Professional Documents
Culture Documents
Internal Labelling by Diels Alder Modification
Internal Labelling by Diels Alder Modification
Uploaded by
Tom FlemingOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Internal Labelling by Diels Alder Modification
Internal Labelling by Diels Alder Modification
Uploaded by
Tom FlemingCopyright:
Available Formats
TETRAHEDRON LETTERS
Pergamon
Tetrahedron Letters 43 (2002) 47854788
Internal labeling of oligonucleotide probes by DielsAlder cycloaddition
Duncan Graham,* Antonio Grondin, Callum McHugh, Ljiljana Fruk and W. Ewen Smith
Department of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow G1 1XL, UK
Received 4 March 2002; revised 30 April 2002; accepted 13 May 2002
AbstractA new method of adding uorescent labels to the middle of oligonucleotides is reported. DielsAlder cycloaddition was used to add ve uorescent maleimides to an oligonucleotide containing a 2%-deoxyuridine modied at the 5-position with a spaced furan. This is a new approach to internal oligonucleotide chemistry that opens up a large range of possibilities for further conjugation. 2002 Elsevier Science Ltd. All rights reserved.
Addition of external labels to oligonucleotides is necessary to satisfy the need of current DNA assays and allow the development of new approaches. The most common current approach for DNA analysis is the use of uorescence spectroscopy.13 Most uorophores are added to the 5% or 3% terminus by a number of different methods. Internal labeling is more complex to achieve and a number of approaches have been employed. These include modication of the backbone, sugar or base. Addition of uorophores to the base are most popular. Current approaches include the use of functional groups such as alkyl amines that react with activated dyes after oligonucleotide synthesis4 or the use of a pre-labeled nucleoside phosphoramidite.3 The post synthesis approach suffers from low yields and side reactions and the use of dye nucleoside phosphoramidites requires complex chemistry and expensive monomers. Brown et al.5 recently reported the use of an Fmoc protected alkyl alcohol modied thymidine phosphoramidite that could be used to add commercially available dye phosphoramidites in high yield to the middle of an oligonucleotide. The aim of the research reported here was to produce a nucleoside phosphoramidite that could be used to add a label to the middle of an oligonucleotide after solid phase synthesis in a highly efcient manner. We report the use of DielsAlder cycloaddition for the addition of uorescent dyes to a modied 2%deoxyuridine residue after oligonucleotide synthesis. Hill et al.6 recently reported the use of DielsAlder
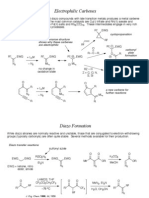
cycloadditions as a method of adding uorophores to the 5%-terminus of oligonucleotides. This demonstrated the advantages of using DielsAlder reactions for conjugations to oligonucleotides, namely, a fast, quantitative reaction occurring in an aqueous buffer system. Additionally, as DielsAlder reactions occur between a diene and a dienophile there is no need for use of selective protecting groups to prevent reaction with common nucleophiles and hence do not suffer from any side reactions. In this approach one phosphoramidite can be used to add any number of different dyes. In this study we report the addition of a diene to the 5 position of 2%-deoxyuridine. The diene was chosen to be added to the nucleoside, as a number of commercially available dienophiles in the form of uorescent maleimides can be used without further chemical modication. A range of dienes was considered for addition to a nucleoside and their reactions with a maleimide examined.7 Furan was chosen due to the number of readily available derivatives, the speed and efciency of the reaction with maleimides and stability to the conditions used for oligonucleotide synthesis. The starting material for the synthesis was 5-iodo-2%deoxyuridine which was initially protected on the 5%hydroxyl with dimethoxytrityl 1. A palladium catalyzed Sonogashira coupling was used to add an acetylenic furan derivative 2 prepared by the condensation of pentynoic acid and furfuryl amine. This protected furan 2%-deoxyuridine 3 was then phosphitylated using standard procedures to give the monomer 4 ready for solid phase oligonucleotide synthesis (Scheme 1).
* Corresponding author. E-mail: duncan.graham@strath.ac.uk
0040-4039/02/$ - see front matter 2002 Elsevier Science Ltd. All rights reserved. PII: S 0 0 4 0 - 4 0 3 9 ( 0 2 ) 0 0 9 3 0 - 9
4786
D. Graham et al. / Tetrahedron Letters 43 (2002) 47854788
Scheme 1. (i) Pyridine, 4,4%-dimethoxytrityl chloride (1.2 equiv.), DMAP (0.1 equiv.), 80%; (ii) DMF, carbonyl diimidazole (1.1 equiv.), 40C, 95%; (iii) DMF, triethylamine (5 equiv.), CuI (0.2 equiv.), 2 (1.5 equiv.), Pd (PPh3)4 (0.1 equiv.), 79%; (iv) THF, 2-cyanoethyl-N,N -diisopropylchlorophosphine (1.1 equiv.), DIPEA (3 equiv.), 98%.
The monomer was then used as a 50 mg/ml solution (THF/MeCN 1:1) on an Expedite 8909 oligonucleotide synthesizer with a 15-minute coupling cycle. The HPLC
trace indicated excellent incorporation of the modied monomer (Fig. 1). A 20mer that has been used as an antisense oligonucleotide to inhibit MDR1 gene expres-
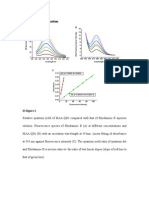
Figure 1. HPLC trace obtained for the crude modied 20mer after deprotection using a Chromolith reverse phase column running at 4 ml/min and a buffer system of A=0.1 M ammonium acetate pH 7.0, B=MeCN. (CV=column volume 1.662 ml) 5 CV 5% B, 25 CV 530% B, 5 CV 30% B, 5 CV 30100% B, 3 CV 100% B.
D. Graham et al. / Tetrahedron Letters 43 (2002) 47854788
4787
sion was synthesized.8 The position marked with X represents the furan modied uridine. 5%CCT CGC GCX CCA GCC CTA CC DielsAlder cycloadditions were then attempted using this oligonucleotide and ve uorophore maleimides. The following dye maleimides were purchased from Molecular Probes and used without further manipulation, FAM, Texas red, TAMRA-5, TAMRA-6 and Alexa Fluor 532 (Fig. 2). The two isomers of TAMRA i.e. 5 and 6 were examined to see if the different isomers affected the cycloaddition. The oligonucleotide was dissolved in a phosphate buffer (25 mM, pH 5.5) and the uorophore maleimides added in an acetonitrile buffer mixture that gave a nal acetonitrile concentration of 30% v/v. The cycloadditions were left in the dark at 40C for 3 h prior to analysis by reverse phase HPLC. The TAMRA dyes were not heated due to the thermal instability of the dye but were left to react for 4 h instead of 3 h. In each case 3 equiv. of dye maleimide were used to ensure complete reaction. The HPLC traces of the cycloadditions showed clear differences between the furan oligonucleotide peak and the cycloadduct peak indicating that the cycloadduct had formed in excellent yield. Fig. 3 shows the HPLC trace of the oligonucleotides produced by cycloaddition of three of the maleimides. All of the cycloadducts displayed a reduced retention time due to the increased polarity of the conjugated oligonucleotides. Only three are shown in Fig. 3 as the Alexa Fluor 532 and FAM conjugates had the same retention time as did the TAMRA 6 isomer and Texas Red conjugates.
The labeled oligonucleotides were then examined for uorescence. The emission wavelengths observed for the oligonucleotides differed slightly from those quoted. This is presumed to be due to the use of an aqueous buffer as opposed to methanol apart from the value quoted for FAM,9 which is in aqueous buffer and is identical (Table 1). Interestingly we found that the two
Figure 3. HPLC of the cycloadducts using a Chromolith reverse phase column at 4 ml/min and a buffer system of: A=0.1 M ammonium acetate pH 7.0, B=MeCN. (CV= column volume 1.662 ml) 05 CV 5% B, 515 CV 510% B, 1535 CV 1025% B, 3545 CV 25100% B, 4548 CV 100% B. Peak 1=TAMRA 5, Peak 2=Texas Red, Peak 3=FAM, Peak 4=furan oligo. (The peaks have been scaled for clarity.)
Figure 2. The uorophore maleimides used in the cycloadditions.
4788
D. Graham et al. / Tetrahedron Letters 43 (2002) 47854788
Table 1. Comparison of the emission wavelengths of the labeled oligonucleotides with the literature values
Fluorophore FAM TAMRA-5 TAMRA-6 Alexa Fluor 532 Texas Red Emission (aq.) (nm) 515 571 558 533 606 Lit. value9 (nm) 515 567 567 552 600 (aq.) (MeOH) (MeOH) (MeOH) (MeOH)
Acknowledgements The authors would like to thank the BBSRC for the award of a David Phillips fellowship to D.G. and for funding the work through grant number E11015. References
1. Wojczewski, C.; Stolze, K.; Engels, J. W. Synlett 1999, 1667. 2. Christopoulos, T. K. Anal. Chem. 1999, 71, 425R. 3. Davies, M. J.; Shah, A.; Bruce, I. J. Chem. Soc. Rev. 2000, 29, 97. 4. Randolph, J. B.; Waggoner, A. S. Nucleic Acids Res. 1997, 25, 2923. 5. Brown, L. J.; May, J. P.; Brown, T. Tetrahedron Lett. 2001, 42, 2587. 6. Hill, K. W.; Taunton-Rigby, J.; Carter, J. D.; Kropp, E.; Vagle, K.; Pieken, W.; McGee, D. P. C.; Husar, G. M.; Leuck, M.; Anziano, D. J.; Sebesta, D. P. J. Org. Chem. 2001, 66, 5352. 7. Grondin, A.; Robson, D. C.; Smith, W. E.; Graham, D. J. Chem. Soc., Perkin Trans. 2 2001, 2136. 8. Astriab-Fisher, A.; Sergueev, D. S.; Fisher, M.; Shaw, B. R.; Juliano, R. L. Biochem. Pharmacol. 2000, 60, 83. 9. http://www.probes.com/
TAMRA isomers gave emission wavelengths that differed by 13 nm where the literature values are identical. This proved that the oligonucleotides were uorescent and that the cycloaddition does not interfere with the uorescence emission. In conclusion, this letter demonstrates a new method of internally labeling oligonucleotides that is fast, simple and high yielding. Different types of uorophore were used to demonstrate the versatility of this approach and demonstrate the selectivity of the chemistry. Additionally the one modied phosphoramidite can be used with any uorescent maleimide and is therefore a generic modication.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5810)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (844)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (346)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- IB Chem2 5 Assess T6Document3 pagesIB Chem2 5 Assess T6Trúc HồNo ratings yet
- Ammonia Synthesis KineticsDocument4 pagesAmmonia Synthesis KineticsYulianto Triyono HadiNo ratings yet
- Chemical Reaction Stoichiometry I: Cheme 101 - 8.3 Worksheet 1 Semester Ay 2020-2021 Department of Chemical EngineeringDocument7 pagesChemical Reaction Stoichiometry I: Cheme 101 - 8.3 Worksheet 1 Semester Ay 2020-2021 Department of Chemical EngineeringAcademicBMNo ratings yet
- PyMol PracticalDocument7 pagesPyMol PracticalTom FlemingNo ratings yet
- Sulphonamide FragmentDocument5 pagesSulphonamide FragmentTom FlemingNo ratings yet
- Synthesis of ImidazolesDocument5 pagesSynthesis of ImidazolesMuhammad SalehNo ratings yet
- Levulinyl Deprotection MethodDocument9 pagesLevulinyl Deprotection MethodTom FlemingNo ratings yet
- QD Molecular Beacon For FISH in E Coli - Pang, Biosensors Bioelectr 2010 - SUPP INFODocument1 pageQD Molecular Beacon For FISH in E Coli - Pang, Biosensors Bioelectr 2010 - SUPP INFOTom FlemingNo ratings yet
- Fluorescent Organic LabellingDocument23 pagesFluorescent Organic LabellingTom Fleming100% (1)
- How Fast MSDocument5 pagesHow Fast MSsaadNo ratings yet
- Lecture 11 - Heat Effects of Industrial Reactions - May16Document22 pagesLecture 11 - Heat Effects of Industrial Reactions - May16Ysabela Angela FloresNo ratings yet
- Exercise and Question CH3681 Final 15 16Document9 pagesExercise and Question CH3681 Final 15 16SuNo ratings yet
- Carbenoids 2Document15 pagesCarbenoids 2costea0028No ratings yet
- Elimination Reactions: E1, E2Document11 pagesElimination Reactions: E1, E2Susobhan ghoshNo ratings yet
- Redox Practice HLDocument5 pagesRedox Practice HLSere FernandezNo ratings yet
- Fig. The Basis of The Steady-StateDocument18 pagesFig. The Basis of The Steady-StateRany Feby SyafitriNo ratings yet
- Chemistry Module Ii Physical Chemistry Ii For Iit Jee Main and Advanced Ranveer Singh Mcgraw Hill Education - Ebook PDFDocument68 pagesChemistry Module Ii Physical Chemistry Ii For Iit Jee Main and Advanced Ranveer Singh Mcgraw Hill Education - Ebook PDFrobert.davidson233100% (30)
- CP504Lecture - 06 - OK (Enzyme Reactor Design)Document12 pagesCP504Lecture - 06 - OK (Enzyme Reactor Design)siskaNo ratings yet
- Chemical Kinetics: Gist of The LessonDocument34 pagesChemical Kinetics: Gist of The Lessonanshikahp1No ratings yet
- A Metal-Organic Framework With Exceptional Activity For C-H Bond AminationDocument5 pagesA Metal-Organic Framework With Exceptional Activity For C-H Bond AminationAdam MNo ratings yet
- Cre QuestionDocument2 pagesCre QuestionChinu Routa100% (1)
- c4 Rate of Reaction f5Document9 pagesc4 Rate of Reaction f5Rui Er LiewNo ratings yet
- A Primer To Designing Organic SynthesisDocument42 pagesA Primer To Designing Organic SynthesisMohammed100% (1)
- Enantioselective Enzymatic Desymmetrizations in Organic SynthesisDocument71 pagesEnantioselective Enzymatic Desymmetrizations in Organic SynthesisVipul ValaNo ratings yet
- CHM 231 Organi Chem 1Document101 pagesCHM 231 Organi Chem 1Adams DeborahNo ratings yet
- Review de L-Prolina y Sus Derivados en Sintesis AsimetricaDocument31 pagesReview de L-Prolina y Sus Derivados en Sintesis Asimetricatalero22No ratings yet
- CHEM 40 Problem Set 1.2 PDFDocument2 pagesCHEM 40 Problem Set 1.2 PDFNeil Kenneth MabalotNo ratings yet
- A2 Photosynthesis Homework StudyDocument3 pagesA2 Photosynthesis Homework StudystjustinsNo ratings yet
- CRE-CIA 2 - A QUESTION PAPER 2023 OddDocument1 pageCRE-CIA 2 - A QUESTION PAPER 2023 OddA.RAJKUMAR (HICET) HICET STAFFCHEMNo ratings yet
- Retrosynthesis Practice Problems: O O OR O N O O BR O O OR 1)Document10 pagesRetrosynthesis Practice Problems: O O OR O N O O BR O O OR 1)butko88No ratings yet
- Bio PresentationDocument18 pagesBio Presentationapi-583318017No ratings yet
- Dr. Sana Aslam Assistant Professor Department of Chemistry Government College Women University FaisalabadDocument14 pagesDr. Sana Aslam Assistant Professor Department of Chemistry Government College Women University Faisalabadrimsha mansoorNo ratings yet
- SCH 504 MetathesisDocument11 pagesSCH 504 MetathesisYıldız ÇeltikNo ratings yet
- Exercise On Order of ReactionDocument4 pagesExercise On Order of ReactionGopi KupuchittyNo ratings yet
- 21.8 (A) Lindermann-Hinshelwood Mechanism: (Slow, RDS) + +Document18 pages21.8 (A) Lindermann-Hinshelwood Mechanism: (Slow, RDS) + +cmc107No ratings yet
- Thermochemistry, Chemical Kinetics, Electrochemistry Phase Transition, Colloids in FoodDocument120 pagesThermochemistry, Chemical Kinetics, Electrochemistry Phase Transition, Colloids in FoodVo Trung Kien B2100780No ratings yet