Professional Documents
Culture Documents
Notes On Materials Science and Engineering: Unit: Iii
Notes On Materials Science and Engineering: Unit: Iii
Uploaded by
gautham10c13Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Notes On Materials Science and Engineering: Unit: Iii
Notes On Materials Science and Engineering: Unit: Iii
Uploaded by
gautham10c13Copyright:
Available Formats
NOTES ON MATERIALS SCIENCE AND ENGINEERING UNIT: III
Heat Treatment Annealing and its types, Normalizing, Hardening tempering, Aus-tempering and Mar-tempering Microstructure observation - Surface Heat treatment processes Carburizing, Nitriding, cyaniding, carbonitriding, flame and induction hardening.
Compiled & Prepared by K. Devendranath Ramkumar
Assistant Professor (Senior)
SCHOOL OF MECHANICAL AND BUILDING SCIENCES
Chapter: 1
Heat Treatment of Steels
Heat Treatment is the controlled heating and cooling of metals to alter their physical and mechanical properties without changing the product shape. Heat treatment is sometimes done inadvertently due to manufacturing processes that either heat or cool the metal such as welding or forming. Heat Treatment is often associated with increasing the strength of material, but it can also be used to alter certain manufacturability objectives such as improve machining, improve formability, restore ductility after a cold working operation. Thus it is a very enabling manufacturing process that can not only help other manufacturing process, but can also improve product performance by increasing strength or other desirable characteristics. The operation consists of:
Heating the steel to a certain temperature, "Soaking" at this temperature for a time sufficient to allow the necessary changes to occur, Cooling at a predetermined rate.
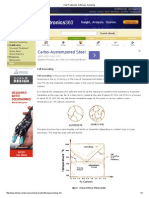
Full Annealing Full annealing is the process of slowly raising the temperature about 50C (90F) above the Austenitic temperature line A3 or line ACM in the case of Hypo eutectoid steels (steels with < 0.77% Carbon) and 50C (90F) into the AusteniteCementite region in the case of Hypereutectoid steels (steels with > 0.77% Carbon).
It is held at this temperature for sufficient time for all the material to transform into Austenite or Austenite-Cementite as the case may be. It is then slowly cooled at the rate of about 20 C/hr (36 F/hr) in a furnace to about 50 C (90 F) into the Ferrite-Cementite range. At this point, it can be cooled in room temperature air with natural convection. The grain structure has coarse Pearlite with ferrite or Cementite (depending on whether hypo or hyper eutectoid). The steel becomes soft and ductile. The purpose of annealing may involve one or more of the following aims: 1. To soften the steel and to improve machinability. 2. To relieve internal stresses induced by some previous treatment (rolling, forging, uneven cooling). 3. To remove coarseness of grain. The treatment is applied to forgings, cold-worked sheets and wire, and castings. Normalizing Normalizing is the process of raising the temperature to over 60 C (108 F), above line A3 or line ACM fully into the Austenite range. It is held at this temperature to fully convert the structure into Austenite, and then removed form the furnace and cooled at room temperature under natural convection. This results in a grain structure of fine Pearlite with excess of Ferrite or Cementite. The resulting material is soft; the degree of softness depends on the actual ambient conditions of cooling. This process is considerably cheaper than full annealing since there is not the added cost of controlled furnace cooling. The main difference between full annealing and normalizing is that fully annealed parts are uniform in softness (and machinability) throughout the entire part; since the entire part is exposed to the controlled furnace cooling. In the case of the normalized part, depending on the part geometry, the cooling is non-uniform resulting in nonuniform material properties across the part. This may not be desirable if further machining is desired, since it makes the machining job somewhat unpredictable. In such a case it is better to do full annealing. Process Annealing Process Annealing is used to treat work-hardened parts made out of low-Carbon steels (< 0.25% Carbon). This allows the parts to be soft enough to undergo further cold working without fracturing. Process annealing is done by raising the temperature to just below the Ferrite-Austenite region, line A1 on the diagram. This temperature is about 727 C (1341 F) so heating it to about 700 C (1292 F) should suffice. This is held long enough to allow recrystallization of the ferrite phase, and then cooled in still air. Since the material stays in the same phase through out the process, the only change that occurs is the size, shape and distribution of the grain structure. This
process is cheaper than either full annealing or normalizing since the material is not heated to a very high temperature or cooled in a furnace. Stress Relief Annealing Stress Relief Anneal is used to reduce residual stresses in large castings, welded parts and cold-formed parts. Such parts tend to have stresses due to thermal cycling or work hardening. Parts are heated to temperatures of up to 600 - 650 C (1112 - 1202 F), and held for an extended time (about 1 hour or more) and then slowly cooled in still air. Spheroidization Spheroidization is an annealing process used for high carbon steels (Carbon > 0.6%) that will be machined or cold formed subsequently. This is done by one of the following ways:
1. Heat the part to a temperature just below the Ferrite-Austenite line, line A1 or below the Austenite-Cementite line, essentially below the 727 C (1340 F) line. Hold the temperature for a prolonged time and follow by fairly slow cooling. Or 2. Cycle multiple times between temperatures slightly above and slightly below the 727 C (1340 F) line, say for example between 700 and 750 C (1292 - 1382 F), and slow cool. Or 3. For tool and alloy steels heat to 750 to 800 C (1382-1472 F) and hold for several hours followed by slow cooling. All these methods result in a structure in which all the Cementite is in the form of small globules (spheroids) dispersed throughout the ferrite matrix. This structure allows for improved machining in continuous cutting operations such as lathes and screw machines. Spheroidization also improves resistance to abrasion. Tempering Tempering is a process done subsequent to quench hardening. Quench-hardened parts are often too brittle. This brittleness is caused by a predominance of Martensite. This brittleness is removed by tempering. Tempering results in a desired combination of hardness, ductility, toughness, strength, and structural stability. Tempering is not to be confused with tempers on rolled stock-these tempers are an indication of the degree of cold work performed.
The mechanism of tempering depends on the steel and the tempering temperature. The prevalent Martensite is a somewhat unstable structure. When heated, the Carbon atoms diffuse from Martensite to form a carbide precipitate and the concurrent formation of Ferrite and Cementite, which is the stable form. Tool steels for example, lose about 2 to 4 points of hardness on the Rockwell C scale. Even though a little strength is sacrificed, toughness (as measured by impact strength) is increased substantially. Springs and such parts need to be much tougher these are tempered to a much lower hardness. Tempering is done immediately after quench hardening. When the steel cools to about 40 C (104 F) after quenching, it is ready to be tempered. The part is reheated to a temperature of 150 to 400 C (302 to 752 F). In this region a softer and tougher structure Troostite is formed. Alternatively, the steel can be heated to a temperature of 400 to 700 C (752 to 1292 F) that results in a softer structure known as Sorbite. Sorbite has less strength than Troostite but more ductility and toughness. The heating for tempering is best done by immersing the parts in oil, for tempering up to 350 C (662 F) and then heating the oil with the parts to the appropriate temperature. Heating in a bath also ensures that the entire part has the same temperature and will undergo the same tempering. For temperatures above 350 C (662 F) it is best to use a bath of nitrate salts. The salt baths can be heated up to 625 C (1157 F). Regardless of the bath, gradual heating is important to avoid cracking the steel. After reaching the desired temperature, the parts are held at that temperature for about 2 hours, then removed from the bath and cooled in still air. Chapter: 2 Surface Hardening Methods
Depth of Hardening: There is no technical limit to the depth of hardening with carburizing techniques, but it is not common to carburize to depths in excess of 0.050 in. Carburizing Time: 4 to 10 hours Carburizing Temperature: 1750
o
(above
the
upper
critical
temperature-Austenite area) Quenching: All of the carburizing processes (pack, gas, liquid) require quenching or from a the lower
carburizing
temperature
temperature or reheating and quenching. Parts are then tempered to the desired hardness.
Figure 1. Case depth versus Carburizing time.
PACK CARBURIZING In this process, the part that is to be carburized is packed in a steel container so that it is completely surrounded by granules of charcoal. The charcoal is treated with an activating chemical such as Barium Carbonate (BaCO3) that promotes the formation of Carbon Dioxide (CO2). PACK CARBURIZING PROCESS This gas in turn reacts with the excess carbon in the charcoal to produce carbon monoxide, CO. Carbon Monoxide reacts with the lowcarbon steel surface to form atomic carbon which diffuses into the steel. Carbon Monoxide supplies the carbon gradient that is necessary for diffusion. The carburizing process does not harden the steel. It only increases the carbon content to some predetermined depth below the surface to a sufficient level to allow subsequent quench hardening. Carbon Monoxide reaction: CO2 + C ---> 2 CO Reaction of Cementite to Carbon Monoxide: 2 CO + 3 Fe --->Fe3C + CO2 Quenching Process: It is difficult to quench the part immediately, as the sealed pack has to be opened and the part must be removed from the pack. One technique that is used often is to slow cool the entire pack and subsequently harden and temper the part after it is removed from the sealed pack. Depth of Hardening: There is no technical limit to the depth of hardening with carburizing techniques, but it is not common to carburize to depths in excess of 0.050 in. Carburizing Time: 4 to 10 hours GAS CARBURIZING Can be done with any carbonaceous gas, such as methane, ethane, propane, or natural gas. Most carburizing gases are flammable and controls are needed to keep carburizing gas at 1700 oF from
contacting air (oxygen). The advantage of this process over pack carburizing is an improved ability to quench from the carburizing temperature. Conveyor hearth furnaces make quenching in a controlled atmosphere possible. LIQUID CARBURIZING Can be performed in internally or externally heated molten salt pots. Carburizing salt contains cyanide compounds such as sodium cyanide (NaCN). Cycle times for liquid cyaniding are much shorter (1 to 4 hours) than gas and pack carburizing processes. Disadvantage is the disposal of salt. (Environmental problems) and cost (safe disposal is very expensive). NITRIDING In this process, nitrogen is diffused into the surface of the steel being treated. The reaction of nitrogen with the steel causes the formation of very hard iron and alloy nitrogen compounds. The resulting nitride case is harder than tool steels or carburized steels. The advantage of this process is that Figure 3 Nitriding hardness is achieved without the oil, water or air quench. As an added advantage,
hardening is accomplished in a nitrogen atmosphere that prevents scaling and discoloration. Nitriding temperature is below the lower critical temperature of the steel and it is set between 925
o
F and 1050oF. The nitrogen source is usually Ammonia (NH3). At the nitriding temperature the
ammonia dissociates into Nitrogen and Hydrogen.
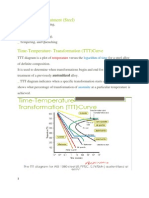
Figure 4. Nitriding time for various types of alloy steels 2NH3 ---> 2N + 3H2 The nitrogen diffuses into the steel and hydrogen is exhausted. A typical nitriding setup is illustrated in Figure 3.
The white layer shown in Figure 4 has a detrimental effect on the fatigue life of nitrided parts, and it is normally removed from parts subjected to severe service. Two stage gas-nitriding processes can be used to prevent the formation of white layer. White layer thickness may vary between 0.0003 and 0.002 in which depends on nitriding time. The most commonly nitrided steels are chromium-molybdenum alloy steels and Nitralloys. Surface hardness of 55 HRC to 70 HRC can be achieved with case depths varying from 0.005 in to 0.020 in. Nitrided steels are very hard and grinding operations should not be performed after nitriding. White layer is removed by lapping. Carbonitriding This process involves with the
diffusion of both carbon and nitrogen into the steel surface. The process is performed in a gas atmosphere furnace using a carburizing gas such as propane or methane mixed with several percent (by volume) of ammonia. Methane or propane serve as the source of carbon, the ammonia serves as the source of nitrogen. Quenching is done in a gas which is not as severe as water quench. As a result of les severe quench, there is less distortion on the material to be treated. A typical carbonitriding system is shown in the following slide. Case hardnesses of HRC 60 to 65 are achieved at the surface.( Not as high as nitrided surfaces.) Case depths of 0.003 to 0.030 in can be accomplished by carbonitriding. One of the advantages of this process is that it can be applied to plain carbon steels, which give significant case depths. Carbonitriding gives less distortion than carburizing. Carbonitriding is performed at temperatures above the transformation temperature of the steels (1400F to 1600F) Cyaniding It is similar to carbonitriding, and involves the diffusion of both carbon and nitrogen into the surface of the steel. The source of the diffusing element in this method is a molten cyanide salt such as sodium cyanide. It is a supercritical treatment involving temperatures in the range of 1400oF to 1600oF. Case depths are between 0.010 in. and 0.030 in. Diffusion times are less than one hour. Water or oil quench is required. This type of cases presents a significant distortion. Advantage of this method is the short time it requires to complete the diffusion; otherwise it should be avoided because of high distortion. INDUCTION HARDENING
In this process an electric current flow is induced in the work piece to produce a heating action. Every electrical conductor carrying a current has a magnetic field surrounding the conductor. Since the core wire is a dead-end circuit, the induced current cannot flow anyplace, so the net effect is heating of the wire. The induced current in the core conductor alternates at frequencies from 60 cycles per second (60 Hz) to millions of Hertz. The resistance to current flow causes very rapid heating of the core material. Heating occurs from the outside inward. Induction hardening process includes water quench after the heating process. The big advantage of this system is its Figure 7. Induction hardening small parts. The major disadvantage is the cost. Flame Hardening Flame hardening is the process of selective hardening with a combustible gas flame as the source of heat for austenitizing. (The material should have at least 0.40 % Carbon content to allow hardening.) Water quenching is applied as soon as the transformation temperature is reached. The heating media can be oxygen acetylene, propane, or any other combination of fuel gases that will allow reasonable heating rates. This procedure is applied to the gear teeth; shear blades, cams, ways on the lathes, etc. Flame hardening temperatures are around 1500oF. Up to HRC 65 hardness can be achieved. For best results the hardness depth is 3/16 inch. There are three methods: (1) SPOT Flame Hardening: Flame is directed to the spot that needs to be heated and hardened. (2) SPIN Flame Hardening: The work piece is rotated while in contact with the flame (3) PROGRESSIVE Flame Hardening: The torch and the quenching medium move across the surface of the work piece. Laser and Electron Beam Hardening These methods can be used to perform selective hardening of hardenable steels. They perform the same function as the flame in flame hardening or the induction coil in induction hardening. It is only applicable to steels that have sufficient carbon and alloy content to allow quench hardening. The laser or electron bam is used to raise the surface temperature of the part. Electron Beam Hardening requires vacuum. Laser beam do not require vacuum and quenching can be done by using a gas. Electron beam spot sizes are about 0.010 to 0.015 in2. Lasers can be larger but usually no larger than about 0.150 in2. Both methods present two disadvantages: (1) Equipment cost speed and ability to confine heating on
(2) High alloys may not respond. These methods are limited to plain carbon steels and low alloy steels and irons.
How to Select the Right Surface Hardening Method (1) Carburizing is the best method for low carbon steels. (2) Nitriding is a lower distortion process than carburizing but it can be used for certain type of steels such as chromium-molybdenum alloy steels or Nitralloy-type steels. (3) Flame hardening is preferred for heavy cases or selective hardening of large machine components. (4) Induction hardening works best on parts small enough and suitable in shape to be compatible with the induction coil. (5) Electron beam and laser hardening are limited to the low alloy steels and plain carbon steels only.
You might also like
- Heat Treatment of SteelDocument35 pagesHeat Treatment of Steelakhilsyam21No ratings yet
- Annealing, Normalizing, Quenching, Martensitic TransformationDocument22 pagesAnnealing, Normalizing, Quenching, Martensitic TransformationAboo BackerNo ratings yet
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo ratings yet
- Heat Treatment of SteelDocument59 pagesHeat Treatment of SteelNaman ShethNo ratings yet
- Unit 2 Heat TreatmentDocument59 pagesUnit 2 Heat TreatmentAmutha PSGRKCWNo ratings yet
- Annealing Normalizing QuenchingDocument22 pagesAnnealing Normalizing QuenchingManish GuptaNo ratings yet
- Heat Treatment LectureDocument58 pagesHeat Treatment LectureKeith Tanaka Magaka100% (1)
- HEAT TREATMENT of SteelDocument33 pagesHEAT TREATMENT of Steelparamaguru vNo ratings yet
- Heat TreatmentDocument59 pagesHeat TreatmentINSTECH Consulting100% (1)
- Iron - Carbon Phase DiagramDocument33 pagesIron - Carbon Phase Diagramvishnu anand100% (2)
- ERP Travelling Block - Hook BlockDocument9 pagesERP Travelling Block - Hook BlocknobodymagdesignNo ratings yet
- C45 Chemical and Mechanical Properties PDFDocument2 pagesC45 Chemical and Mechanical Properties PDFabhics67100% (2)
- Steel Defect GuideDocument15 pagesSteel Defect Guidegrd4100% (3)
- Manufacturer - Approved Saudi Aramco Data Sheet APCS-1A/1B/1C/1D/1E//1FDocument3 pagesManufacturer - Approved Saudi Aramco Data Sheet APCS-1A/1B/1C/1D/1E//1FjinujoseNo ratings yet
- Introduction To Heat TreatmentDocument10 pagesIntroduction To Heat TreatmentAzhar AliNo ratings yet
- A941Document7 pagesA941rohit kumarNo ratings yet
- Heat Transfer: Non-Stationary Heat Transfer Through Walls, Measurement of Thermal Conductivity, Heat Transfer with Two Phase RefrigerantsFrom EverandHeat Transfer: Non-Stationary Heat Transfer Through Walls, Measurement of Thermal Conductivity, Heat Transfer with Two Phase RefrigerantsRating: 5 out of 5 stars5/5 (1)
- Chapter 4 Heat Treatment of SteelDocument29 pagesChapter 4 Heat Treatment of SteelDa Champ Cena100% (2)
- Heat Treatment of Metals Module 4Document88 pagesHeat Treatment of Metals Module 4Palliyil UjwalNo ratings yet
- Chapter 10-Casting IDocument38 pagesChapter 10-Casting Iking slayerNo ratings yet
- Heat Treatment of Steel PDFDocument8 pagesHeat Treatment of Steel PDFkaviatchennai100% (2)
- Report Heat Treatment Eng Lab 3Document7 pagesReport Heat Treatment Eng Lab 3khalifawhan43% (7)
- Wire Ropes, Elevators & Machine Shop (PSE Module 31.1) SolutionDocument7 pagesWire Ropes, Elevators & Machine Shop (PSE Module 31.1) SolutionChen-chenLaydaPerezMontesNo ratings yet
- Heat TreamentDocument9 pagesHeat TreamentAtul GaurNo ratings yet
- Heat TreatmentDocument22 pagesHeat Treatmentansh_k9250% (2)
- Heat Treatment Part 2Document46 pagesHeat Treatment Part 2Naman DaveNo ratings yet
- Heat Treatment GC - 08Document54 pagesHeat Treatment GC - 08kr_abhijeet72356587No ratings yet
- Unit 2: Heat Treatment of Iron and SteelsDocument24 pagesUnit 2: Heat Treatment of Iron and SteelsRahul kumarNo ratings yet
- Industrial Materials and ProcessesDocument19 pagesIndustrial Materials and ProcessesRoland EmersonNo ratings yet
- Heat TreatmentDocument9 pagesHeat TreatmentsvsddsdsNo ratings yet
- Heat Treatment: Unit - IiDocument94 pagesHeat Treatment: Unit - Iisenthilkumar tsNo ratings yet
- Heat Treatments - Softening - Annealing2Document3 pagesHeat Treatments - Softening - Annealing2JiteshPbhujbalNo ratings yet
- Full Annealing Full Annealing Is The Process of Slowly Raising The Temperature About 50Document10 pagesFull Annealing Full Annealing Is The Process of Slowly Raising The Temperature About 50scorpionarnoldNo ratings yet
- Tratamente Termice F4Document6 pagesTratamente Termice F4andreeaoana45No ratings yet
- Annealing, Normalizing, Quenching, Martensitic TransformationDocument22 pagesAnnealing, Normalizing, Quenching, Martensitic TransformationAboo Backer100% (1)
- Heat Treatment of Metals-SmrDocument39 pagesHeat Treatment of Metals-SmrsultanrandhawaNo ratings yet
- Heat Treatment ProcessesDocument7 pagesHeat Treatment Processessonu100% (1)
- Chapter V - Heat TreatmentDocument24 pagesChapter V - Heat TreatmentHoàng Minh LongNo ratings yet
- Document 7Document6 pagesDocument 7Roy mugendi100% (1)
- Anealing TypesDocument29 pagesAnealing TypesPratheep AddrinNo ratings yet
- Bab 6 Heat Treatment of SteelsDocument23 pagesBab 6 Heat Treatment of SteelsReynard LisanNo ratings yet
- Heat TreatmentDocument4 pagesHeat TreatmentAshish BoraNo ratings yet
- Lab PHY 2Document16 pagesLab PHY 2Shivraj ChouguleNo ratings yet
- Presentation On Heat TreatmentDocument38 pagesPresentation On Heat Treatmentamit gajbhiyeNo ratings yet
- Capili Jefferson 12Document9 pagesCapili Jefferson 12Christian Al EncarnacionNo ratings yet
- Lecture Week 11 Annealing, Stress Releiving, Normalizing, HardeningDocument38 pagesLecture Week 11 Annealing, Stress Releiving, Normalizing, HardeningMahit HuilgolNo ratings yet
- Lecture-C2-No - 3Document12 pagesLecture-C2-No - 3abasnovakingNo ratings yet
- Stainless Steel Heat TreatmentDocument4 pagesStainless Steel Heat TreatmentJuan Carlos Fernandez LoveraNo ratings yet
- Heat TreatmentDocument40 pagesHeat TreatmentFavour LawrenceNo ratings yet
- EMAT 2K23 (9th)Document52 pagesEMAT 2K23 (9th)haristariq2004No ratings yet
- Objectives of Heat TreatmentDocument6 pagesObjectives of Heat TreatmentAdaitaChowdhury100% (1)
- Modified HT NotesDocument16 pagesModified HT NotesBeesam Ramesh KumarNo ratings yet
- Thermal Processing of Metals & AlloysDocument10 pagesThermal Processing of Metals & AlloysOyedotun Tunde0% (1)
- Heat Treatment of SteelsDocument29 pagesHeat Treatment of SteelsGraham KundaiNo ratings yet
- HT ManualDocument17 pagesHT ManualAkhilesh KumarNo ratings yet
- Heat Treatment of SteelsDocument6 pagesHeat Treatment of SteelsSrinivas LaishettyNo ratings yet
- Annealing, Normalizing, Quenching, Martensitic TransformationDocument22 pagesAnnealing, Normalizing, Quenching, Martensitic Transformationmukesh_ganganiNo ratings yet
- Heat Treatment: Unit - 2Document75 pagesHeat Treatment: Unit - 2reza chamanfarNo ratings yet
- L8 Heat TreatmentsDocument20 pagesL8 Heat TreatmentsQIU QIUNo ratings yet
- Heat treatment of materials - نسخةDocument7 pagesHeat treatment of materials - نسخةHmwDyNo ratings yet
- Heat treatment of materials - نسخةDocument7 pagesHeat treatment of materials - نسخةHmwDyNo ratings yet
- Over View Heat Treatment Processes: by MadhubalanDocument61 pagesOver View Heat Treatment Processes: by Madhubalanjohn powerNo ratings yet
- Heat TreatmentDocument29 pagesHeat TreatmentGUNTA LOHITHNo ratings yet
- 3 - Heat Treatment & Engineering ApplicationDocument24 pages3 - Heat Treatment & Engineering ApplicationHussein SaeedNo ratings yet
- Heat Treatments of Ferrous AlloysDocument19 pagesHeat Treatments of Ferrous AlloysAd Man GeTigNo ratings yet
- 4 - Heat TreatmentDocument29 pages4 - Heat TreatmentNomor SatuNo ratings yet
- Heat Treatment ProcessDocument46 pagesHeat Treatment ProcessMallappa KomarNo ratings yet
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesFrom EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesNo ratings yet
- ManufacturingDocument17 pagesManufacturingPadmaNo ratings yet
- FLCT - Lesson - Module 3Document3 pagesFLCT - Lesson - Module 3Cherry DerramasNo ratings yet
- ESS DEE RX Spectrum Technical Data SheetDocument4 pagesESS DEE RX Spectrum Technical Data Sheetjai soniNo ratings yet
- E Program Files An ConnectManager SSIS TDS PDF Interzone 954 Eng A4 20180313Document9 pagesE Program Files An ConnectManager SSIS TDS PDF Interzone 954 Eng A4 20180313José Roberto Moreno MoisantNo ratings yet
- Prepare Your Research InstrumentDocument18 pagesPrepare Your Research InstrumentSohad ElnagarNo ratings yet
- A Presentation ON Prototype: Dimensional Accuracy: Dr. D.Y PATIL College of Engineering Akurdi, Pune Maharashtra - 411044Document10 pagesA Presentation ON Prototype: Dimensional Accuracy: Dr. D.Y PATIL College of Engineering Akurdi, Pune Maharashtra - 411044AkshayJadhavNo ratings yet
- GSB-Form-255 Visual Inspection ReportDocument1 pageGSB-Form-255 Visual Inspection ReportMade GileeNo ratings yet
- IS 1875: 1992 Carbon Steel Billets, Blooms, Slabs AND Bars For Forgings - SpecificationDocument10 pagesIS 1875: 1992 Carbon Steel Billets, Blooms, Slabs AND Bars For Forgings - SpecificationSubhrakanta DehuryNo ratings yet
- Processes and Printers 3DDocument6 pagesProcesses and Printers 3DFresnel FisicoNo ratings yet
- Jetblast™ Copper SlagDocument4 pagesJetblast™ Copper SlaglambtranNo ratings yet
- 2017-0014 DEW Cryodur 2990 GBDocument8 pages2017-0014 DEW Cryodur 2990 GBLeandro Fortunato GomesNo ratings yet
- Working Knowledge How Organizations Manage What THDocument16 pagesWorking Knowledge How Organizations Manage What THNabil AchouriNo ratings yet
- 38CrSi DatasheetDocument3 pages38CrSi DatasheetKashif MohiuddinNo ratings yet
- Seminar Report On Gas WeldingDocument17 pagesSeminar Report On Gas WeldingMohd Sohail AliNo ratings yet
- Surface Preparation StandardDocument5 pagesSurface Preparation StandardKarthikeyan ShanmugavelNo ratings yet
- Metallurgical EngineeringDocument3 pagesMetallurgical EngineeringAPPI NAIDUNo ratings yet
- Bangla Report SampleDocument19 pagesBangla Report SamplefmrashedNo ratings yet
- Pt. Sepuluh Sumber Anugerah: Summary Daily Manpower ReportDocument32 pagesPt. Sepuluh Sumber Anugerah: Summary Daily Manpower ReportbayuNo ratings yet
- Catalogue of ProductsDocument40 pagesCatalogue of ProductsHai lm5No ratings yet
- Sample Questions Mem560 Chapter 5: Joining & Assembly ProcessDocument1 pageSample Questions Mem560 Chapter 5: Joining & Assembly ProcessAhmadIzzatFahmiNo ratings yet
- Asme IxDocument95 pagesAsme Ixjoseph.maquez24No ratings yet