Professional Documents
Culture Documents
Production of Cyclohexane
Uploaded by
sushant kadamOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Production of Cyclohexane
Uploaded by
sushant kadamCopyright:
Available Formats
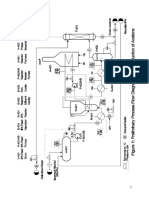
Production of Cyclohexane through Catalytic Hydrogenation of Benzene
Background
Cyclohexane is industrially produced from Benzene as it is not a naturally available
resource. Cyclohexane undergoes oxidation reactions yielding Cyclohexanone and
Cyclohexanol which are precursors for the production of Adipic acid and Caprolactum.
Caprolactum is the raw material used for producing polymer Nylon-6. Benzene reacts with
a mixture of hydrogen and methane in contact with a Nickel based catalyst producing
Cyclohexane. The conversion of this vapour phase reaction is almost 99%.
Reaction involved: Benzene + Hydrogen Cyclohexane (Vapour Phase)
Reactor Used: Catalytic Packed Bed Conversion Reactor
Reactor conditions: Outlet Temperature = 497 K, Pressure Drop = 1.02 atm
Catalyst Used: Nickel Based
Process Description
Fresh benzene (370 kmol/h) and excess hydrogen (1470 kmol/h) is preheated to a
temperature of 422 K and sent to a packed bed reactor. A vapour phase reaction occurs in
the reactor at 497 K which converts benzene to cyclohexane through catalytic
hydrogenation of benzene. The conversion of this reaction is about 99%. The reactor
products are cooled to 370 K and sent through a pressure reduction valve which reduces
the pressure of the stream from 30 atm to 24 atm. A two stage separator separates the
product cyclohexane from unreacted hydrogen and methane- first at a high pressure (24
atm) and then at a lower pressure (3 atm). The unreacted hydrogen-methane mixture is
recovered from the top of the flash column and is sent to a splitter having a splittling ratio
of 9:1. The smaller stream is sent as a recycle stream and mixes with fresh hydrogen, while
the rest is drawn out as fuel gas for incinerators. The bottom stream of the flash column
having 99% (wt/wt) cyclohexane is sent as feed to a distillation column for further
purification. The column consists of 12 stages operating at a reflux ratio of 5:1. The residue
from the column has our desired product with a purity of 99.5% (wt/wt) cyclohexane.
Assumptions
1. Side reactions were ignored.
2. A one pass conversion of 99% was considered.
3. The reactor outlet temperature was considered to be 497 K and pressure drop 1.02
atm.
4. Benzene used is mostly used up and hence, the unreacted benzene is not recovered.
5. The splitting ratio is mainly controlled by the composition of hydrogen in the feed
to the reactor. This was set by trial and error.
6. Custom units were followed for the simulation and not SI unit system
Results
INLET OUTLET
Parameter Unit Benzene Hydrogen Cyclohexane
Temperature K 311 311 427.708
Pressure atm 37.7 37.7 6
Mass Flow kg/h 28936.6 3417.51 23555.6
Mass Fraction
Hydrogen 0 0.816298 1.06E-15
Methane 0 0.183702 1.69E-12
Benzene 1 0 0.00520527
Cyclohexane 0 0 0.994795
Conclusion
Cyclohexane is obtained with a purity of 99.5% on weight basis through hydrogenation of
Benzene.
References
1. Cyclohexane
ARCO Technology Inc.
Hydrocarbon Processing, November 1977, p 143
2. Soave G.
"Equilibrium constants from a modified Redlich-Kwong equation of state" C.E.S.,
27, 6,1197-1203 (1972)
You might also like
- Project 2 CumeneDocument9 pagesProject 2 CumeneUmar Alijandro50% (2)
- Final Design - Assignment IIIDocument67 pagesFinal Design - Assignment IIIAmilcarwalter67% (3)
- Cumene ManufactringDocument74 pagesCumene ManufactringTan JieSheng100% (1)
- Production of Cyclohexanone From Phenol HydrogenationDocument157 pagesProduction of Cyclohexanone From Phenol Hydrogenationnoman100% (2)
- Senior ReportDocument113 pagesSenior ReportAnkit VermaNo ratings yet
- Manufacturing Process of NitrobenzeneDocument74 pagesManufacturing Process of NitrobenzenePankaj Borana100% (1)
- Chemical Design EthylbenzeneDocument32 pagesChemical Design Ethylbenzeneafnan_lion94No ratings yet
- Phenol Production from Cumene & TolueneDocument9 pagesPhenol Production from Cumene & TolueneAnonymous RJkpep7D0rNo ratings yet
- Hydrodealkylation SimulationDocument8 pagesHydrodealkylation SimulationSchaieraNo ratings yet
- Process Design of Monoethanolamine ProductionDocument83 pagesProcess Design of Monoethanolamine ProductionArpit Patel100% (1)
- PROCESS 3 (Chlorobenzene and Caustic Process) PDFDocument42 pagesPROCESS 3 (Chlorobenzene and Caustic Process) PDFPatricia Miranda100% (1)
- Process Design and Economics Assignment Development of PFD and Process Concept DiagramDocument9 pagesProcess Design and Economics Assignment Development of PFD and Process Concept Diagramshailaja chowdhuryNo ratings yet
- Systemdesignfinalreport CumeneDocument57 pagesSystemdesignfinalreport Cumenebiondimi0% (1)
- Design of Acetone HYSYSDocument6 pagesDesign of Acetone HYSYSlockas222100% (1)
- Optimal Acetone Production via IPA DehydrogenationDocument221 pagesOptimal Acetone Production via IPA DehydrogenationYasser AshourNo ratings yet
- Cumene to Phenol ProcessDocument2 pagesCumene to Phenol ProcessaliNo ratings yet
- Project Report at Cumene PDFDocument103 pagesProject Report at Cumene PDFDiv Savaliya100% (2)
- Although This Process Is No Longer in Common UseDocument15 pagesAlthough This Process Is No Longer in Common Usedia_aldy100% (1)
- AcetoneDocument7 pagesAcetoneGeorgiana AndreeaNo ratings yet
- Mini DP ChlorobenzeneDocument103 pagesMini DP ChlorobenzeneMuhammad sherazNo ratings yet
- Hydrodealkylation Process (HDA)Document6 pagesHydrodealkylation Process (HDA)Mohammad Bin Fahad100% (1)
- Manfacture OF: Cyclo HexaneDocument91 pagesManfacture OF: Cyclo HexaneNikhil Kumar Chennuri100% (4)
- CUMENEDocument24 pagesCUMENEhiteshNo ratings yet
- Overall Flowsheet Simulation Benzene Cyclohexane TW6Document7 pagesOverall Flowsheet Simulation Benzene Cyclohexane TW6Mitesh ParmarNo ratings yet
- Presentation CumeneDocument39 pagesPresentation Cumeneممدوح الرويليNo ratings yet
- Cumene Properties UsesDocument4 pagesCumene Properties UsesC.Çağrı Yekeler50% (2)
- A Project Report Submitted By: in Partial Fulfilment For The Award of The DegreeDocument91 pagesA Project Report Submitted By: in Partial Fulfilment For The Award of The DegreeHari BharathiNo ratings yet
- Project 6 - Ethylene Oxide PDFDocument13 pagesProject 6 - Ethylene Oxide PDFStephanie Hawkins100% (1)
- Hydrogenation of Nitrobenzene To AnilineDocument8 pagesHydrogenation of Nitrobenzene To AnilineYu HuiNo ratings yet
- Design of EthylbenzeneDocument5 pagesDesign of Ethylbenzenesahar vahdatifarNo ratings yet
- Cumene Energy 2520balanceDocument13 pagesCumene Energy 2520balanceismailchoughule50% (2)
- CPE639 Mini Project - Production of Acetonitrile Using Fluidized Bed Reactor PDFDocument41 pagesCPE639 Mini Project - Production of Acetonitrile Using Fluidized Bed Reactor PDFnoorNo ratings yet
- 8-Plant Design - Separation Units Part 4Document189 pages8-Plant Design - Separation Units Part 4MrHemFunNo ratings yet
- Cumene Mass & Energy Balance PDFDocument33 pagesCumene Mass & Energy Balance PDFMeet Khunt100% (1)
- Hydrodealkylation ProcessesDocument6 pagesHydrodealkylation ProcessesCluisantony Jayco Dize100% (1)
- Process Simulation and Optimization of Cyclohexane Manufacturing Plant Using Unisim and Hint PDFDocument23 pagesProcess Simulation and Optimization of Cyclohexane Manufacturing Plant Using Unisim and Hint PDFKhadeejaNo ratings yet
- Basics of plantwide process controlDocument2 pagesBasics of plantwide process controlCluisantony Jayco Dize0% (1)
- Benzene Production Using Hydrodealkylation RouteDocument3 pagesBenzene Production Using Hydrodealkylation RouteCluisantony Jayco DizeNo ratings yet
- Cumene To PhenolDocument73 pagesCumene To Phenolvpsrpuch67% (3)
- For Hysys UsersDocument5 pagesFor Hysys UsersZohaib RanaNo ratings yet
- Project On NitrobenzeneDocument65 pagesProject On NitrobenzeneAmit Khosla80% (10)
- Cumene Production Robert SchmidtDocument14 pagesCumene Production Robert SchmidtVatsalNo ratings yet
- Cumene Production Process DescriptionDocument1 pageCumene Production Process DescriptionAudrey Patrick KallaNo ratings yet
- Mtbe PDFDocument47 pagesMtbe PDFYayee LalainheavenNo ratings yet
- Kuwait University Chemical Engineering Plant Design Hysys ReportDocument20 pagesKuwait University Chemical Engineering Plant Design Hysys ReportCrazy HelloNo ratings yet
- CSTRDocument11 pagesCSTRfarahanisiliasNo ratings yet
- Acetaldehyde Production by Ethanol DehydrogenationDocument9 pagesAcetaldehyde Production by Ethanol DehydrogenationHugo Gerdulli AlbertinNo ratings yet
- Production of Ethylbenzene by Liquid-Phase Benzene Alkylation (Thesis)Document26 pagesProduction of Ethylbenzene by Liquid-Phase Benzene Alkylation (Thesis)Kiran Kumar100% (1)
- Simulation Design Project 2013 PDFDocument167 pagesSimulation Design Project 2013 PDFNhut NguyenNo ratings yet
- Cumene Process, Prod - CBIDocument2 pagesCumene Process, Prod - CBIChris LindseyNo ratings yet
- Chlorobenzene Plant TutorialDocument21 pagesChlorobenzene Plant Tutorialdjona lokimaNo ratings yet
- Material and Balance For Sohio Process That Produce AcrytonitrileDocument2 pagesMaterial and Balance For Sohio Process That Produce Acrytonitrileafnan_lion940% (1)
- Cumene A PDFDocument4 pagesCumene A PDFdanena88No ratings yet
- CiclohexanoDocument6 pagesCiclohexanoSebastian BelloNo ratings yet
- AADocument30 pagesAAAhmed MajidNo ratings yet
- Hysys SimulationDocument24 pagesHysys SimulationNeybil100% (1)
- Process Control CompleteDocument71 pagesProcess Control CompleteJanusNo ratings yet
- Abstract:: Pinacol Pinacolone Rearrangement Reaction (Preparation of Benzopinacolone)Document6 pagesAbstract:: Pinacol Pinacolone Rearrangement Reaction (Preparation of Benzopinacolone)Bryan Gerard GuillermoNo ratings yet
- First Review Report On Production of Phenol: Done by GuideDocument27 pagesFirst Review Report On Production of Phenol: Done by GuideRuban RkNo ratings yet
- exp 2 (Autosaved)Document7 pagesexp 2 (Autosaved)zanjinyadzaNo ratings yet
- SyllabusDocument194 pagesSyllabusabNo ratings yet
- Adv For The Post of Apprentices 2021Document6 pagesAdv For The Post of Apprentices 2021sushant kadamNo ratings yet
- Jaro Education JDDocument4 pagesJaro Education JDsushant kadamNo ratings yet
- Sem 8 SyallbusDocument16 pagesSem 8 Syallbussushant kadamNo ratings yet
- Union Bank Recruitment ApplicationDocument3 pagesUnion Bank Recruitment Applicationsushant kadamNo ratings yet
- 123-Zero Method For Net-Zero Carbon Manufacturing at Net-Zero CostDocument12 pages123-Zero Method For Net-Zero Carbon Manufacturing at Net-Zero Costsushant kadamNo ratings yet
- CR RemovalDocument4 pagesCR Removalsushant kadamNo ratings yet
- Understanding Energy FootprintsDocument5 pagesUnderstanding Energy FootprintsChrisNo ratings yet
- Industrial Processes Chapter SummaryDocument22 pagesIndustrial Processes Chapter SummaryMayank VisalparaNo ratings yet
- A Review On Phenolic Resin and Its Composites: Current Analytical Chemistry October 2017Document14 pagesA Review On Phenolic Resin and Its Composites: Current Analytical Chemistry October 2017sushant kadamNo ratings yet
- Cyclohexane PDFDocument14 pagesCyclohexane PDFsushant kadamNo ratings yet
- 08 - Chapter 2 PDFDocument20 pages08 - Chapter 2 PDFsushant kadamNo ratings yet
- Brief Summary Subject: Proposed Coal Tar Distillation Project To Manufacture 84,000 MT/Annum Coal TarDocument2 pagesBrief Summary Subject: Proposed Coal Tar Distillation Project To Manufacture 84,000 MT/Annum Coal Tarsushant kadamNo ratings yet
- 12 - Chapter 3 PDFDocument122 pages12 - Chapter 3 PDFsushant kadamNo ratings yet
- Comparative studies on adsorptive removal of chromium from contaminated waterDocument7 pagesComparative studies on adsorptive removal of chromium from contaminated watersushant kadamNo ratings yet
- PF Resin PDFDocument40 pagesPF Resin PDFpradeep4545No ratings yet
- Multiphase ReactorDocument37 pagesMultiphase ReactorMaria Charlene Caraos TapiaNo ratings yet
- Toothpickase Lab ActivityDocument6 pagesToothpickase Lab Activityrachel4151No ratings yet
- Kinetics exam questionsDocument9 pagesKinetics exam questionsridithaNo ratings yet
- DataDocument54 pagesDataferperez90No ratings yet
- Catalysis - Petroleum Technology Quarterly - 2014. Refining, Gas Processing and PetrochemicalsDocument68 pagesCatalysis - Petroleum Technology Quarterly - 2014. Refining, Gas Processing and PetrochemicalsjravisrinivasNo ratings yet
- Integrated Pollution Prevention and Control Draft Reference Document On Best Available Techniques in The Large Volume Inorganic Chemicals, Ammonia, Acids and Fertilisers Industries Draft March 2004Document332 pagesIntegrated Pollution Prevention and Control Draft Reference Document On Best Available Techniques in The Large Volume Inorganic Chemicals, Ammonia, Acids and Fertilisers Industries Draft March 2004WellfroNo ratings yet
- General Organic Chemistry - Iii: Section (A) : Solvents, Reagents and Leaving GroupsDocument20 pagesGeneral Organic Chemistry - Iii: Section (A) : Solvents, Reagents and Leaving GroupsGOURISH AGRAWALNo ratings yet
- Heterogeneous Kinetic Study For Esterification of Acetic Acid With EthanolDocument8 pagesHeterogeneous Kinetic Study For Esterification of Acetic Acid With EthanolSarang GohNo ratings yet
- Enzymes Speed Up Chemical ReactionsDocument16 pagesEnzymes Speed Up Chemical Reactionstaryll_01No ratings yet
- Brannaka J DissertationDocument140 pagesBrannaka J DissertationJoseph BrannakaNo ratings yet
- Guard Bed CatalystsDocument7 pagesGuard Bed CatalystsargachoNo ratings yet
- Tech Data - Resin CraftDocument1 pageTech Data - Resin Craftkriskee13No ratings yet
- Biodiesel Production With Green Technologies - Islam e Ravindra (2017) PDFDocument142 pagesBiodiesel Production With Green Technologies - Islam e Ravindra (2017) PDFDiego Bittencourt MachadoNo ratings yet
- Chemical EngineeringDocument287 pagesChemical EngineeringSleek KastrowNo ratings yet
- Enzymes ReviewerDocument2 pagesEnzymes ReviewerAshley MalolotNo ratings yet
- Enzymes Cell Biology Lecture PowerPoint VCBCDocument27 pagesEnzymes Cell Biology Lecture PowerPoint VCBCAnsh PalNo ratings yet
- General Biology 1: Quarter I - Module 7 Biological Molecules-EnzymesDocument27 pagesGeneral Biology 1: Quarter I - Module 7 Biological Molecules-EnzymesJohn Joseph Jalandoni100% (2)
- Biochemistry I BSC 211: Thomson Sanudi Basic Sciences DepartmentDocument17 pagesBiochemistry I BSC 211: Thomson Sanudi Basic Sciences DepartmentKelvin ChipezeniNo ratings yet
- 2020 Specimen Paper 2Document18 pages2020 Specimen Paper 2sarabNo ratings yet
- Mayank Ate PaperDocument19 pagesMayank Ate PaperAbhishekSinghNo ratings yet
- International As: CHEMISTRY (9620)Document24 pagesInternational As: CHEMISTRY (9620)任思诗No ratings yet
- REACTOR DESIGN FOR AMMONIA OXIDATIONDocument5 pagesREACTOR DESIGN FOR AMMONIA OXIDATIONabdul rehmanNo ratings yet
- Enzyme ReviewDocument2 pagesEnzyme ReviewSamura HasibNo ratings yet
- Che 511 Lecture Note 2023Document5 pagesChe 511 Lecture Note 2023Bright ChimezieNo ratings yet
- Kinetics of EnzymesDocument2 pagesKinetics of EnzymesJohnNo ratings yet
- Reactor Design For PetrochemicalDocument40 pagesReactor Design For Petrochemicalsinwei93No ratings yet
- CBE NewsMagazine2015 02162016Document40 pagesCBE NewsMagazine2015 02162016Lmd LmdNo ratings yet
- Proposal Rough 4Document10 pagesProposal Rough 4api-316780587No ratings yet
- Applied Catalysis B: Environmental: Matej Hu S, Venkata D.B.C. Dasireddy, Neja Strah Stefan Ci C, Bla Z LikozarDocument12 pagesApplied Catalysis B: Environmental: Matej Hu S, Venkata D.B.C. Dasireddy, Neja Strah Stefan Ci C, Bla Z LikozarAminNo ratings yet