Professional Documents
Culture Documents
MedChem Exams F2001-Metabolism
Uploaded by
api-37072970 ratings0% found this document useful (0 votes)
33 views11 pagesOriginal Title
MedChem Exams > F2001-Metabolism
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
33 views11 pagesMedChem Exams F2001-Metabolism
Uploaded by
api-3707297Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 11
i
|
Basic Medicinal Chemistry Principles NAME Py Edhuceet Rent, re (print)
Noveriueresti onlay signature: &r ScbomA Fl re" (sign)
Lab Sec: a T WwW @® F (circle one)
PART I. Application Multiple Choice. Circle the single most appropriate letter (3 pts each]
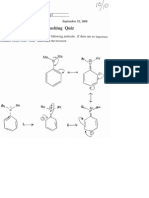
1, PAPS (3'-phosphoadenosine-s'-phosphosulfate; shown below) is a cofactor (or co-substrate)
involved in which metabolic reaction?
avoed iain Ney
cing
® acy! sulfate formation.
B Glucuronide formation. X
C. Ketone reduction to the corresponding alcohol,
D. Alcohol oxidation to the corresponding aldehyde
E. Phosphorylation (phosphate formation).
2. We are going to predict the metabolism of the following oral medication for Huntingtons’s Disease.
In the following sequential series of statements, which statement represents the first time a false
statement is made? a
‘0
A. This compound could undergo aromatic hydroxylation, N-dealkylation, benzylic hydroxylatiok,
ketone reduction, w-hydroxylation (omega-hydroxylation) or w-1 hydroxylation (omega-1
hydroxylation). ‘That is, all of these reactions are possible
B, Let's assume the parent compound undergoes w-hydroxylation (omega hydroxylation) to form
metabolite “B”, Metabolite “B” could then undergo further metabolism, by glucuronidation, 6
7. Sulfate formation, or oxidation by alcohol dehydrogenase.
(ues assume that the metabolite “B” underwent oxidation by alcohol dehydrogenase to form en
‘metabolite “C", Metabolite “C” could then undergo further metabolism, either by
glucuronidation, or oxidation by aldehyde dehydrogenase.
D. Let's assume that the metabolite “\C” underwent oxidation by aldehyde dehydrogenase to form
Metabolite “D”. Metabolite “D” could then undergo further metabolism by glucuronidation
E. None of the above; ie., there are no errors in statements A-D above.
‘The metabolism of the local anesthetic procaine (shown below) represents:
= an Coe HN: in ae
Bioinactivation
Bioactivation
C. Active metabolite formation
D. Toxification (toxic metabolite formation) *
E. A pathway thatis incorrectly shown in the scheme above (the metabolites are wrong). <
4, The anticoagulant warfarin undergoes ketone reduction to two steroisomeric alcohols. The S-aleohol
is an active metabolite, and the R-alcohol is an inactive metabolite. Which of the following
Structures represents the active metabolite resulting from ketone reduction? (circle the letter)
9
5. During the development of the new drug shown below, animal studies show that litle or no parent
drug appears in the bloodstream after oral administration. You suggest that this may be due 10 2
profound first pass effect’ with this drug, but no one believes you. Which one of che following
Experiments would be the most appropriate to prove whether you are right or wrong?
Hx AR
= °
|A. Destroy the gut flora in the animals (using a broad spectrum antibiotic) and then re-administer
the drug orally.
B. Destroy the gut flora in the animals (using a broad spectrum antibiotic) and then re-administer
the drug, but parenterally this time.
& Pretreat the animals with a P-450 inducer and then re-administer the drug orally.
Pretreat the animals with a P-450 inhibitor and then re-administer the drug orally.
SK Re-administer the drug parenterally.
“rage
‘Which one of the following statements about enzyme superfamilies is false (incorrect)?
A. Humans have 30-40 distinct P-450 isoforms. ©
Humans have P-450 isoforms for endobiotic and isoforms for xenobiotic metabolism.”
Humans, rats, and mice all have the same CYP2D6 isoforms (number and specificity)
D. Humans have multiple glucuronyl transferase enzymes (which differ in their substrate
specificity)
7. A patient stabilized on the anticoagulant warfarin develops epilepsy, and so is put on Phenobarbital
(aclassic CYP3A1 inducer). No one pays attention to the obvious drug interaction that will
undoubtedly occur in this patient, and as a result
‘A. the patient bleeds. We need to increase the dose of warfarin,
the patient bleeds. We need to decrease the dose of warfarin,
6 the patient starts developing clots. We need to increase the dose of warfarin.
. the patient starts developing clots. We need to decrease the dose of warfarin.
E. nothing happens. Although there is a potential drug interaction here, it is highly over-rated and
is unlikely to occur; therefore, we don’t need to adjust anything. (Note: if you chose this one,
your fired!)
8. We discussed a recent example in which patients on the immunosuppressive drug cyclosporin
suffered transplant rejections. It was later determined that these patients were on St. John’s Wort, an
herbal medication used for depression. The reason there was a drug interaction was that
© The active component in St. John’s Wort represents a CYP3A4 inducer.
The active component in St. John’s Wort represents a CYP3A¢ inhibitor.
C. Cyclosporin represents a CYP3A4 inducer.
D. Cyclosporin represents a CYP3A¢ inhibitor.
Note: don’t think you needed to have memorized anything in order to answer the question above. Just
think it through logically.
PART II. Mechanistic Multiple Choice. Circle the most ‘single appropriate letter unless otherwise
directed. [2 pts each]
1, The drug illustrated by the partial structure below is expected to undergo aromatic hydroxylation via
the typical arene oxide mechanism. Look at the partial pathways proposed, and identify which
would be most likely (if any).
f t
i C “i ; 5
t f f
y ¢ r y
C. Both pathways are likely.
D. Neither pathway is likely
You might also like
- MedChem Quiz Visual Quiz-DalbyDocument1 pageMedChem Quiz Visual Quiz-Dalbyapi-3707297No ratings yet
- MedChem Quiz F2003-ArrowPushQuiz 2Document1 pageMedChem Quiz F2003-ArrowPushQuiz 2api-3707297No ratings yet
- MedChem Quiz F2003-HPLC QuizDocument1 pageMedChem Quiz F2003-HPLC Quizapi-3707297No ratings yet
- MedChem Quiz F2003-A&P LAB QuizDocument1 pageMedChem Quiz F2003-A&P LAB Quizapi-3707297No ratings yet
- MedChem Quiz F2000-HPLC QuizDocument1 pageMedChem Quiz F2000-HPLC Quizapi-3707297100% (1)
- MedChem Quiz F2000-CrownEther QuizDocument1 pageMedChem Quiz F2000-CrownEther Quizapi-3707297No ratings yet
- MedChem Quiz F2000-ArrowPushQuiz 1Document1 pageMedChem Quiz F2000-ArrowPushQuiz 1api-3707297No ratings yet
- MedChem Exams F2003-FinalDocument13 pagesMedChem Exams F2003-Finalapi-3707297No ratings yet
- MedChem Exams Summary Exam1-MedChem PartDocument2 pagesMedChem Exams Summary Exam1-MedChem Partapi-3707297No ratings yet
- MedChem Exams F2003-MetabolismDocument14 pagesMedChem Exams F2003-Metabolismapi-3707297No ratings yet
- MedChem Exams F2002-MetabolismDocument12 pagesMedChem Exams F2002-Metabolismapi-3707297No ratings yet
- MedChem Exams F2002-A&P PartDocument11 pagesMedChem Exams F2002-A&P Partapi-3707297No ratings yet
- MedChem Exams F2001-A&P PartDocument5 pagesMedChem Exams F2001-A&P Partapi-3707297No ratings yet
- MedChem Exams F2000-MetabolismDocument8 pagesMedChem Exams F2000-Metabolismapi-3707297No ratings yet