7 pagesAcid - Base Lab 2016 BegleyIsaac SnitkoffNo ratings yet
- Determination Acetic AcidDocument21 pagesDetermination Acetic Acidameyakem100% (1)
- ABcurves SP 19Document8 pagesABcurves SP 19Sakshi BangarwaNo ratings yet
- Sarthak Dadkar PIIAL Prac 4 PDFDocument5 pagesSarthak Dadkar PIIAL Prac 4 PDFAjuba AbujaNo ratings yet
- Lab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, KaDocument10 pagesLab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, Kaenock yegonNo ratings yet
- Module Anachem Acid-Base 2Document9 pagesModule Anachem Acid-Base 2arejay castroNo ratings yet
- Chem 18.1 Experiment 6 Formal ReportDocument5 pagesChem 18.1 Experiment 6 Formal Reportlouize_1496No ratings yet
- Titration of A Diprotic Acid Identifying An Unknown: ObjectiveDocument9 pagesTitration of A Diprotic Acid Identifying An Unknown: ObjectivePuji WulandariNo ratings yet
- Summary: Phthalate (KHP) Solution Which The Molarity Is Already Known. Expressing The Chemical ReactionDocument15 pagesSummary: Phthalate (KHP) Solution Which The Molarity Is Already Known. Expressing The Chemical ReactionDayledaniel SorvetoNo ratings yet
- Lab Report IonizationDocument6 pagesLab Report IonizationJasmeetSinghNo ratings yet
- Determining The Amount of Acetic Acid in VinegarDocument2 pagesDetermining The Amount of Acetic Acid in VinegarAadhi JNo ratings yet
- WINSEM2022-23 BBIT206P LO VL2022230503900 Reference Material I 23-12-2022 Experiment 2 Acid-Base Titration PH Meter FADocument4 pagesWINSEM2022-23 BBIT206P LO VL2022230503900 Reference Material I 23-12-2022 Experiment 2 Acid-Base Titration PH Meter FAGravity JaiNo ratings yet
- ProjectDocument2 pagesProjectCindy Mae A. PogoyNo ratings yet
- Lab Report 3 KotDocument15 pagesLab Report 3 KotNikMuhammadIzzatNo ratings yet
- Lab Format:: Lab 2: Determination of Carbonate/BicarbonateDocument5 pagesLab Format:: Lab 2: Determination of Carbonate/BicarbonateAnaya FatimaNo ratings yet