Professional Documents
Culture Documents
Lupin Alkaloid
Uploaded by
iynaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lupin Alkaloid
Uploaded by
iynaCopyright:
Available Formats
The Lupin Alkaloids
Ian Bass Seiple
10/11/2006
The Lupin Alkaloids
I.B. Seiple
Baran Group Meeting
10/11/2006
Lupin
Grain legume, high in protein, commonly used for livestock feed in Europe, Africa, Australia, and Asia
Also used in cereals, baby formula, pasta, soups and salads in the United States (22 states, 42,000 pounds/year)
Seeds used in traditional Chinese medicine
Has been cultivated for over 2000 years
Over 500 species of the genus Lupinus known
In its raw form, the mildly toxic lupin alkaloids present in the plants causes a bitter taste, and are used as a defensive mechanism against herbivores
Alkaloids are commonly removed (or reduced) by soaking the raw seeds in water prior to use
In the 1920's, German plant breeders produced the first alkaloid-free, "sweet" lupin
Lupin Alkaloids - Biological Properties
(-)-Cytisine has been identified as a selective partial nicotinic receptor agonist (nicotinic acetylcholine recepters are affected by Parkinson's and
Alzheimer's diseases) (Nicotine is a full agonist at neuronal nAChR's, and has additional undesirable biological effects)
Alkaloid extracts from Lupinus species have recently shown antimicrobial activity
L. albus showed inhibitory effects on Gram negative bacteria
L. varius and L. densiflorus strongly inhibited Gram positive bacteria
Many known lupin alkaloids show significant antifungal activity
matrine has shown antiulcerogenic and anticancer activities.
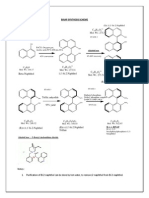
Quinolizidine Core Structure
5
4
7
6
1
2
10
The Lupin Alkaloids
I.B. Seiple
HO
HO
(-)-Lupinine
(+)-Epilupinine
OH
N
Albine
H
N
OH
(+)-Lupanine
Retamine
Aphyllidine
Aphylline
(-)-Sparteine
O
Angustifoline
NH
(-)-Cytisine (R = H)
(many alkylated
derivatives)
Lusitanine
NH
NR
NHAc
Baran Group Meeting
10/11/2006
Baptifoline
Multiflorine
O
N
O
N
H
H
Camoensidine
H
H
(+)-Matrine
H
H
H
H
(-)-Sophocarpine
H
H
H
H
(-)-Sophoridine
(-)-Sophoramine
H
H
(+)-Allomatrine
N
H H
(+)-aloperine
The Lupin Alkaloids
I.B. Seiple
Baran Group Meeting
10/11/2006
Biosynthesis
NH2
CO2H
H
NH2
Decarboxylation
NH2
H'
H
NH2
NH
NH+
NH2
NH
L-lysine
O
NH2
H
NH
H
N+
N+
[O], condensation
N+
NH+
H- delivery
O
H
H
N
N+
H
H
N
W. M. Golebiewski, I. D. Spenser, Can. J. Chem., 1988, 66, 1734
(-)-sparteine
HO
reduction
H
N
(-)-Lupinine
NH+
The Lupin Alkaloids
I.B. Seiple
EtO2C
Br
NH2
HIO4, pH = 5
buffer, rt
rt to reflux, Et2
EtO2C
CO2Et Na, xylenes, reflux
N
Vibramixer (41%)
K2CO3 (28%)
(after two distillations)
LiAlH4, Et2O
H
N
O
Bn
N
OH
1. LiAlH4
2. H2, Pd/C
OH
OH
(82%)
Bn
N
HO
O
O
Baran Group Meeting
10/11/2006
epilupinine
paraformaldehyde
acetic acid
acetone
N
H HCl
Hg(OAc)2, acetic acid
reflux, K2CO3
N+
O
N
reflux
multiple xtalizations
(13%)
KOH, diethylene glycol
hydrazine hydrate, 75 C
to 200 C
N+
N
N
()-sparteine
E. E. van Tamelen and R. L. Foltz, J. Am. Chem. Soc. 1969, 91 (26), 7372-7377
The Lupin Alkaloids
I.B. Seiple
BnO
BnO
LiAlH4, THF, 0 C
formaldehyde (37% aq)
28 h, 65 C (82%)
Baran Group Meeting
10/11/2006
BnO
BnO
H2 (50 psi), Pd/C,
HCl, EtOH (100%)
BnO
H
H2N
Lupinine
NH2
(separable)
1.6
H2 (50 psi), Pd/C,
HCl, EtOH (100%)
P. A. Grieco and D. T. Parker, J. Org. Chem. 1988, 53, 3325-3330
BnO
H
N
Epilupinine
O
N
Tol
LDA, THF
ethylcyanoformate
-78 to rt, 2 h
LDA, THF,
LDA, THF
-20 to -10 C (92%)
S
Tol
O S
CO2Et
H
O
S
O S
I
Tol -78 to rt, 29 h (62%)
1. LiAlH , THF
4
Tol
Et2O, 0 C, 3 h (88%)
O S
CO2Et 2. Raney-Ni, EtOH (90%)
H
+
N
11%
84%
Tol
CeCl3
7 H2O
NaBH4
MeOH
O S
H
Tol
O S
H
Tol
+
N
30%
58%
OH
H
Also, Al-Hg removal of the sulfer was
attempted before reduction, resulting in a
1:3.6 ratio, with a slightly reduced yield.
(+)-epilupinine
1. LiAlH4, THF
Et2O, 0 C, 3 h (88%)
2. Raney-Ni, EtOH (90%)
OH
H
N
(-)-lupinine
D. H. Hua et al, Synthesis, 1991, 970-974
It is also important to note that starting with
the enantiomer of the menthyl sulfinate
yields (-)-lupinine as the major product in
similar ratios.
The Lupin Alkaloids
I.B. Seiple
OAc
1. (SOCl)2, DMSO, Et3N, (91%)
2. (-)-B-methoxydiisopinocampheylborane,
allyltrimethylsilane, Et2O, -78 C, then
NaOH, H2O2 (76%)
3. MsCl, Et3N, CH2Cl2 (99%)
OH
OAc
Grubb's catalyst
CH2Cl2, reflux (79%)
HN
H
OAc
O
N
DDQ, dioxane
reflux (50%)
Cbz
Cbz
CH2OAc
CH2OAc SiMe
3
90%
Cbz
TFA, 0 C
72%
N
OH
H
N
Silvani et al, Org. Lett., 2004, 6 (4), 493-496
H
(-)-Cytisine
OH
N
H
1. (COCl)2, DMSO, Et3N,
CH2Cl2, -65 C to rt
2. KMnO4, ButOH-phosphate
buffer (pH 7.2)
Cbz
NaH, THF, rt (89%)
HN
H
Cbz
H
N
OMs
6 N HCl, reflux (78%)
HN
H
1. PPh3, THF, then H2O (66%)
2. acryloyl chloride, Et3N,
CH2Cl2 (89%)
Cbz
O
1. NaOH, THF (98%)
2. MsCl, Et3N
CH2Cl2 (67%)
Cbz
N3
Cbz
NaN3, DMF, 80 C
SiMe3
OAc
Cbz
Cbz
HO
OMs
OAc
Baran Group Meeting
10/11/2006
Cbz
Me3Si
1. DCC, HOBt, MeCN, rt
2. NaOH, MeOH, rt
62% aldehyde
26% cyclized product
HN
Cbz
H2N
1. Dess-Martin
CH2Cl2, rt
CH2OH
83%
Cbz
2. K2CO3, MeOH
10% Pd-C, Et3N-THF (2:8)
120 C, sealed tube, 8 h
N
N
D. Lesma et al., Eur. J. Org. Chem., 2001, 1377
Cbz
H2, 10% Pd-C, EtOH
65%
(-)-Virgilidone
The Lupin Alkaloids
I.B. Seiple
1. (Boc)2O, Et3N, CH2Cl2 (quant.)
2. (COCl)2, DMSO, Et3N, CH2Cl2
-40 to rt (91%)
HO
CO2Me
O
CO2Me
Baran Group Meeting
10/11/2006
LiHMDS, N-(5-chloro-2-pyridyl)triflimide
THF, -78 C to -20 C (89%)
TfO
CO2Me
N
H
Boc
Pd(PPh3)4, LiCl, CuI
2-tributylstannyl-6-methoxypyridine
THF, 65 C (88%)
Boc
1. H2, Pd/C, MeOH, rt (quant.)
2. TFA, CH2Cl2, 0 C (quant.)
N
MeO
CO2Me
MeO
N
Boc
1. NaH, MeI or BnBr, THF-HMPA
0 C to rt (quant.)
2. LDA, ClCO2Et, THF
-78 C (quant.)
SmI2, THF-HMPA
MeOH, 0 C to rt (78%)
MeO
MeO
N
N
H
CO2Et
OH
CO2Me
NH
N
R
LiAlH4, THF, 0 C to rt (96% total)
OH
OH
OH
TMSCl, NaI, MeCN, reflux
MeO
MeO
N
R
N
R
50%
(R = Me)
46%
O
(R = Bn)
H
H
N
Bn
N
H
N
Me
83%
quant.
(-)-kuraramine
(-)-isokuraramine
H2, Pd(OH)2,
ammonium formate
MeOH, reflux (81%)
+
N
Me
(-)-jussiaeiine A (R = Me)
MsCl, Et3N, CH2Cl2
0 C, then toluene
reflux (89%)
N
H
T. Honda et al, J. Org. Chem. 2005, 70, 499-504
N
H
H
N
H
(+)-cytisine
The Lupin Alkaloids
I.B. Seiple
Baran Group Meeting
10/11/2006
Br-
Bn
Br
(Bu3Sn)2, BnPd(Ph3P)2Cl
DMF 130 C
1. LiAlH4, Et2O
2. BnBr, MeCN
CO2Me
Br
CO2Me
40 - 50%
70 - 80%
OMe
O'Neil, B. T. et. al. Org. Lett. 2000, 2, 4201
OMe
OH
OMe
1. BnBr, MeCN
2. Na2S2O4
H2 (1 atm), PtO2
MeOH/Et3N
76%
100%
85:15 trans:cis
N
O
Bn
Bn
100%
1. LiHMDS, THF, 0 C
2. ClP(O)(OEt)2, THF, -78 to 20 C
H2 (1 atm), PtO2
MeOH
CO2Me
82%
4:1 trans:cis
OMe
OH
OMe
OPO(OEt)2
N
1. MeSO2Cl, Et3N, CH2Cl2
2. PhMe, reflux
3. H2, Pd(OH)2, NH4HCO2, MeOH
P(OAc)2 (2.5 mol %)
P(o-tol)3 (5 mol %)
Et3N (5 eq), MeCN, 60 C
57%
Coe, J. W. Org. Lett. 2000, 2, 4205
1. Me3NO2H2O, OsO4 (cat.)
2. NaIO4, EtOH-H2O (3:1)
3. H2 (50 psi), Pd(OH)2, aq. NH4OH
MnO2, C6H6, 80 C
N
O
58%
74%
O
48% over 3 steps
NH
N
O
()-Cytisine
The Lupin Alkaloids
I.B. Seiple
1. HSiCl3, [(allyl)PdCl]2
(-)-S-MOP
1. Ethylene glycol, TsOH
2. LDA, BnO(CH2)3CHO
2. H2O2, KI, KHCO3
3. Swern
TiCl4
(62%)
3. MsCl
O 4. DBU
(64%)
1. Lawesson's reagent
2. Raney Ni
H2, Pd/C, Pd(OH)2
Zn(N3)22pyr, DEAD, PPh3
O
OBn
O
O
BocNHOBoc,
K2CO3, DMF
Baran Group Meeting
10/11/2006
(78%)
N3
O
O
TFA, 4 MS, then
NaHCO3
(95%)
3. LDA, I(CH2)4Cl
O 4. NaI, acetone
(76%)
(74 - 98%)
N Boc
Cl
BocO
hv (254 nm)
benzene
N
-O
N+
LiAlH4, THF, reflux
N
N
(76%)
O
(95%)
N
N
(+)-sparteine
15.7% overall
J. Aube et al., Org. Lett., 2002, 4 (15), 2577-2579
Note: by starting with the other
norbornadione enantiomer, (-)sparteine could also be
synthesized, but it is
commercially available.
The Lupin Alkaloids
I.B. Seiple
1. LHMDS, EtOCH2Cl,
THF -78 C to rt, 87%
2. KOtBu, THF, -78 C
84%
BnN
1. MeCO2tBu, LDA, THF, -78 C 68%
2. 10% Pd/C, H2 (1 atm), 100%
3. AcOH, toluene, reflux, 73%
BnN
CO2Et
CO2Et
Baran Group Meeting
10/11/2006
O
N
1. LiAlH4, THF
2. PBr3, toluene
70%
CO2Et
O
2-pyridone, K2CO3, Bu4NBr
toluene, reflux
N
O
66%
H
LDA (2 eq), THF, rt, 3 h
44%
MnO2, CH2Cl2
70%
H
Br
N
O
N
N
BH3THF, 0 C to rt
N
85%
O
H
()-thermopsine
()-anagyrine
BocN
LHMDS, (EtO2C)2C=CH2
THF, -78 to rt
BocN
as above
1. TFA, CH2Cl2, THF, 0 C, 100%
2. AcOH, toluene, reflux, 89%
3. NaCl, H2O, DMSO, 130 C 72 h, 72%
EtO2C
CO2Me
79%
EtO2C
CO2Me
Gallagher, T.; Gray, D. Angew. Chem. Int. Ed. 2006, 45, 2419-2423
O
N
CO2Me
The Lupin Alkaloids
I.B. Seiple
OEt
O
OEt
CO2Et
1. H2N
benzene, reflux, -H2O
O
N
Pd/C, AcOH, 50 psi
O
CN NC
2. Adam's cat., 2000 psi
(55%)
EtOH, acetic acid
CO2Et
3. 100 C, neat (lactamization)
CO2Et
(65%)
acrylonitrile, base
1. NaH, benzene, reflux
2. acetic acid, reflux
Baran Group Meeting
10/11/2006
L. Mandell et al, J. Am. Chem. Soc. 1963, 85, 2683-2684
d,l-matrine
CN
O
O
1. LiAlH4, Et2O, rt
2. glutaric anhydride, CHCl3
3. Ac2O, CHCl3, reflux
L-Selectride, CH2Cl2, -30 C
then MeSO3H, CHCl3 rt 20 h
(46% total)
O
N
O O
(56% total)
N
1. 33% H2SO4
2. L-Selectride
H
O
Jen Chen, J. Chem. Soc.,Chem. Comm. 1986, 905-907
3. 1,1'-thiocarbonyldiimidazole, ClCH2CH2Cl
4. Bu3SnH, xylene reflux
d,l-matrine
(23% overall)
The Lupin Alkaloids
I.B. Seiple
MeO2C
CO2Me
CO2Me
Na+
C
N
CO2Me
NH
(86%)
MeO2C
1. Et3N,
2. B(OH)3
toluene, reflux
(100%)
MeO2C
O
N
H
2. EtOCSSK, acetone
(72%)
N
H
EtO
N
H
MeO2C
O
CO2Me
TFA, CH2Cl2
CO2tBu
(90%)
CO2Me
N
H
CO2H
MeO2C
O
MeO2C
O
(COCl)2, CH2Cl2;
N-hydroxy-4-methylthiazolinethione,
Et3N; tert-dodecanethiol, Chx,
AIBN, reflux
H
(56%)
O
CO2tBu
CO2Me
MeO2C
O
1. BH3Me2S, THF
H
N
H
2. 2 M HCl, reflux
(85%)
N
E
N
O
MeO2C
O
N
Propogation
CO2Me
S
OEt
CO2tBu S
N
O
()-matrine
CO2Me
CO2tBu
CO2Me
MeO2C
CO2Me
H
CO2tBu
MeO2C
CO2Me
to
benzene, then
2-propanol, heat
(27% total)
S. Z. Zard et al, Angew. Chem. Int. Ed. 1998, 37 (8), 1128-1131
MeO2C
O
CO2tBu
lauroyl peroxide
CO2tBu 1. ClCH2COCl, Et N
3
(90%)
CO2Me
CO2Me
CO2tBu
CO2tBu H2, Pd/C
Baran Group Meeting
10/11/2006
N
O
You might also like
- CO 301 Heterocyclic ChemistryDocument31 pagesCO 301 Heterocyclic ChemistryMohini BajajNo ratings yet
- Homogeneous Catalysis PDFDocument99 pagesHomogeneous Catalysis PDFevsgoud_goudNo ratings yet
- Rapid and Efficient Reduction of Aliphatic Nitro Compounds To AminesDocument4 pagesRapid and Efficient Reduction of Aliphatic Nitro Compounds To AminesKybernetikumNo ratings yet
- Mitsos Aug 04Document9 pagesMitsos Aug 04fersg12No ratings yet
- Borrowing HydrogenDocument7 pagesBorrowing HydrogenVinayak KhairnarNo ratings yet
- EpscDocument6 pagesEpscVohinh NgoNo ratings yet
- Synthesis and Evaluation of Subglutinol Analogs as Immunomodulatory AgentsDocument36 pagesSynthesis and Evaluation of Subglutinol Analogs as Immunomodulatory AgentsJagjeet GujralNo ratings yet
- 1,7-Cyclogermacra-1 (10), 4-Dien-15-Al, A Sesquiterpene With A Novel Skeleton, and Other Sesquiterpenes From Haitian Vetiver OilDocument23 pages1,7-Cyclogermacra-1 (10), 4-Dien-15-Al, A Sesquiterpene With A Novel Skeleton, and Other Sesquiterpenes From Haitian Vetiver OilРусланNo ratings yet
- 1 ReductionDocument17 pages1 ReductionNajiya JamshyNo ratings yet
- Hoshino 1996Document7 pagesHoshino 1996ivanjavierlozadayala178No ratings yet
- 1 NaphtholDocument7 pages1 NaphtholWalid Ebid ElgammalNo ratings yet
- Simple One-Step Synthesis of Organotin HydridesDocument2 pagesSimple One-Step Synthesis of Organotin HydridesgeliliNo ratings yet
- Erna Fitriana AlfantiDocument6 pagesErna Fitriana AlfantiIzam M. FalahNo ratings yet
- Synthesis and Antimuscarinic Activity of 2-WEthyl-N-lhydroxyethylaminoethyl 22diphenylpropionate A Metaboliteof Aprophen jps.2600820603Document2 pagesSynthesis and Antimuscarinic Activity of 2-WEthyl-N-lhydroxyethylaminoethyl 22diphenylpropionate A Metaboliteof Aprophen jps.2600820603THEUSER0001No ratings yet
- CHJV04I02P0104Document6 pagesCHJV04I02P0104chemistryjournalNo ratings yet
- Synthesis of (3Z) Dodecenyl (E) 2 ButenoateDocument6 pagesSynthesis of (3Z) Dodecenyl (E) 2 ButenoatedangchihienNo ratings yet
- Synthesis of the Steroidal Natural Product Ouabain and Aglycone OuabageninDocument23 pagesSynthesis of the Steroidal Natural Product Ouabain and Aglycone OuabageninNober Sandy LayukNo ratings yet
- Synthesis, Spectral Characterization and In-Vitro Analysis of Some Novel Title Chalcones DerivativesDocument5 pagesSynthesis, Spectral Characterization and In-Vitro Analysis of Some Novel Title Chalcones DerivativesSudhanshu Kumar JhaNo ratings yet
- Appendix Attempts Towards Synthesis of IbuprofenDocument12 pagesAppendix Attempts Towards Synthesis of IbuprofenJayNo ratings yet
- AKD BuchwaldhartwigDocument39 pagesAKD BuchwaldhartwigMurali Venkat NagNo ratings yet
- Reduction of Acids, Esters, Acid Chlorides, Amides, and Nitriles To Amines or Alcohols With NaBH4-BF3-Et2ODocument3 pagesReduction of Acids, Esters, Acid Chlorides, Amides, and Nitriles To Amines or Alcohols With NaBH4-BF3-Et2OalchymystNo ratings yet
- ) - 6a-Hydroxy - ) - 6b-Hydroxycarvotanacetone ) - CarvoneDocument3 pages) - 6a-Hydroxy - ) - 6b-Hydroxycarvotanacetone ) - CarvoneРусланNo ratings yet
- Derivative Vitamin C Application Synthesis of Labelled Ascorbic AcidDocument3 pagesDerivative Vitamin C Application Synthesis of Labelled Ascorbic Acidزياد الحسناويNo ratings yet
- Binap Synthesis SchemeDocument2 pagesBinap Synthesis SchemechidambaramrNo ratings yet
- Synthesis of Ruthenium Nitrosyl Complexes of Bipyridine and Phenanthroline.Document4 pagesSynthesis of Ruthenium Nitrosyl Complexes of Bipyridine and Phenanthroline.Benjamín Marc Ridgway de SassouNo ratings yet
- Reconstruction of Lavandulol Carbon Skeleton Based On Limonene. Synthesis of IsotetrahydrolavandulolDocument2 pagesReconstruction of Lavandulol Carbon Skeleton Based On Limonene. Synthesis of IsotetrahydrolavandulolРусланNo ratings yet
- Cyano ToxinsDocument90 pagesCyano ToxinshackenbergerNo ratings yet
- Reduction of 6 Beta Methoxy 3 Alpha 5 CyDocument4 pagesReduction of 6 Beta Methoxy 3 Alpha 5 Cyvictorubong404No ratings yet
- Rhodium-Catalyzed Triarylphosphine Synthesis Via Cross-Coupling ofDocument43 pagesRhodium-Catalyzed Triarylphosphine Synthesis Via Cross-Coupling ofGabriela ArequipaNo ratings yet
- CHEM 180: Christian MANAHAN Anna Esperanza LEGASPIDocument19 pagesCHEM 180: Christian MANAHAN Anna Esperanza LEGASPIAnna LegaspiNo ratings yet
- PROTECTING GROUPS Carey & Sundberg Chapter 13.1 problems # 1; 2; 3a, b, c ; Smith: Chapter 7Document15 pagesPROTECTING GROUPS Carey & Sundberg Chapter 13.1 problems # 1; 2; 3a, b, c ; Smith: Chapter 7Yogesh ChandrasekaranNo ratings yet
- Alcano 7Document3 pagesAlcano 7Antônio Neto MachadoNo ratings yet
- Chem 215 Myers: Birch ReductionDocument7 pagesChem 215 Myers: Birch ReductionPrasanna AndojuNo ratings yet
- Herbicide 2,4-D Analysis and FormulationsDocument6 pagesHerbicide 2,4-D Analysis and FormulationsKitty van VuurenNo ratings yet
- Carbon-Carbon Bond Formation: Comprehensive Organic Synthesis 1991, Vol. 2, 99Document31 pagesCarbon-Carbon Bond Formation: Comprehensive Organic Synthesis 1991, Vol. 2, 99mmiliyasNo ratings yet
- 39179-Article Text-141298-1-10-20181227 PDFDocument6 pages39179-Article Text-141298-1-10-20181227 PDFNexi anessaNo ratings yet
- Synthesis, Antibacterial and Antifungal Evlaution of Novel Pyrazoline DerivativesDocument6 pagesSynthesis, Antibacterial and Antifungal Evlaution of Novel Pyrazoline DerivativesNexi anessaNo ratings yet
- Inorganic Chemistry Volume 50 Issue 20 2011Document12 pagesInorganic Chemistry Volume 50 Issue 20 2011Lee ToulouseNo ratings yet
- Anti Parasitic Agents From Australian Marine EnvironmentDocument33 pagesAnti Parasitic Agents From Australian Marine EnvironmentEd LiuNo ratings yet
- F Ac 18 1 2012 0510Document36 pagesF Ac 18 1 2012 0510Handugan Quinlog NoelNo ratings yet
- AlkaloidsDocument71 pagesAlkaloidskebajikan7972No ratings yet
- SMNR 2005 Scheerer Jonathan R.Document37 pagesSMNR 2005 Scheerer Jonathan R.Vikneswaran VîçkýNo ratings yet
- Synthesis of 3-sulfonyloxypyridines: Oxidative ring expansion of α-furylsulfonamides and N→O sulfonyl transferDocument5 pagesSynthesis of 3-sulfonyloxypyridines: Oxidative ring expansion of α-furylsulfonamides and N→O sulfonyl transferlapsNo ratings yet
- Alcohol and Biological Markers of Alcohol Abuse - Gas ChromatDocument11 pagesAlcohol and Biological Markers of Alcohol Abuse - Gas ChromatSohail MMNNo ratings yet
- Scifinder®: Mild and Efficient Iodination of Aromatic and Heterocyclic Compounds With The Naclo2/Nai/Hcl SystemDocument2 pagesScifinder®: Mild and Efficient Iodination of Aromatic and Heterocyclic Compounds With The Naclo2/Nai/Hcl SystemJesus Garcia PatiñoNo ratings yet
- Ultrasonic Allylation of Aromatic Aldehydes and KetonesDocument3 pagesUltrasonic Allylation of Aromatic Aldehydes and KetonesTúlio CoutoNo ratings yet
- 1 s2.0 S0040402011012105 MainDocument7 pages1 s2.0 S0040402011012105 MainANTHERSONRNo ratings yet
- Sulfur-Facilitated Organic Synthesis TechniquesDocument58 pagesSulfur-Facilitated Organic Synthesis TechniquesVasudevan SubramaniyanNo ratings yet
- Research Article: Synthesis of New Benzofuran-2-Carboxylic Acid DerivativesDocument8 pagesResearch Article: Synthesis of New Benzofuran-2-Carboxylic Acid Derivativesfatriani smakNo ratings yet
- Ma 8012477Document7 pagesMa 8012477Friska SiamiNo ratings yet
- Vitamin B: Structure & ModificationsDocument42 pagesVitamin B: Structure & ModificationsDhika ArdiansyahNo ratings yet
- The Oxidation of Alcohols and Ethers Using Calcium HypochloriteDocument3 pagesThe Oxidation of Alcohols and Ethers Using Calcium HypochloriteVictor VikeneNo ratings yet
- Halogenated Benzenes, Toluenes and Phenols with Water: Solubility Data SeriesFrom EverandHalogenated Benzenes, Toluenes and Phenols with Water: Solubility Data SeriesAri L. HorvathRating: 5 out of 5 stars5/5 (1)
- Low Molecular Weight Sulphur Containing Natural Products: Plenary Lectures Presented at the International Symposium on Low Molecular Weight Sulphur Containing Natural Products, Jablonna, Warsaw, 12-16 July 1976From EverandLow Molecular Weight Sulphur Containing Natural Products: Plenary Lectures Presented at the International Symposium on Low Molecular Weight Sulphur Containing Natural Products, Jablonna, Warsaw, 12-16 July 1976J. WróbelNo ratings yet
- Fourth International Conference on Non-Aqueous Solutions: Vienna 1974From EverandFourth International Conference on Non-Aqueous Solutions: Vienna 1974V. GutmannNo ratings yet
- Transition Metal-Catalyzed Benzofuran Synthesis: Transition Metal-Catalyzed Heterocycle Synthesis SeriesFrom EverandTransition Metal-Catalyzed Benzofuran Synthesis: Transition Metal-Catalyzed Heterocycle Synthesis SeriesNo ratings yet
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsFrom EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsNo ratings yet
- Compound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesFrom EverandCompound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesNo ratings yet
- Intrauterine Growth RestrictionDocument42 pagesIntrauterine Growth RestrictionOgirahma T. NaidNo ratings yet
- Andrews Straight Wire Appliance (SWA)Document201 pagesAndrews Straight Wire Appliance (SWA)Emad Ahmad Anis50% (2)
- Forming NounsDocument1 pageForming NounsHà TrangNo ratings yet
- Efektivitas Format Pendokumentasian Keperawatan Model PORDocument11 pagesEfektivitas Format Pendokumentasian Keperawatan Model PORSyahri FaziraNo ratings yet
- The Pittston Dispatch 08-05-2012Document62 pagesThe Pittston Dispatch 08-05-2012The Times LeaderNo ratings yet
- Medical Device Regulatory Affairs Specialist in Austin TX Resume Gary BakerDocument3 pagesMedical Device Regulatory Affairs Specialist in Austin TX Resume Gary BakerGaryBakerNo ratings yet
- Butyrophenone Analog As A Potential Atypical Antipsychotic Agent (J) .Bioorganic & Medicinal Chemistry, 2008, 16 (15) .7291-7301Document11 pagesButyrophenone Analog As A Potential Atypical Antipsychotic Agent (J) .Bioorganic & Medicinal Chemistry, 2008, 16 (15) .7291-7301Qi JacksonNo ratings yet
- Mathematical Literacy p1 Sep 2022 Addendum Eastern CapeDocument4 pagesMathematical Literacy p1 Sep 2022 Addendum Eastern CapeRexNo ratings yet
- CE (Ra) F (SH) PF1 (MJ GG) PFA (PR SS)Document4 pagesCE (Ra) F (SH) PF1 (MJ GG) PFA (PR SS)Krishna DubeyNo ratings yet
- 2013 Cholesterol Guidelines at A Glance: - Robert Barnes, M3Document15 pages2013 Cholesterol Guidelines at A Glance: - Robert Barnes, M3CoolrobertizNo ratings yet
- Chethan Pandarinath, PH.D.: Education and ResearchDocument4 pagesChethan Pandarinath, PH.D.: Education and ResearchChethan PandarinathNo ratings yet
- Cupping The Great Missing Therapy (Dr. Sahbaa M. Bondok)Document240 pagesCupping The Great Missing Therapy (Dr. Sahbaa M. Bondok)Teerth KrishnanandNo ratings yet
- How Stem Cells Helped Restore a Man's SightDocument2 pagesHow Stem Cells Helped Restore a Man's Sightamitmanju44No ratings yet
- Modified Release Dosage FormDocument24 pagesModified Release Dosage FormSuraj ChoudharyNo ratings yet
- Best Practice Diagnostic Guidelines For Patients Presenting With Breast SymptomsDocument60 pagesBest Practice Diagnostic Guidelines For Patients Presenting With Breast SymptomsPratamasari Insani100% (2)
- Pharma PrelimDocument8 pagesPharma PrelimNom NomNo ratings yet
- Blood Groups, Abo and RHDocument6 pagesBlood Groups, Abo and RHromeoenny4154No ratings yet
- Abdominal Organs Essay SolutionDocument77 pagesAbdominal Organs Essay SolutionEmmanuel IshiomaNo ratings yet
- Cytochemistry Chapter SummaryDocument3 pagesCytochemistry Chapter SummaryNathaniel SimNo ratings yet
- Combiflam Tablets PI - 08072019Document13 pagesCombiflam Tablets PI - 08072019ArunNo ratings yet
- Osteopathic Considerations in Systemic Dysfunction (2nd Ed)Document296 pagesOsteopathic Considerations in Systemic Dysfunction (2nd Ed)Natan Babek100% (1)
- Explosive Booster BI-Directional (HMX) MSDSDocument6 pagesExplosive Booster BI-Directional (HMX) MSDSHuong WkNo ratings yet
- 70 04 62 PDFDocument1 page70 04 62 PDFFadhli Warsito95No ratings yet
- Sinomarin (R)Document3 pagesSinomarin (R)mesaimeerNo ratings yet
- Blood Group & CoagulationDocument24 pagesBlood Group & CoagulationKalpeshPatilNo ratings yet
- GBV NovDocument60 pagesGBV NovGCMediaNo ratings yet
- Applied Paramedic Law and Ethic - Townsend & LuckDocument362 pagesApplied Paramedic Law and Ethic - Townsend & LuckCherie Harward100% (3)
- ThegreatgatsbyDocument6 pagesThegreatgatsbyapi-287109554No ratings yet
- DOH STAR AWARDS 2019 APPLICATIONDocument2 pagesDOH STAR AWARDS 2019 APPLICATIONLeland C. DagoyNo ratings yet
- DR Ahmed Abar CVDocument7 pagesDR Ahmed Abar CVAnonymous H1EZvy1yQNo ratings yet