Professional Documents
Culture Documents
Material Balances Project Ethylbenzene: Process Description
Material Balances Project Ethylbenzene: Process Description
Uploaded by
cedwOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Material Balances Project Ethylbenzene: Process Description
Material Balances Project Ethylbenzene: Process Description
Uploaded by
cedwCopyright:
Available Formats
Material Balances Project Ethylbenzene
Ethyl benzene (EB) is used as a chemical intermediate in making styrene, the building block for manufacturing polystyrene. It is a major commodity chemical that is produced throughout the world. A byproduct of the process is diethyl benzene (DEB) that is an intermediate in divinyl benzene manufacture. Since the demand for styrene is far greater than the demand for divinyl benzene, the selectivity for our process should favor ethyl benzene production. Ethyl benzene is produced by coupling ethylene and benzene with an acidic catalyst. Diethyl benzene forms when ethylene reacts with ethyl benzene. The formation of multiply-substituted benzenes is limited by running the reaction with a large excess of benzene. The reactions that produce EB and DEB are C6H6 + C2H4 C6H5C2H5 C6H6 + 2C2H4 C6H4(C2H5)2 2 1
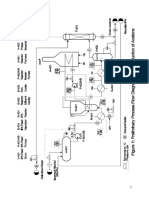
where is the extent of reaction. The selectivity of these reactions is determined by the feed ratio and processing conditions. A simplified process flow diagram is shown in the appended figure. Process Description Fresh benzene (Stream 1) and ethylene (Stream 2) are combined with a recycle stream containing unreacted benzene and a small amount of ethyl benzene. The combined stream is fed to a reactor where all of the ethylene in the feed reacts. The reactor effluent (Stream 4) is cooled so that most of the benzene, ethyl benzene, and diethyl benzene condenses. An ethane impurity from the ethylene feed as well as some benzene and ethyl benzene vapor are purged from the process and used as fuel gas. The condensed liquid is fed to the first distillation column. A high purity benzene stream is removed from the top of the column and recycled. The bottoms from the first column are sent to a second distillation column. The second column produces high-purity ethyl benzene in the top stream and diethyl benzene in the bottom. Data on the composition of these streams are provided in a following section. Additional Process Information Stream 1 Stream 2 Assume that benzene is pure. The ethylene feed contains 7.0 mol% ethane as an impurity. Ethane does not react but moves through the process as an inert gas until it is purged in Stream 5. Stream 3 Benzene-to-ethylene molar ratio is adjusted to control reaction selectivity. Use a 10:1 ratio when making hand calculation. R-301 The limiting reactant achieves 100% conversion. Reaction selectivity is determined by B3/E3 ratio. See relationship below. Stream 5 Fuel gas composition is 40 mol% ethane, 55 mol% benzene and 5 mol% ethyl benzene. Stream 7 Recycle stream composition is 99.2 mol% benzene and 0.8 mol% ethyl benzene.
Stream 9 Overhead stream composition is 99.6 mol% ethyl benzene, 0.4 mol% benzene. Stream 10 Bottoms stream composition is 90 mol% diethyl benzene, 10 mol% ethyl benzene. Reaction Information The selectivity for ethyl benzene production is a function of benzene-to-ethylene ratio. This relationship is expressed as
2 E3 = 1 B3
1. 2
However, the size of T-301 limits the B3/E3 ratio to a maximum value of 12. Operating Costs Much of the expense in manufacturing ethyl benzene is associated with utility costs like compressing gases to reaction pressure and evaporating liquids for separation in distillation towers. These costs cannot be estimated well in a first chemical engineering course. Therefore, utility costs may be ignored this semester. The difference between product price and feedstock cost should be called revenue. It should not be called profit, since operating and other expenses have not been included. ethylene cost = $ 0.77 /kg benzene cost = $ 1.22 /kg EB price = $ 1.54 /kg DEB price = $ 0.76 /kg Fuel gas value = $ 0.54 /kg Problem You, the engineering team, are to optimize the operation of the EB process in order to produce 100,000 metric-tons/yr (100,000,000 kg/yr). Your goal is to minimize operating costs and maximize revenue. You are constrained by the selectivity of the reaction and by the size of T-301 used for separating and recycling excess benzene. Group Formation A design group is to consist of two members. You are encouraged to make groups by yourselves. When you have formed a group, please turn in the names of group members to Dr. Kugler. He will combine groups to make 3- or 4-person design teams. A list of design teams will be provided on November 12. Computations You are expected to use a spreadsheet for material balance and cost calculations. The first step should be solving the material balance by hand calculations. Use a basis of n2 = 100 kmol/h and the reactor-feed ratio of B3/E3 = 10. After completing the hand calculation, set up the spreadsheet
2
to do the material balance when you specify B3/E3 ratio. Copy results into a stream table. These first steps, including pencil-written hand calculations, should be included as an appendix to your report to demonstrate that calculations were done correctly. After producing a stream table on your spreadsheet using kmol units, convert kmol units to mass on a second stream table, then scale so that EB meets production goal. Profit or loss for selected operating conditions can be calculated from scaled-stream-table data. Reports Each team will be expected to prepare a written report recommending the best operating procedures for the EB process. This report is due at 3:00 PM, Wednesday, December 7. The report should follow the Departments design-report guidelines. Data should be in the form of graphs and tables, since this serves both to condense results and make them easily understandable. The appendix should include your spreadsheet and a hand calculation of the 10/1 case. Report Authors Although work on a group report can never be divided equally, only those members making substantial contributions to the final report should be listed as authors.
You might also like
- Handbook of Ship Calculations, Construction and OperationDocument776 pagesHandbook of Ship Calculations, Construction and OperationYuriyAK91% (11)
- Shim Catalogue To PrintDocument32 pagesShim Catalogue To PrintAkram SiddigNo ratings yet
- Energy Saving of A Methyl Methacrylate Separation Process PDFDocument11 pagesEnergy Saving of A Methyl Methacrylate Separation Process PDFClaudia CelestinoNo ratings yet
- InstruCalc 9 Product BulletinDocument9 pagesInstruCalc 9 Product Bulletinmpica100% (1)
- Acetylene, the Principles of Its Generation and Use A Practical Handbook on the Production, Purification, and Subsequent Treatment of Acetylene for the Development of Light, Heat, and PowerFrom EverandAcetylene, the Principles of Its Generation and Use A Practical Handbook on the Production, Purification, and Subsequent Treatment of Acetylene for the Development of Light, Heat, and PowerNo ratings yet
- Reiki Self AttunementDocument3 pagesReiki Self AttunementFangedNimphNo ratings yet
- Hydrogenation of Nitrobenzene To AnilineDocument8 pagesHydrogenation of Nitrobenzene To AnilineYu HuiNo ratings yet
- Acetaldehyde Production by Ethanol Dehydrogenation PDFDocument1 pageAcetaldehyde Production by Ethanol Dehydrogenation PDFLuis Enrique Bautista BalderasNo ratings yet
- Pulse Tube Cryocooler-SeminarDocument26 pagesPulse Tube Cryocooler-SeminarUtsav RaoNo ratings yet
- Naval Equipment GB PDFDocument16 pagesNaval Equipment GB PDFFernando Raúl LADINONo ratings yet
- Maxwell's EquationDocument27 pagesMaxwell's Equationimanarrovi100% (3)
- Plant DesignDocument42 pagesPlant Designmuhammad ilyasNo ratings yet
- Eimt NciiDocument11 pagesEimt NciiEdgar Martin GuevarraNo ratings yet
- IEC61869-5 Instrument Transformers Pt.5 Additional Requirements For CVTDocument19 pagesIEC61869-5 Instrument Transformers Pt.5 Additional Requirements For CVTGabriel Zenarosa LacsamanaNo ratings yet
- LECTURE - 6: Ethylene Derivatives: Ethylene Oxide and Ethanol Amines 6.1 Ethylene OxideDocument7 pagesLECTURE - 6: Ethylene Derivatives: Ethylene Oxide and Ethanol Amines 6.1 Ethylene Oxideمحمود محمدNo ratings yet
- Project Ethyl Benzene .. 2019-20 .. Jay RSDocument100 pagesProject Ethyl Benzene .. 2019-20 .. Jay RSBhatu Devare100% (1)
- (Group 2) Plant Design Assignment 1 Task 2Document39 pages(Group 2) Plant Design Assignment 1 Task 2Lee Jian100% (1)
- Lecture 18 Ethylene GlycolDocument6 pagesLecture 18 Ethylene GlycolJayraj DaymaNo ratings yet
- Ethylbenzene MSDS PDFDocument6 pagesEthylbenzene MSDS PDFyuanitaNo ratings yet
- Project Group 9 CC01 PDFDocument24 pagesProject Group 9 CC01 PDFHuy NinhNo ratings yet
- Cumene A PDFDocument4 pagesCumene A PDFdanena88No ratings yet
- Production of Ethylene OxideDocument22 pagesProduction of Ethylene OxideShahabuddin Khan Niazi100% (1)
- Chemical Design EthylbenzeneDocument32 pagesChemical Design Ethylbenzeneafnan_lion94No ratings yet
- Preventive Maintenance Electric Service EquipmentDocument25 pagesPreventive Maintenance Electric Service EquipmentDanielAlejandroRamosQueroNo ratings yet
- British Petroleum Marketing Strategy AnalysisDocument19 pagesBritish Petroleum Marketing Strategy AnalysisShivaani Aggarwal0% (1)
- Eb 12Document25 pagesEb 12amms9988No ratings yet
- Final Project2Document135 pagesFinal Project2Mr NU KHANNo ratings yet
- Ethyl Benzene Project ReportDocument88 pagesEthyl Benzene Project ReportRahul Srivastava92% (26)
- Ethyl Benzene Plant Design PDFDocument31 pagesEthyl Benzene Plant Design PDFKaul PatrickNo ratings yet
- Ethyl-Benzene Process DescriptionDocument6 pagesEthyl-Benzene Process DescriptionAhsan Raza100% (3)
- B2 Group 1..acetone Production PDFDocument21 pagesB2 Group 1..acetone Production PDFElif TaşdövenNo ratings yet
- Project 5 Ethyl BenzeneDocument13 pagesProject 5 Ethyl Benzenefearoth87100% (3)
- Design of EthylbenzeneDocument5 pagesDesign of Ethylbenzenesahar vahdatifarNo ratings yet
- 5 6251216941030047774Document41 pages5 6251216941030047774Salihah AbdullahNo ratings yet
- Acetaldehyde Production by Ethanol DehydrogenationDocument9 pagesAcetaldehyde Production by Ethanol DehydrogenationHugo Gerdulli AlbertinNo ratings yet
- Ethylbenzene ProductionDocument30 pagesEthylbenzene ProductionUum LukmanNo ratings yet
- Presentation CumeneDocument39 pagesPresentation Cumeneممدوح الرويليNo ratings yet
- Production of N Octane From Ethylene and I ButaneDocument2 pagesProduction of N Octane From Ethylene and I ButaneRamyaNo ratings yet
- Kinetics of Catalytic Dehydrogenation of Ethylbenzene To StyreneDocument5 pagesKinetics of Catalytic Dehydrogenation of Ethylbenzene To Styreneibrahim3318No ratings yet
- Ethyl Benzene Plant DesignDocument45 pagesEthyl Benzene Plant DesignfaridzawiNo ratings yet
- Methyl Methacrylate Plant CostDocument3 pagesMethyl Methacrylate Plant CostIntratec Solutions50% (2)
- Project 6 - Ethylene Oxide PDFDocument13 pagesProject 6 - Ethylene Oxide PDFStephanie Hawkins100% (1)
- Styrene Production From EthylbenzeneDocument10 pagesStyrene Production From EthylbenzeneChegg ChemNo ratings yet
- Ethyl Benzene ProductionDocument6 pagesEthyl Benzene ProductionsoheilsedNo ratings yet
- Hydrodealkylation SimulationDocument8 pagesHydrodealkylation SimulationSchaieraNo ratings yet
- Ethylbenzene Production ReportDocument17 pagesEthylbenzene Production ReportVamsidhar Gannavarapu100% (1)
- Ethyl BenzeneDocument14 pagesEthyl Benzenectqmqyo0% (1)
- Cumene ProductionDocument26 pagesCumene ProductionAMOGH JHANWARNo ratings yet
- Ethyl BenzeneDocument11 pagesEthyl BenzeneIan Jasper SabordoNo ratings yet
- Ethyl Benzene Production ReactionsDocument2 pagesEthyl Benzene Production ReactionsMohd Hakimie0% (1)
- ETHYLBENZENEDocument19 pagesETHYLBENZENEolaNo ratings yet
- Ethylbenzene ProductionDocument30 pagesEthylbenzene ProductionVishal Dhapa100% (1)
- EnnnDocument9 pagesEnnnSajid AliNo ratings yet
- Design Report 1Document31 pagesDesign Report 1arif arifinNo ratings yet
- Reactor ModelDocument12 pagesReactor ModelTanuja ThanuNo ratings yet
- Historical ProfileDocument90 pagesHistorical Profilefaridzawi100% (1)
- Art:10 1134/S0965544111010038Document10 pagesArt:10 1134/S0965544111010038CátiaLuzNo ratings yet
- Literature Survey On 2EH ProductionDocument3 pagesLiterature Survey On 2EH Productionkilldeadman100% (2)
- 2 Ethyl 2520hexanol Methods 2520of 2520 ProductionDocument10 pages2 Ethyl 2520hexanol Methods 2520of 2520 Productionapi-3714811No ratings yet
- Project: Design of A Reactor For The Aniline ProductionDocument19 pagesProject: Design of A Reactor For The Aniline ProductionLUIS ESTEBAN VÁSQUEZ CASTANEDANo ratings yet
- CEPSA Good Reference For ZeoliteDocument29 pagesCEPSA Good Reference For Zeolitedie_1No ratings yet
- Styrene From Ethane and BenzeneDocument6 pagesStyrene From Ethane and BenzeneAmy Puah100% (2)
- Manufacturing AcetoneDocument3 pagesManufacturing AcetoneSiran Marayo100% (1)
- AcetoneDocument7 pagesAcetoneGeorgiana AndreeaNo ratings yet
- CumeneDocument5 pagesCumeneNasmiyeth Rodriguez VittaNo ratings yet
- Production of CyclohexaneDocument2 pagesProduction of Cyclohexanesushant kadamNo ratings yet
- EthylbenzeneDocument4 pagesEthylbenzenejulien.bol3No ratings yet
- Operating Cost Ethylbenzene 1Document1 pageOperating Cost Ethylbenzene 1JonesHutaurukNo ratings yet
- CHE655 - Plant Design Project #5 Summer 2010 Design of An Ehtyl Benzene Production ProcessDocument13 pagesCHE655 - Plant Design Project #5 Summer 2010 Design of An Ehtyl Benzene Production ProcessAyşe ÖztürkNo ratings yet
- Ilovepdf MergedDocument39 pagesIlovepdf MergedmoheedNo ratings yet
- Eb 12Document25 pagesEb 12SrewaBenshebilNo ratings yet
- WEG Fire Pump Motor Usa375 Brochure EnglishDocument12 pagesWEG Fire Pump Motor Usa375 Brochure EnglishZet LaimeNo ratings yet
- 0510 - 12 - IGCSE2 - MT - 2 - QP - Sep 23Document15 pages0510 - 12 - IGCSE2 - MT - 2 - QP - Sep 23BENZVI KARTHAR SINGHNo ratings yet
- Maulana Mukhtar Ahmad Nadvi Technical Campus, Malegaon Department of Electrical EngineeringDocument5 pagesMaulana Mukhtar Ahmad Nadvi Technical Campus, Malegaon Department of Electrical EngineeringRohini HaridasNo ratings yet
- Water Hammer - What Is It and How Does It Impact FirefightingDocument1 pageWater Hammer - What Is It and How Does It Impact FirefightingMohamed Abou El hassanNo ratings yet
- Joint Initiative of Govt of India and OrissaDocument70 pagesJoint Initiative of Govt of India and OrissaPuspak MohapatraNo ratings yet
- Enhanced Biodiesel Production From Jatropha Oil Using Calcined Waste Animal Bones As CatalystDocument10 pagesEnhanced Biodiesel Production From Jatropha Oil Using Calcined Waste Animal Bones As CatalystFarah TalibNo ratings yet
- Sace Tmax XT: Circuit-Breaker Terminals and ConnectionDocument28 pagesSace Tmax XT: Circuit-Breaker Terminals and ConnectionPatran ValentinNo ratings yet
- Calculations of Buck Boost ConverterDocument3 pagesCalculations of Buck Boost ConverterUmar AkhtarNo ratings yet
- IECEx BAS 10.0045X 000Document6 pagesIECEx BAS 10.0045X 000Marcos SiqueiraNo ratings yet
- Ultracapacitor Seminar ReportDocument24 pagesUltracapacitor Seminar ReportMällí MíssílèNo ratings yet
- Electrical Installation Guide ExtractDocument6 pagesElectrical Installation Guide ExtractnaveedfndNo ratings yet
- OCR A Physics A Level: Topic 6.2: Electric FieldsDocument8 pagesOCR A Physics A Level: Topic 6.2: Electric FieldsjmsonlNo ratings yet
- Chapter 13: Using The Mixing Plane ModelDocument36 pagesChapter 13: Using The Mixing Plane ModelramgopaljhaNo ratings yet
- Minimizing Penalty in Industrial Power Consumption by Engaging APFC Unit: A ReviewDocument4 pagesMinimizing Penalty in Industrial Power Consumption by Engaging APFC Unit: A ReviewAnonymous kw8Yrp0R5r100% (1)
- All About ViscosityDocument20 pagesAll About ViscosityaamendarNo ratings yet
- Coolant Level Sensor and j1939 Harness SectionDocument11 pagesCoolant Level Sensor and j1939 Harness SectionHamilton Miranda0% (1)
- Sailun CatalogDocument6 pagesSailun CatalogAtif EngNo ratings yet
- Quadropol QMR : Industrial SolutionsDocument5 pagesQuadropol QMR : Industrial SolutionstonyNo ratings yet
- Mercedes R129-DiagramsDocument772 pagesMercedes R129-DiagramsRH MotorNo ratings yet