Professional Documents
Culture Documents
Equilibria Quiq
Uploaded by
SahanNivanthaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Equilibria Quiq
Uploaded by
SahanNivanthaCopyright:
Available Formats
A2 CHEMISTRY
1.
EQULIBRIA
SAHAM
A saturated solution of calcium hydroxide, Ca(OH) 2(aq), has a pH of 9.6.
(a)
Write an expression linking hydrogen ion concentration and pH. Use this to calculate
the concentration of hydrogen ions in this solution.
(3)
(b)
(i)
The ionisation constant for water, Kw = 1.0 10
14
mol dm .
Write the expression for Kw.
Kw =
(1)
(ii)
Calculate the concentration of hydroxide ions in the saturated solution of
calcium hydroxide.
(1)
(iii)
Calculate the concentration of calcium hydroxide in the saturated solution.
(1)
A2 CHEMISTRY
(iv)
EQULIBRIA
SAHAM
Calculate the solubility of calcium hydroxide in g dm .
Give your answer to three significant figures.
(1)
(v)
Suggest why your calculated value may differ significantly from the value in
chemistry reference books.
.........................................................................................................................
.........................................................................................................................
(1)
(c)
An alternative method for finding the solubility of calcium hydroxide is to titrate 100
3
cm of the saturated solution with hydrochloric acid of concentration 0.00100 mol dm
.
Ca(OH)2 (aq) + 2HCl (aq) CaCl2 (aq) + 2H2O (l)
(i)
Calculate the pH of the hydrochloric acid.
(1)
(ii)
Use your answer to (b)(iii) and the information above to calculate the volume of
3
hydrochloric acid needed to neutralise 100 cm of the saturated calcium
hydroxide solution.
(3)
A2 CHEMISTRY
(iii)
EQULIBRIA
SAHAM
Sketch the titration curve for this reaction.
(2)
(iv)
Suggest why phenolphthalein is not a suitable indicator for this reaction.
..........................................................................................................................
..........................................................................................................................
(1)
(Total 15 marks)
2.
In the Ostwald process for the production of nitric acid, ammonia is oxidised at 900 C over
a platinum/ rhodium alloy catalyst according to the equation:
4NH3(g) + 5O2(g)
4NO(g) + 6H2O(g)
The reaction is very exothermic.
(a)
(i)
Why do the concentrations of the substances in an equilibrium mixture remain
constant?
....
....
(1)
A2 CHEMISTRY
(ii)
EQULIBRIA
SAHAM
State with a reason the effect of an increase in pressure on this equilibrium
system.
....
....
....
(2)
(b)
The mixture obtained from the catalyst chamber contains excess oxygen.
Write the equation for the further reaction that occurs on cooling this mixture.
....
(1)
(ii)
Show, by means of an equation, how the product in (b) (i) is used to make
nitric acid.
....
(2)
(c)
(i)
Sketch a Maxwell-Boltzmann distribution that could represent the energies of
the molecules in the Ostwald reaction system at a given temperature.
(2)
A2 CHEMISTRY
(ii)
EQULIBRIA
SAHAM
Use your diagram and any necessary explanation to show how the presence of a
catalyst leads to an increase in reaction rate at the same temperature.
....
....
....
....
....
....
(4)
(Total 12 marks)
3.
(a)
Define the following terms.
(i)
pH .....................................................................................................................
(1)
(ii)
Kw ......................................................................................................................
(1)
(b)
Explain the meaning of the term strong, as applied to an acid or a base.
......
......
(1)
(c)
Calculate the pH of the following solutions.
(i)
HCl(aq) of concentration 0.200 mol dm .
A2 CHEMISTRY
EQULIBRIA
SAHAM
(1)
A2 CHEMISTRY
(ii)
EQULIBRIA
SAHAM
NaOH (aq) of concentration 0.800 mol dm (Kw = 1.00 10
14
mol dm ).
(2)
(d)
HA is a weak acid with a dissociation constant Ka = 5.62 10 mol dm .
(i)
Write an expression for the dissociation constant, Ka, of HA.
(1)
(ii)
Calculate the pH of a 0.400 mol dm solution of HA.
(3)
A2 CHEMISTRY
(e)
EQULIBRIA
SAHAM
A buffer solution contains HA(aq) at a concentration of 0.300 mol dm , and its
3
sodium salt, NaA, at a concentration of 0.600 mol dm . Calculate the pH of this
buffer solution.
(3)
(Total 13 marks)
4.
Consider the following equation:
2SO2 + O2
2SO3
3
2.0 moles of SO2 and 1.0 mole of O2 were allowed to react in a vessel of volume 60 dm .
At equilibrium 1.8 moles of SO3 had formed and the pressure in the flask was 2 atm.
(a)
(i)
Write the expression for Kc for this reaction between SO2 and O2.
(1)
(ii)
Calculate the value of Kc, with units.
(3)
A2 CHEMISTRY
(b)
EQULIBRIA
SAHAM
The reaction between SO2 and O2 is exothermic. State the effect on the following, if
the experiment is repeated at a higher temperature:
(i)
Kc ..
(1)
(ii)
the equilibrium position ....................................................................................
(1)
(c)
State the effect of a catalyst on:
(i)
Kc ..
(1)
(ii)
the equilibrium position ....................................................................................
(1)
(d)
(i)
Write the expression for Kp for the reaction between SO2 and O2.
(1)
(ii)
Calculate the mole fractions of SO2, O2 and SO3 at equilibrium.
(2)
A2 CHEMISTRY
(iii)
EQULIBRIA
SAHAM
Calculate the partial pressures of SO2, O2 and SO3 at equilibrium.
(1)
(iv)
Calculate the value of Kp, with units.
(2)
(Total 14 marks)
111. (a)
The electronic configuration of a cobalt atom can be written as [Ar]3d 4s .
Give the electronic configuratin of the Co
3+
ion.
......
(1)
(b)
(i)
By reference to the standard electrode potentials given below, suggest a
reducing agent which might reduce aqueous Co
your reasoning.
3+
ions to cobalt metal. Give
E /V
2+
Zn(s)
0.76
2+
Fe(s)
0.44
2+
Co(s)
0.28
2+
Sn(s)
0.14
Zn (aq) + 2e
Fe (aq) + 2e
Co (aq) + 2e
Sn (aq) + 2e
10
A2 CHEMISTRY
EQULIBRIA
+
O2(g) + 2H (aq) + 2e
3+
Co (aq) + e
H2O2(aq)
2+
Co (aq)
SAHAM
+0.68
+1.82
Suitable reducing agent ....
Reasoning .....
.......
(3)
(ii)
Suggest two factors that might prevent a reducing agent from being as effective
as the electrode potentials might seem to suggest.
.......
.......
.......
.......
(2)
(c)
(i)
Write the formula of the hexaaquacobalt(II) ion.
.......
(1)
(ii)
Give an equation, involving the hexaaquacobalt(II)ion, to illustrate the process
of ligand exchange.
.......
(2)
(Total 9 marks)
11
A2 CHEMISTRY
5.
(a)
EQULIBRIA
SAHAM
A mixture of hydrogen iodide, hydrogen and iodine (all in the gaseous state)
establishes dynamic equilibrium if a constant temperature is maintained.
2HI (g)
(i)
H2 (g) + I2 (g)
H = +9.6 kJ mol
Explain the meaning of the term dynamic equilibrium.
............................................................................................................................
............................................................................................................................
............................................................................................................................
(2)
(ii)
How, if at all, would the proportion of hydrogen iodide present at equilibrium
change if the temperature were to be increased? Justify your answer.
............................................................................................................................
............................................................................................................................
............................................................................................................................
............................................................................................................................
(2)
(iii)
The reaction is catalysed by metals such as gold and platinum. How, if at all,
would the proportion of hydrogen iodide present at equilibrium change if the
reaction were to be catalysed? Justify your answer.
............................................................................................................................
............................................................................................................................
(1)
12
A2 CHEMISTRY
(b)
EQULIBRIA
SAHAM
Part of an energy profile for this reaction is shown below. It is not intended to be to
scale.
Complete the profile showing:
the products;
the progress of both uncatalysed and catalysed reactions;
labelled arrows to indicate the activation energies of both the uncatalysed and
catalysed reactions.
(4)
(Total 9 marks)
6.
Thermochemical data, at 298 K, for the equilibrium between zinc carbonate, zinc oxide and
carbon dioxide is shown below.
ZnCO
3(s)
ZnO(s) + CO2(g)
H = +71.0 kJ mol
1
S [ZnO(s)] =
+43.6 J mol K
S [ZnCO3(s)] =
+82.4 J mol K
13
A2 CHEMISTRY
EQULIBRIA
SAHAM
S [CO2(g)] = +213.6 J mol K
(a)
(i)
Suggest reasons for the differences between the three standard entropies.
............................................................................................................................
............................................................................................................................
............................................................................................................................
............................................................................................................................
(2)
(ii)
Calculate the entropy change for the system, S
system
, for this reaction. Include
the sign and units in your answer.
(2)
(b)
Calculate the entropy change for the surroundings, S
your method clearly.
surroundings,
at 298 K, showing
(2)
14
A2 CHEMISTRY
(c)
(i)
EQULIBRIA
SAHAM
Calculate the total entropy change for this reaction, S
total,
at 298 K.
(1)
(ii)
What does the result of your calculation in (c)(i) indicate about the natural
direction of this reaction at 298 K?
Justify your answer.
............................................................................................................................
............................................................................................................................
(1)
(d)
(i)
Write an expression for the equilibrium constant, Kp, for this reaction.
(1)
15
A2 CHEMISTRY
(ii)
EQULIBRIA
SAHAM
State how you would alter ONE condition to increase the yield of carbon
dioxide from this equilibrium reaction.
Justify your answer.
...........................................................................................................................
...........................................................................................................................
...........................................................................................................................
...........................................................................................................................
(2)
(Total 11 marks)
7.
The reaction in the Haber Process that is used to produce ammonia is a homogeneous
dynamic equilibrium:
N2(g) + 3H2(g)
(a)
2NH3(g)
H = 92 kJ mol
State the meaning of the terms:
(i)
dynamic equilibrium;
............................................................................................................................
............................................................................................................................
(2)
(ii)
homogeneous.
............................................................................................................................
(1)
(b)
Give, with a reason in each case, the effect of the following on the position of the
equilibrium above:
16
A2 CHEMISTRY
EQULIBRIA
17
SAHAM
A2 CHEMISTRY
(i)
EQULIBRIA
SAHAM
an increase in pressure;
............................................................................................................................
............................................................................................................................
............................................................................................................................
(2)
(ii)
an increase in temperature.
............................................................................................................................
............................................................................................................................
............................................................................................................................
(2)
(Total 7 marks)
8.
(a)
Methane reacts with steam in a reversible reaction. In industry this reaction, carried
out at a pressure of 30 atm, is used to produce hydrogen for the manufacture of
ammonia
CH4(g) + H2O(g)
(i)
CO(g) + 3H2(g)
H = +210 kJ mol
Define the term partial pressure as applied to a gas mixture.
............................................................................................................................
............................................................................................................................
(1)
(ii)
Write an expression for the equilibrium constant, Kp, for this reaction.
18
A2 CHEMISTRY
EQULIBRIA
SAHAM
(1)
19
A2 CHEMISTRY
(iii)
EQULIBRIA
SAHAM
State and explain the effect of increasing the total pressure on the position of
this equilibrium;
............................................................................................................................
............................................................................................................................
............................................................................................................................
............................................................................................................................
............................................................................................................................
(2)
(b)
State the effect on the value of Kp for this equilibrium of the following.
(i)
Increasing the total pressure.
............................................................................................................................
(1)
(ii)
Increasing the temperature.
............................................................................................................................
(1)
(iii)
Adding a catalyst.
............................................................................................................................
(1)
(c)
There is a theory that methane, CH4, constantly leaks from the earths crust. This is not
noticeable on land but at the bottom of a cold sea, such as off the Canadian coast, the
methane is trapped in a solid cage of water molecules.
CH4(g) + 6H2O(s)
20
[CH4(H2O)6](s)
methane hydrate
A2 CHEMISTRY
EQULIBRIA
SAHAM
At 29 C the equilibrium pressure of the methane is 101.3 kPa.
(i)
Write an expression for Kp for this equilibrium.
(1)
(ii)
Deduce the value of Kp at 29 C, stating its units.
(1)
(iii)
At 0 C the equilibrium pressure of methane rises to 2600 kPa. What does this
tell you about the effect of temperature change on the position of equilibrium
and about the enthalpy change for this reaction?
............................................................................................................................
............................................................................................................................
............................................................................................................................
(2)
21
A2 CHEMISTRY
(iv)
EQULIBRIA
SAHAM
Some people have suggested collecting the methane hydrate from the bottom of
the sea and allowing it to warm up to 0 C on board a ship. Comment on
whether this would be a useful method for collecting methane.
............................................................................................................................
............................................................................................................................
............................................................................................................................
(1)
(Total 12 marks)
9.
Ethanoic acid, CH3COOH, is a weak acid which can be used, with its salts, to make buffer
solutions.
(a)
Explain what is meant by the term weak acid.
....................................................................................................................................
....................................................................................................................................
(1)
(b)
Explain what is meant by the term buffer solution.
....................................................................................................................................
....................................................................................................................................
(2)
22
A2 CHEMISTRY
(c)
EQULIBRIA
SAHAM
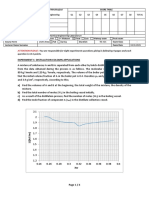
An aqueous solution of ethanoic acid of concentration 1.00 mol dm
has a pH of 2.8.
3
Sketch, with care, how the pH changes during the titration of 25.0 cm 1.00 mol dm
aqueous ethanoic acid with aqueous sodium hydroxide of the same concentration.
14
pH
12
10
8
6
4
2
0
10
20
30
40
50
V o lu m e o f a lk a li a d d e d /c m 3
(4)
(d)
Indicate on your sketch the portion of the curve where the mixture is behaving as a
buffer.
(1)
(e)
(i)
Write an expression for the dissociation constant, Ka, for ethanoic acid.
(1)
(ii)
Explain how the pKa of ethanoic could be found from the graph.
............................................................................................................................
............................................................................................................................(2)
(Total 11 marks)
23
A2 CHEMISTRY
EQULIBRIA
24
SAHAM
You might also like
- Criminal EvidenceDocument6 pagesCriminal EvidenceSahanNivanthaNo ratings yet
- Civil ProcedureDocument28 pagesCivil ProcedureSahanNivanthaNo ratings yet
- Cie Structured Quiz Practice AnswersDocument7 pagesCie Structured Quiz Practice AnswersSahanNivanthaNo ratings yet
- Cie A2 ElectrochemistryDocument20 pagesCie A2 ElectrochemistrySahanNivanthaNo ratings yet
- Bisnus LogoDocument1 pageBisnus LogoSahanNivanthaNo ratings yet
- Chapter Summary Worksheet: Chapter 4 Application of Rates and EquilibriumDocument2 pagesChapter Summary Worksheet: Chapter 4 Application of Rates and EquilibriumSahanNivanthaNo ratings yet
- Chapter Summary WorksheetDocument2 pagesChapter Summary WorksheetSahanNivanthaNo ratings yet
- Constitutional Law - AssignmentDocument42 pagesConstitutional Law - AssignmentSahanNivanthaNo ratings yet
- Fndÿ J! A SH Yd JHDMDSSL CD SL Ixioh: YeoskaúuDocument5 pagesFndÿ J! A SH Yd JHDMDSSL CD SL Ixioh: YeoskaúuSahanNivanthaNo ratings yet
- Chapter Summary Worksheet: Chapter 2 Entropy: How Far?Document1 pageChapter Summary Worksheet: Chapter 2 Entropy: How Far?SahanNivanthaNo ratings yet
- Chapter 1 PDFDocument2 pagesChapter 1 PDFSahanNivanthaNo ratings yet
- Advanced English Paper 1Document8 pagesAdvanced English Paper 1SahanNivanthaNo ratings yet
- HRM AssignmentDocument61 pagesHRM AssignmentSahanNivantha0% (1)
- Employment Law AssignmentDocument37 pagesEmployment Law AssignmentSahanNivantha0% (1)
- Assignment-Law of ContractDocument44 pagesAssignment-Law of ContractSahanNivantha100% (1)
- Legal System 1Document20 pagesLegal System 1SahanNivanthaNo ratings yet
- Assignment Criminal LawDocument72 pagesAssignment Criminal LawSahanNivantha100% (1)
- (A) (B) (C) (D) (E) (F) (A) (B) (C) (D) (E) (F) (G) : First YearDocument1 page(A) (B) (C) (D) (E) (F) (A) (B) (C) (D) (E) (F) (G) : First YearSahanNivanthaNo ratings yet
- Organic Short Notes: Functiona L Group Formula - Suffi XDocument2 pagesOrganic Short Notes: Functiona L Group Formula - Suffi XSahanNivanthaNo ratings yet
- Athma PooõwaDocument2 pagesAthma PooõwaSahanNivanthaNo ratings yet
- Agrarian Development Act, No. 46 of 2000Document82 pagesAgrarian Development Act, No. 46 of 2000SahanNivanthaNo ratings yet
- Unit 5 Mock Paper1Document78 pagesUnit 5 Mock Paper1SahanNivanthaNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Unit 3 Jan 2019 QPDocument28 pagesUnit 3 Jan 2019 QPThe Game SlackerNo ratings yet
- Flash and Fire Point of Lubricant ExperimentDocument5 pagesFlash and Fire Point of Lubricant ExperimentShanti Kiran Z100% (1)
- Atomic Physics Using Short-Wavelength Coherent RadiationDocument10 pagesAtomic Physics Using Short-Wavelength Coherent RadiationmukphyzicsNo ratings yet
- Lesson 2 Matter in The Liquid PhaseDocument27 pagesLesson 2 Matter in The Liquid PhaseDarren Daniel InfanteNo ratings yet
- PWLS 1.0.1 ToolsDocument222 pagesPWLS 1.0.1 Toolspot100% (1)
- Principles of General Chemistry 3rd Edition Silberberg Test Bank 1Document14 pagesPrinciples of General Chemistry 3rd Edition Silberberg Test Bank 1william100% (47)
- Sameer Khan ProjectDocument113 pagesSameer Khan ProjectSameer KhanNo ratings yet
- Compressible Gas Flow in PipelinesDocument3 pagesCompressible Gas Flow in PipelinesRahul ChandrawarNo ratings yet
- Heat and Mass Formula SheetDocument14 pagesHeat and Mass Formula Sheetramy100% (2)
- CSEC Physics June 2014 P2Document19 pagesCSEC Physics June 2014 P2Kriston KhanNo ratings yet
- Self-Cleaning Cotton FabricsDocument6 pagesSelf-Cleaning Cotton FabricsAliAkbarPamungkasNo ratings yet
- Isolobal AnalogyDocument4 pagesIsolobal Analogyindu priyaNo ratings yet
- Sas7 STM-005Document6 pagesSas7 STM-005mayasNo ratings yet
- QB PDFDocument18 pagesQB PDFShivani0% (1)
- Hydrolysis of Cellulose To Glucose by Solid Acid CatalystsDocument17 pagesHydrolysis of Cellulose To Glucose by Solid Acid CatalystsCaesar SiregarNo ratings yet
- Waterborn Silicate PaintsDocument98 pagesWaterborn Silicate PaintsJane Ashworth100% (1)
- Liposomes 181012045701Document55 pagesLiposomes 181012045701Ravi ParasharNo ratings yet
- Saft MieDocument93 pagesSaft MiePatrice PariNo ratings yet
- Chemistry Form 1Document14 pagesChemistry Form 1MORRIS ANUNDANo ratings yet
- Propulsion PDFDocument20 pagesPropulsion PDFAmritansh RanjanNo ratings yet
- Chemistry Interim Assessment 2 Teacher Booklet: Kipp New Orleans SchoolsDocument20 pagesChemistry Interim Assessment 2 Teacher Booklet: Kipp New Orleans SchoolsTyneishah SpearsNo ratings yet
- FinalDocument4 pagesFinalSimge DemirNo ratings yet
- Chem 31 NotesDocument4 pagesChem 31 NotesEvernim OmpacanNo ratings yet
- Lecture 6 STMDocument29 pagesLecture 6 STMROHITM RA1811002040067No ratings yet
- Sample Paper HMT EME504Document1 pageSample Paper HMT EME504manidev25% (4)
- Preparation of DEETDocument4 pagesPreparation of DEETdevbones18No ratings yet
- Concept of PH and BufferDocument27 pagesConcept of PH and BufferRolling Coast100% (1)
- Corrosion of Constructional Steels in Marine and Industrial Environment - 2013Document188 pagesCorrosion of Constructional Steels in Marine and Industrial Environment - 2013lei huangNo ratings yet
- Govpub C13Document54 pagesGovpub C13Matty WrobelNo ratings yet
- Haloalkanes Q BankDocument7 pagesHaloalkanes Q BankVishnuNo ratings yet