Professional Documents
Culture Documents
MedChem Exams F2000-Metabolism
Uploaded by
api-37072970 ratings0% found this document useful (0 votes)
17 views8 pagesOriginal Title
MedChem Exams > F2000-Metabolism
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
17 views8 pagesMedChem Exams F2000-Metabolism
Uploaded by
api-3707297Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 8
joo
Basic Medicinal Chemistry Principles NAME _) -z
Metabolism (Dr. Davis) ; ae
November 21, 2000 SSN 32 Gow
PART L. True/Palse, Indicate whether each ofthe following questions are “tue” by circling the reer TO
t's or false” by circling the leter "F". (2 pts each).
Alp 1, ‘The oxygen species generated by cytochrome P-450 is highly electrophilic (cleetron-
U deficient).
T@». In order for a phase-2 metabolic reaction to occur, a phase-1 metabolic reaction must occur
first.
T®)3. The presystemic ‘frst pass effect’ is based upon the gut flora metabolizing a phase-2
conjugate excreted in the bile
“The three major P-450 isozymes responsible for drag metabolism in humans are CYP2D6,
CYP2E1, and CYP3A4.
‘Genetic polymorphism’ in metabolism occurs if >1% of the population shows differences in
tee meataboliz pathway being considered in comparison to the general population
Genetic polymorphism in the metabolism of isonicotinic acid hydrazide (INH, below) is based
on differences in the rate of N-acetylation by different populations.
—-NHNH,
aS
Zo
N’
0 F 7. The process of induction is an adaptive process in which enzyme levels can be increased by
The process of inc dorepression of transcription and (2) post-translational stebilization of
mRNA.
t(B)8. Changes in metabolism based on induction and inhibition are typically qualitative rather than
quantitative.
tion of the P-450 isoform(s) responsible for the metabolism of
T Bp. A likely consequence of inhibitior
pregnancy.
contraceptive steroids woul
QF 10. A iikely consequence of induction of the -450 isoform(s) responsible for the metabolism of
the anticoagulant warfarin would be clotting.
PART
Page ;
Il. Multiple Choice. Circle the single most appropriate letter [3 pts each]
1. Halogens have a very predictable effect on the hydroxylation of aromatic rings.
A
&
c
D.
Since they are ring deactivating by an inductive effect, but are gy ca directing by a
resonance effect, rings with halogens undergo the ortho/para position.
Since they are ortho/para directing by a resonance effect” Dut are ring deactivating by an
inductive effect, aromatic rings with halogens generally do not undergo hydroxylation.
Since they are ring acivating by an inductive effect, and are ortho/para directing by a resonance
effect, rings with halogens undergo aromatic hydroxylation in the ortho/para position.
Since they are ring deactivating by an inductive effect, and are meta directing by a resonance
effect, rings with halogens undergo aromatic hydroxylation in the meta position.
2. Approximately how many cytochrome P-450 isoforms are present in humans?
A
D.
One.
Between five and seven.
Between thirty-five and forty.
Greater than one hundred.
3. The metabolically-based interaction between alcohol (ethanol) and acetaminophen is based on:
c.
D.
Ethanol-based induction of a P-450 isoform responsible for the metabolism of acetaminophen.
Ethanol-based inhibition of a P-450 isoform responsible for the metabolism of acetaminophen.
Acetaminophen-based induction of a P-450 isoform responsible for the metabolism of ethanol.
Acetaminophen-based inhibition of a P-450 isoform responsible for the metabolism of ethanol.
4, Which one of the following statements concerning the ‘prodrug’ concept is incorrect (false)?
A
B.
D.
A prodrug is frequently designed to overcome problems of ‘localized toxicity’ seen with the
parent drug (i., the parent drug is toxic while it is present in a particular part of the body; the
prodrug is not).
A prodrug is frequently designed to overcome problems in tissue distribution seen with the
parent drug (e.g., the parent compound cannot enter the requisite tissue or organ; the prodrug
can),
Prodrug design based on esters is generally a poor idea because of the high variability in esterase
activity between individuals (i., there would be a highly variable response between
individuals).
A prodrug requires metabolism to convert it to the parent drug.
5. Anew drug with an ester functionality has an unacceptably short half-life. Four of your chemist
colleagues are coming up with suggestions to prolong the half-life through various molecular
modifications. Which one of your colleagues is making an incorrect statement.
A
B.
c.
6)
4
George says “We can slow down the metabolism of the drug if we convert the ester to an amide
(provided the amide derivative is still active).”
Susan says “We can slow down the metabolism of the drug if we place methyl groups near the
ester functionality to increase steric hindrance (provided the derivative is still active).
Pam says “We'd better figure out exactly how the drug is metabolized first. If ester hydrolysis
isn’t a major pathway for metabolism for this drug, then slowing down ester hydrolysis by
chemical modification isn’t going to appreciably increase the half-life.”
\None of the above. All of your colleagues are correct in their statements.
Page 3
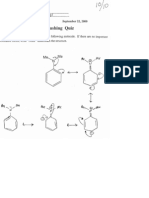
PART Il. PREDICTING METABOLIC PATHWAYS.
Directions (READ CAREFULLY): For the reactions specified below, you are to indicate the
metabolite(s) formed from the reaction or enzyme specified in the space provided. You need not dravh.
the complete structure, only that part which has been changed. If the reaction is not possible, simply
write “no reaction” in the box and explain why not. ~
So that there is no confusion (e.g,, “I’m not sure whether the reaction would go because there is already
a polar functional group present on the other side of the molecule”) you should use the following
eriteria: If the functional group required is present and the reaction is mechanistically feasible, then you
can perform the metabolic reaction. If the necessary functional group is not present and/or the reaciion
is not mechanistically feasible, you cannot do the reaction.
‘You may find these structures helpful in formulating some of your responses to some of these questions.
°
t
no.
COOH me I
HO: SH
HO Upp
1. Shown below is the structure of Trimetrexate, a folate antagonist which is approved as an orphan
drag for the treatment of Pneumocystis carinii in patients with AIDS. Indicate the structure of the
final metabolite that would result from the reaction specified on the parent drug, Trimetrexate. If
the reaction is not possible, simply write “no reaction” in the box and explain why not, You need
not draw the complete structure, only that part which has been changed (2 pts each].
i
H
Hi NJ N
A None Z
|
o., CH, Ni
HCO fn iy NHy
OCH,
B. The product resulting from Denaylic
hydroxylation of Trimetrexate, followed by the
action of alc/aldehyde dehdrogenases:
“y
‘A, The product(s) resulting from oxidative
deamination of Trimetrexate:
Baca 0
a QD
6) +e
& i 3
You might also like
- MedChem Quiz Visual Quiz-DalbyDocument1 pageMedChem Quiz Visual Quiz-Dalbyapi-3707297No ratings yet
- MedChem Quiz F2003-ArrowPushQuiz 2Document1 pageMedChem Quiz F2003-ArrowPushQuiz 2api-3707297No ratings yet
- MedChem Quiz F2003-HPLC QuizDocument1 pageMedChem Quiz F2003-HPLC Quizapi-3707297No ratings yet
- MedChem Quiz F2003-A&P LAB QuizDocument1 pageMedChem Quiz F2003-A&P LAB Quizapi-3707297No ratings yet
- MedChem Quiz F2000-HPLC QuizDocument1 pageMedChem Quiz F2000-HPLC Quizapi-3707297100% (1)
- MedChem Quiz F2000-CrownEther QuizDocument1 pageMedChem Quiz F2000-CrownEther Quizapi-3707297No ratings yet
- MedChem Quiz F2000-ArrowPushQuiz 1Document1 pageMedChem Quiz F2000-ArrowPushQuiz 1api-3707297No ratings yet
- MedChem Exams F2003-FinalDocument13 pagesMedChem Exams F2003-Finalapi-3707297No ratings yet
- MedChem Exams Summary Exam1-MedChem PartDocument2 pagesMedChem Exams Summary Exam1-MedChem Partapi-3707297No ratings yet
- MedChem Exams F2003-MetabolismDocument14 pagesMedChem Exams F2003-Metabolismapi-3707297No ratings yet
- MedChem Exams F2002-MetabolismDocument12 pagesMedChem Exams F2002-Metabolismapi-3707297No ratings yet
- MedChem Exams F2002-A&P PartDocument11 pagesMedChem Exams F2002-A&P Partapi-3707297No ratings yet
- MedChem Exams F2001-MetabolismDocument11 pagesMedChem Exams F2001-Metabolismapi-3707297No ratings yet
- MedChem Exams F2001-A&P PartDocument5 pagesMedChem Exams F2001-A&P Partapi-3707297No ratings yet