Professional Documents
Culture Documents
Phil. Trans. R. Soc. Lond. B-2004-Forey-639-53 PDF
Uploaded by
Carolina RicárdezOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Phil. Trans. R. Soc. Lond. B-2004-Forey-639-53 PDF
Uploaded by
Carolina RicárdezCopyright:
Available Formats
, published 29 April 2004 , doi: 10.1098/rstb.2003.1453 359 2004 Phil. Trans. R. Soc. Lond.
Peter L. Forey, Richard A. Fortey, Paul Kenrick and Andrew B. Smith
Taxonomy and fossils: a critical appraisal
Email alerting service
here right-hand corner of the article or click
Receive free email alerts when new articles cite this article - sign up in the box at the top
http://rstb.royalsocietypublishing.org/subscriptions go to: Phil. Trans. R. Soc. Lond. B To subscribe to
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
Published online 22 March 2004
Taxonomy and fossils: a critical appraisal
Peter L. Forey
*
, Richard A. Fortey, Paul Kenrick and Andrew B. Smith
Department of Palaeontology, The Natural History Museum, Cromwell Road, London SW7 5BD, UK
(raf@nhm.ac.uk, pauk@nhm.ac.uk, abs@nhm.ac.uk)
Many compendia at the species, genus and family levels document the fossil record, but these are not
standardized, nor usually critical in content, and few are available on the World Wide Web. The sampling
of the available record is good for organisms with fossilizable parts, but preservational constraints on the
entire morphology, life history and geographical distribution lead to difficulties in recognizing and naming
species. We recommend abandoning some of the palaeontological species concepts such as chronospecies
and stratospecies, and we advocate species recognition based on unique combinations of characters. The
compilation of species lists is extremely time consuming, and given the inherent problems we suggest that
compilation of generic lists is a more achievable goal because genera are recognized by definitive morpho-
logical characters. In calculating taxon duration, care must be taken to distinguish between mono-, para-
and polyphyletic groups, the first being the only reliable unit for use in calculating diversity curves. We
support the inclusion of fossils into classifications based on Recent organisms, but we recognize some of
the problems this may pose for standard Linnaean classifications. Web-based taxonomy is the way forward,
having the advantages of speed and currency of information dissemination, universal access with links to
primary literature and increasingly sophisticated imagery. These advantages over conventional outlets will
only be realized with careful Web design and a commitment to maintenance.
Keywords: completeness of the fossil record; diversity; species; parataxa; classification; World Wide Web
1. INTRODUCTION
We have been asked to address the challenges surrounding
the classification of extinct taxa, how these relate to the
taxonomy of extant organisms and our vision of future
taxonomy from the palaeontological perspective. We do
so by starting from what we know; that is, by assessing
what information is currently available. From there we
proceed to discuss how well we know the fossil record so
as to gain some idea of biases that may influence any com-
parison between the extinct and extant worlds (May et al.
1995). Those biases fall into two areas: (i) the nature of
the geological record and how well it yields species counts
faithful to reality; and (ii) the nature of the species that
are named by palaeontologists, including the influence of
taxonomy on our estimations of species numbers. Follow-
ing this, we consider some of the issues surrounding the
use of hierarchical data above the species level. Finally,
we offer some suggestions that may enable palaeonto-
logical data to be used more easily.
2. THE STATE OF OUR KNOWLEDGE
Palaeontologists do not face the extreme problem con-
fronting neontologists who struggle to describe species
before they go extinct. Additionally, palaeontologists have
always paid attention to documenting genera and species,
*
Author for correspondence (plf@nhm.ac.uk).
Author list is strictly alphabetical.
One contribution of 19 to a Theme Issue Taxonomy for the twenty-
first century.
Phil. Trans. R. Soc. Lond. B (2004) 359, 639653 639 2004 The Royal Society
DOI 10.1098/rstb.2003.1453
and this is due, in part, to the use of fossils for strati-
graphic zonation and correlation. Of course, new finds of
fossils will lead to the discovery of new species and per-
haps provide a check for the validity of others. Description
and taxonomic revision are at the heart of palaeontology,
and this has always been so. Many compendia exist, such
as The Fossil record (Harland et al. 1967) and its successor,
The Fossil record 2 (Benton 1993), The treatise on invert-
ebrate paleontology (Moore 1953) and Sepkoskis family
and genus level databases (1982, 1992, 2002). These
databases are not independent of one another, but collec-
tively they do supply a reasonably comprehensive coverage
of fossil taxa above the species level. However, the detail
of coverage and means of disseminating that information
do vary considerably. Table 1 documents a few of the
most commonly used major taxonomic databases for fossil
taxa with some details of their content.
Certain observations on the information contained in
table 1 are pertinent. Virtually none are Web-based and/or
updated on a regular basis. If updated, it is more usual
for wholesale revisions to replace earlier attempts. Because
most are hard copy, any information is always immediately
outdated. Generally, wholesale revisions are performed by
new authors. Because of this there can be different con-
cepts of species and genera and, in particular, very differ-
ent ideas of higher classification leading to minimal
continuity between revisions. For example, two of the
major sources of information on the diversity of foram-
inera are Haynes (1981) and Loeblich & Tappan (1988).
The former recognizes 99 families, the latter 288. The dis-
crepancy is due largely to differing perceptions of rank,
and this may have significant consequences in estimations
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
640 P. L. Forey and others Classication of extinct taxa
T
a
b
l
e
1
.
S
o
m
e
o
f
t
h
e
m
a
j
o
r
d
a
t
a
b
a
s
e
s
,
c
o
m
p
e
n
d
i
a
a
n
d
l
i
s
t
s
o
f
f
o
s
s
i
l
s
w
i
t
h
s
o
m
e
i
n
d
i
c
a
t
i
o
n
o
f
t
h
e
i
r
i
n
f
o
r
m
a
t
i
o
n
c
o
n
t
e
n
t
.
m
o
s
t
m
o
s
t
e
x
c
l
u
s
i
v
e
e
x
c
l
u
s
i
v
e
t
a
x
o
n
o
m
i
c
l
e
v
e
l
o
f
s
p
e
c
i
e
s
s
y
n
o
n
y
m
y
h
i
g
h
e
r
s
t
r
a
t
i
g
r
a
p
h
i
c
g
e
o
g
r
a
p
h
i
c
g
r
o
u
p
l
e
v
e
l
d
i
a
g
n
o
s
i
s
l
i
s
t
e
d
i
n
c
l
u
d
e
d
c
l
a
s
s
i
f
i
c
a
t
i
o
n
r
e
s
o
l
u
t
i
o
n
l
o
c
a
t
i
o
n
h
a
r
d
/
C
D
/
W
e
b
u
p
d
a
t
e
d
r
e
f
e
r
e
n
c
e
a
l
l
t
a
x
a
f
a
m
i
l
y
n
/
a
n
o
n
o
y
e
s
s
t
a
g
e
y
e
s
h
a
r
d
/
W
e
b
n
o
B
e
n
t
o
n
(
1
9
9
3
)
m
a
r
i
n
e
a
n
i
m
a
l
s
g
e
n
u
s
n
/
a
n
o
p
a
r
t
p
a
r
t
s
t
a
g
e
n
o
h
a
r
d
/
C
D
?
S
e
p
k
o
s
k
i
(
2
0
0
2
)
a
m
a
r
i
n
e
a
n
i
m
a
l
s
f
a
m
i
l
y
n
/
a
n
o
n
o
y
e
s
s
t
a
g
e
n
o
h
a
r
d
n
o
S
e
p
k
o
s
k
i
(
1
9
9
2
)
f
u
n
g
a
l
s
p
o
r
e
s
a
n
d
m
y
c
e
l
i
a
s
p
e
c
i
e
s
s
p
e
c
i
e
s
y
e
s
y
e
s
n
o
s
t
a
g
e
n
o
h
a
r
d
n
o
K
a
l
g
u
t
k
a
r
&
J
a
n
s
o
n
i
u
s
(
2
0
0
0
)
d
i
n
o
f
l
a
g
e
l
l
a
t
e
s
s
(
v
a
r
)
n
/
a
y
e
s
y
e
s
n
o
v
a
r
i
a
b
l
e
t
o
s
t
a
g
e
n
o
h
a
r
d
y
e
s
W
i
l
l
i
a
m
s
e
t
a
l
.
(
1
9
9
8
)
b
d
i
n
o
f
l
a
g
e
l
l
a
t
e
s
s
p
e
c
i
e
s
s
p
e
c
i
e
s
y
e
s
y
e
s
n
o
v
a
r
i
a
b
l
e
t
o
s
t
a
g
e
y
e
s
h
a
r
d
y
e
s
C
r
a
m
e
r
&
D
e
z
(
1
9
7
9
)
c
a
c
r
i
t
a
r
c
h
s
s
p
e
c
i
e
s
n
/
a
y
e
s
y
e
s
n
o
v
a
r
i
a
b
l
e
t
o
s
t
a
g
e
n
o
h
a
r
d
n
o
F
e
n
s
o
m
e
e
t
a
l
.
(
1
9
9
0
)
p
a
l
y
n
o
m
o
r
p
h
s
s
p
e
c
i
e
s
s
p
e
c
i
e
s
y
e
s
s
t
a
g
e
y
e
s
h
a
r
d
y
e
s
J
a
n
s
o
n
i
u
s
&
H
i
l
l
s
(
1
9
7
8
)
d
f
o
r
a
m
i
n
i
f
e
r
a
g
e
n
u
s
g
e
n
u
s
y
e
s
y
e
s
y
e
s
s
t
a
g
e
y
e
s
h
a
r
d
n
o
L
o
e
b
l
i
c
h
&
T
a
p
p
a
n
(
1
9
8
8
)
i
n
v
e
r
t
e
b
r
a
t
e
s
s
p
e
c
i
e
s
g
e
n
u
s
t
y
p
e
y
e
s
y
e
s
s
t
a
g
e
y
e
s
h
a
r
d
y
e
s
M
o
o
r
e
(
1
9
5
3
)
e
e
c
h
i
n
o
i
d
s
s
p
e
c
i
e
s
g
e
n
u
s
y
e
s
y
e
s
y
e
s
s
t
a
g
e
y
e
s
W
e
b
y
e
s
w
w
w
.
n
h
m
.
a
c
.
u
k
/
s
c
i
e
n
c
e
/
e
c
h
i
n
o
i
d
s
f
e
c
h
i
n
o
i
d
s
s
p
e
c
i
e
s
n
o
y
e
s
n
o
y
e
s
s
t
a
g
e
y
e
s
h
a
r
d
n
o
K
i
e
r
&
L
a
w
s
o
n
(
1
9
7
8
)
g
f
i
s
h
e
s
s
p
e
c
i
e
s
g
e
n
u
s
y
e
s
p
a
r
t
y
e
s
s
t
a
g
e
y
e
s
h
a
r
d
n
o
S
c
h
u
l
t
z
e
(
1
9
7
8
)
h
r
e
p
t
i
l
e
s
s
p
e
c
i
e
s
s
p
e
c
i
e
s
y
e
s
y
e
s
y
e
s
s
t
a
g
e
y
e
s
h
a
r
d
n
o
K
u
h
n
(
1
9
6
9
)
i
b
i
r
d
s
s
p
e
c
i
e
s
n
o
y
e
s
y
e
s
y
e
s
e
p
o
c
h
y
e
s
h
a
r
d
n
o
B
r
o
d
k
o
r
p
(
1
9
6
3
1
9
7
8
)
m
a
m
m
a
l
s
g
e
n
u
s
n
o
n
o
y
e
s
y
e
s
e
p
o
c
h
y
e
s
C
D
n
o
M
c
K
e
n
n
a
&
B
e
l
l
(
1
9
9
7
)
l
a
n
d
p
l
a
n
t
s
s
p
e
c
i
e
s
s
p
e
c
i
e
s
y
e
s
y
e
s
y
e
s
v
a
r
i
a
b
l
e
t
o
s
t
a
g
e
y
e
s
h
a
r
d
n
o
B
o
u
r
e
a
u
(
1
9
6
4
1
9
7
5
)
p
l
a
n
t
s
s
p
e
c
i
e
s
s
p
e
c
i
e
s
y
e
s
n
o
y
e
s
s
t
a
g
e
y
e
s
W
e
b
n
o
h
t
t
p
:
/
/
i
b
s
.
u
e
l
.
a
c
.
u
k
/
i
b
s
a
S
e
p
k
o
s
k
i
(
2
0
0
2
)
.
A
c
o
m
p
e
n
d
i
u
m
o
f
f
o
s
s
i
l
m
a
r
i
n
e
g
e
n
e
r
a
.
T
h
i
s
v
o
l
u
m
e
h
a
s
b
e
e
n
e
d
i
t
e
d
b
y
D
.
J
a
b
l
o
n
s
k
i
a
n
d
M
.
F
o
o
t
e
a
f
t
e
r
t
h
e
d
e
a
t
h
o
f
J
a
c
k
S
e
p
k
o
s
k
i
(
i
n
1
9
9
9
)
.
T
h
e
c
o
r
e
o
f
t
h
e
d
a
t
a
b
a
s
e
c
o
m
e
s
f
r
o
m
M
o
o
r
e
(
1
9
5
3
)
f
o
r
i
n
v
e
r
t
e
b
r
a
t
e
s
,
R
o
m
e
r
(
1
9
6
6
)
f
o
r
v
e
r
t
e
b
r
a
t
e
s
a
n
d
L
o
e
b
l
i
c
h
&
T
a
p
p
a
n
(
1
9
8
8
)
o
n
f
o
r
a
m
s
,
w
i
t
h
m
a
n
y
e
m
e
n
d
a
t
i
o
n
s
(
e
.
g
.
s
y
n
o
n
y
m
y
,
s
t
r
a
t
i
g
r
a
p
h
i
c
u
p
d
a
t
e
s
)
a
n
d
a
d
d
i
t
i
o
n
s
f
r
o
m
p
r
i
m
a
r
y
l
i
t
e
r
a
t
u
r
e
.
T
h
e
g
e
n
e
r
a
a
r
e
a
s
s
i
g
n
e
d
t
o
o
r
d
e
r
,
c
l
a
s
s
,
p
h
y
l
u
m
a
n
d
k
i
n
g
d
o
m
b
u
t
n
o
t
t
o
f
a
m
i
l
y
.
T
h
i
s
i
s
u
n
f
o
r
t
u
n
a
t
e
b
e
c
a
u
s
e
i
t
i
s
n
o
t
p
o
s
s
i
b
l
e
t
o
r
e
l
a
t
e
t
h
i
s
d
a
t
a
b
a
s
e
w
i
t
h
t
h
e
e
a
r
l
i
e
r
a
n
d
m
u
c
h
u
s
e
d
f
a
m
i
l
y
-
l
e
v
e
l
d
a
t
a
b
a
s
e
(
S
e
p
k
o
s
k
i
1
9
9
2
)
.
b
W
i
l
l
i
a
m
s
e
t
a
l
.
(
1
9
9
8
)
.
T
h
i
s
i
s
u
p
d
a
t
e
d
a
p
p
r
o
x
i
m
a
t
e
l
y
e
v
e
r
y
5
y
e
a
r
s
.
P
l
a
n
s
a
r
e
t
o
i
s
s
u
e
C
D
s
f
o
r
f
u
t
u
r
e
e
d
i
t
i
o
n
s
.
T
h
i
s
s
e
r
i
e
s
,
b
e
g
u
n
i
n
1
9
7
3
,
l
i
s
t
s
g
e
n
e
r
a
a
n
d
s
p
e
c
i
e
s
.
I
t
i
s
i
n
p
a
r
t
c
o
m
p
l
e
m
e
n
t
a
r
y
t
o
S
t
o
v
e
r
&
E
v
i
t
t
(
1
9
7
8
)
a
n
d
S
t
o
v
e
r
&
W
i
l
l
i
a
m
s
(
1
9
8
7
)
w
h
o
g
i
v
e
s
y
n
o
p
t
i
c
d
e
s
c
r
i
p
t
i
o
n
s
a
t
g
e
n
e
r
i
c
l
e
v
e
l
a
n
d
l
i
s
t
s
p
e
c
i
e
s
.
c
C
r
a
m
e
r
&
D
e
z
(
1
9
7
9
)
.
K
n
o
w
n
a
s
t
h
e
E
i
s
e
n
a
c
k
C
a
t
a
l
o
g
u
e
(
s
t
a
r
t
e
d
b
y
A
.
E
i
s
e
n
a
c
k
)
.
C
o
n
t
a
i
n
s
i
l
l
u
s
t
r
a
t
i
o
n
s
o
f
t
y
p
e
s
p
e
c
i
m
e
n
s
.
T
h
i
s
i
s
a
c
a
t
a
l
o
g
u
e
t
h
a
t
t
r
a
n
s
l
a
t
e
s
p
a
p
e
r
s
d
e
s
c
r
i
b
i
n
g
s
p
e
c
i
e
s
i
n
t
o
a
c
o
m
m
o
n
s
t
y
l
e
g
i
v
i
n
g
s
p
e
c
i
e
s
i
n
f
o
r
m
a
t
i
o
n
.
I
t
i
s
c
o
n
s
t
a
n
t
l
y
u
p
d
a
t
e
d
b
y
a
d
d
i
t
i
o
n
r
a
t
h
e
r
t
h
a
n
r
e
v
i
s
i
o
n
.
d
J
a
n
s
o
n
i
o
u
s
&
H
i
l
l
s
(
1
9
7
8
)
.
D
e
a
l
s
w
i
t
h
f
o
s
s
i
l
s
p
o
r
e
s
a
n
d
h
a
s
b
e
e
n
i
s
s
u
e
d
o
n
c
a
r
d
s
,
w
i
t
h
s
u
p
p
l
e
m
e
n
t
s
s
t
i
l
l
c
o
n
t
i
n
u
i
n
g
t
o
a
p
p
e
a
r
,
s
o
m
e
t
i
m
e
s
w
i
t
h
a
d
d
i
t
i
o
n
a
l
a
u
t
h
o
r
s
.
C
o
n
t
a
i
n
s
n
o
t
o
n
l
y
d
i
a
g
n
o
s
e
s
b
u
t
a
l
s
o
t
h
o
s
e
f
e
a
t
u
r
e
s
d
i
s
t
i
n
g
u
i
s
h
i
n
g
a
s
p
e
c
i
e
s
f
r
o
m
t
h
o
s
e
p
r
e
s
u
m
e
d
c
l
o
s
e
r
e
l
a
t
i
v
e
s
.
e
M
o
o
r
e
(
1
9
5
3
)
T
r
e
a
t
i
s
e
o
n
i
n
v
e
r
t
e
b
r
a
t
e
p
a
l
e
o
n
t
o
l
o
g
y
.
T
h
i
s
i
s
t
h
e
m
a
j
o
r
c
o
m
p
e
n
d
i
u
m
f
o
r
f
o
s
s
i
l
i
n
v
e
r
t
e
b
r
a
t
e
s
.
P
u
b
l
i
s
h
e
d
i
n
2
3
p
a
r
t
s
t
h
a
t
c
o
r
r
e
s
p
o
n
d
t
o
t
a
x
o
n
o
m
i
c
g
r
o
u
p
s
.
T
h
e
p
a
r
t
s
h
a
v
e
b
e
e
n
p
r
o
d
u
c
e
d
i
r
r
e
g
u
l
a
r
l
y
o
v
e
r
t
h
e
y
e
a
r
s
;
s
o
m
e
h
a
v
e
b
e
e
n
r
e
v
i
s
e
d
w
h
i
l
e
o
t
h
e
r
s
h
a
v
e
y
e
t
t
o
a
p
p
e
a
r
f
o
r
t
h
e
f
i
r
s
t
t
i
m
e
.
A
W
e
b
-
b
a
s
e
d
v
e
r
s
i
o
n
i
s
p
l
a
n
n
e
d
u
n
d
e
r
t
h
e
n
a
m
e
o
f
P
a
l
e
o
B
a
n
k
(
K
a
e
s
l
e
r
e
t
a
l
.
2
0
0
1
)
.
f
S
m
i
t
h
.
T
h
i
s
W
e
b
-
b
a
s
e
d
d
i
r
e
c
t
o
r
y
l
i
s
t
s
t
h
e
t
y
p
e
s
p
e
c
i
e
s
a
n
d
a
s
m
a
n
y
s
p
e
c
i
e
s
a
s
t
h
e
a
u
t
h
o
r
h
a
s
s
e
e
n
a
n
d
a
s
s
e
s
s
e
d
a
s
v
a
l
i
d
.
g
K
i
e
r
&
L
a
w
s
o
n
(
1
9
7
8
)
.
T
h
i
s
d
i
r
e
c
t
o
r
y
l
i
s
t
s
t
h
e
n
a
m
e
s
o
f
a
l
l
s
p
e
c
i
e
s
o
f
e
c
h
i
n
o
i
d
s
a
n
d
b
u
i
l
d
s
o
n
t
h
a
t
p
r
o
d
u
c
e
d
b
y
L
a
m
b
e
r
t
&
T
h
i
e
r
y
(
1
9
0
9
1
9
2
5
)
.
A
l
t
h
o
u
g
h
t
h
e
g
e
o
g
r
a
p
h
i
c
a
l
l
o
c
a
t
i
o
n
i
s
g
i
v
e
n
t
h
i
s
i
s
o
n
l
y
v
a
l
i
d
f
o
r
t
h
e
t
y
p
e
s
p
e
c
i
m
e
n
.
T
h
e
r
e
i
s
n
o
a
t
t
e
m
p
t
t
o
r
e
a
s
s
e
s
s
e
a
r
l
i
e
r
g
e
n
e
r
i
c
a
s
s
i
g
m
e
n
t
s
.
h
S
c
h
u
l
t
z
e
(
1
9
7
8
)
.
T
h
i
s
s
e
r
i
e
s
w
a
s
b
e
g
u
n
i
n
1
9
7
8
,
s
c
h
e
d
u
l
e
d
f
o
r
1
0
v
o
l
u
m
e
s
,
o
f
w
h
i
c
h
f
i
v
e
h
a
v
e
a
p
p
e
a
r
e
d
a
n
d
o
n
e
i
s
c
u
r
r
e
n
t
l
y
b
e
i
n
g
r
e
v
i
s
e
d
.
T
h
i
s
s
e
r
i
e
s
a
p
p
e
a
r
s
a
t
i
r
r
e
g
u
l
a
r
i
n
t
e
r
v
a
l
s
.
I
t
m
a
y
b
e
o
f
s
i
g
n
i
f
i
c
a
n
c
e
t
h
a
t
a
l
l
t
h
o
s
e
t
h
a
t
h
a
v
e
a
p
p
e
a
r
e
d
h
a
v
e
b
e
e
n
s
i
n
g
l
e
-
a
u
t
h
o
r
e
d
,
w
h
e
r
e
a
s
t
h
o
s
e
r
e
m
a
i
n
i
n
g
a
r
e
s
c
h
e
d
u
l
e
d
a
s
m
u
l
t
i
-
a
u
t
h
o
r
e
d
.
i
K
u
h
n
(
1
9
6
9
)
.
T
h
i
s
s
e
r
i
e
s
a
p
p
e
a
r
s
a
t
i
r
r
e
g
u
l
a
r
i
n
t
e
r
v
a
l
s
a
n
d
t
h
e
r
e
i
s
n
o
p
l
a
n
f
o
r
u
p
d
a
t
i
n
g
.
H
o
w
e
v
e
r
,
a
l
l
o
f
t
h
e
r
e
p
t
i
l
e
g
r
o
u
p
s
a
r
e
c
o
v
e
r
e
d
a
n
d
d
i
a
g
n
o
s
e
s
a
r
e
g
i
v
e
n
t
o
s
p
e
c
i
e
s
l
e
v
e
l
.
Phil. Trans. R. Soc. Lond. B (2004)
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
Classication of extinct taxa P. L. Forey and others 641
of family diversity through time, when foraminiferan data
are included with those of marine organisms in general.
It is quite common for individuals to continue annotat-
ing existing compendia for their own use. For example,
Woodwards Catalogue of fossil fishes (18891901) had
been annotated by different workers at the Natural History
Museum throughout much of the twentieth century. Such
annotation is, however, only available to a few: a difficulty
that may be alleviated by creating a digital facsimile.
The level to which taxa are identified varies between the
family and variety and very few include diagnoses/
descriptions at more than one Linnaean rank. Species-
level compendia do exist for many invertebrate groups but
they are usually uncritical listings of everything that has
been published. For example, Kier & Lawson (1978) list
every echinoid species described between 1925 and 1970
(supplementing an earlier compendium of echinoid spec-
ies by Lambert & Thiery 19091925), but they make no
assessment of whether these are valid taxa and the original
generic assignment is retained whether correct or not.
It is rare that full synonymies at any more than one hier-
archical level are included in the modern compendia,
although reference to primary literature is frequently
given. Nearly all of the compendia, or at least very large
taxonomic partitions, were single-authored, a fact
reflecting the expertise/taxon distribution. Those compen-
dia containing illustrations do so in very different forms.
The treatise on invertebrate paleontology (Moore 1953),
makes extensive use of photographs, whereas the Hand-
book of paleoichthyology (Schultze 1978) and Handbuch der
Palaoherpetologie (Kuhn 1969) use mostly line drawings.
Another consideration concerns the ease of tracing
names back to specimensthe bedrock of taxonomy. For
some compendia (e.g. Sepkoski 1992, 2002; Benton
1993) there is no intention to do so. For most of the
remainder, the literature citation to the original author of
species and genera is given, thus enabling backtracking.
The problem here is that the original literature may be
difficult to access. In some cases (e.g. most of the parts
of Kuhn 1969; table 1) individual holotype specimen
numbers and institutional locations are given, making it
very easy for researchers to isolate their collections of
nomenclatural interest. Increasingly, major holders of type
and figured material are placing information, including
photographs, on the Web, and we see this as a welcome
and indispensable aid. However, the task has barely
begun.
Therefore, although we can claim that palaeontologists
are competent at documenting the fossil record, we have
to admit that there is little interlinking between compen-
dia. They seem to stand alone, to be used by groups of
workers who have historically sorted themselves to special
disciplines within palaeontology. This alone may make it
difficult for a neontologist to use several of these compen-
dia to answer broad questions of past diversity.
3. THE MAGNITUDE OF THE PROBLEM FACING
TAXONOMISTS IN PALAEONTOLOGY:
HOW MANY SPECIES REMAIN UNDESCRIBED?
In some ways palaeontologists face a less daunting task
than neontologists in taxonomy. The fossil record is very
heavily biased towards animals with a robust or resistant
Phil. Trans. R. Soc. Lond. B (2004)
skeleton and plants that are woody, so large parts of the
tree of life are simply absent from the geological record.
Nichol (1977) estimated that ca. 8% of living animal spe-
cies had a skeleton and were therefore likely to be pre-
served. However, if we also acknowledge that scattered
through the geological record there are special deposits
(Konservat-Lagerstatten) where soft-bodied organisms are
fossilized, it is not unreasonable to assume that ca. 10% of
the biota might have entered the fossil record (Paul 1998).
There are some palaeoenvironments that are unlikely to
be preserved in the fossil record. Taxa from such sites will
rarely be sampled, because they have low fossilization
potential. Montane environments may have high biodiv-
ersity today, but being in predominantly erosional settings
their fossil equivalents are infrequent. Oceanic crust is
exceptionally preserved in the record before the break-up
of Pangaea during the Jurassic, so the fossil occurrences
of, for example, mid-ocean ridge biota depend on the rare
obduction of ancient ocean floor. Nonetheless, there are
occasional snapshots of this habitat as far back as the
Devonian (Little 2001). By contrast, habitats ranging
from alluvial plain and rift valley to continental slope have
a better record, from which a good proportion of skel-
etonized species that once existed can in principle be reco-
vered as fossils (Jablonski 1995).
A more pertinent question is how well palaeontologists
have sampled the fossil-bearing rock record for those
groups whose skeleton provides them with a high preser-
vation potential and which were living in suitable environ-
ments? Several methods can be used to estimate this,
including cumulative collection curves, gap analysis,
analysis of stratigraphic ranges (FreqRat), and these all
provide a relatively reassuring picture (Paul 1998). Fur-
thermore, estimates of genus- and family-level diversity
through time appear robust against sampling error. Sepko-
ski (1993) compared his original 1982 compilation of mar-
ine family diversity in the Phanerozoic with one compiled
10 years later, after considerable addition and correction.
Although the absolute numbers had changed, the overall
shape of the diversity graph through time remained
remarkably stable. Furthermore, an independent compi-
lation of families through time (Benton 1995) produced a
similarly shaped diversity plot. Although 10 years is a
rather short time for significant changes to have accumu-
lated, many palaeontologists feel that our knowledge of
taxonomic diversity through time, though not perfect, is
a reasonable representation of the true record.
A similar view emerges from an assessment of the tetra-
pod fossil record by Maxwell & Benton (1990; see also
Benton 1998). They used cumulative collection curves to
demonstrate that for better-studied parts of the world a
large proportion of the fauna has now been collected,
identified and described.
Others have looked at specific fossil assemblages to try
to obtain an estimate of how well sampled the rock record
is. The FreqRat method of Foote & Raup (1996) uses the
ratio of taxa confined to one, two and three stratigraphical
time-units to obtain a measure of the proportion of the
observed to predicted taxonomic range. Applied to spe-
cific monographic studies of trilobites, bivalves and mam-
mals they concluded that possibly 60% of the actual
species diversity has been recorded. Paul (1998) looked at
the proportion of new species in monographs that
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
642 P. L. Forey and others Classication of extinct taxa
Ammonoidea
Conodonta
Trilobita
Graptolithina
Blastozoa
Malacostraca
Polychaeta
Anthozoa
Porifera
Crinoidea
Gastropoda
Brachiopoda
Bryozoa
Echinoida
Bivalvia
Ostracoda
0.2 0.4 0.6 0.8 1.0
1.0
0.8
0.6
0.4
0.2
proportion of living families with a fossil record
p
r
o
b
a
b
i
l
i
t
y
o
f
g
e
n
u
s
p
r
e
s
e
r
v
a
t
i
o
n
p
e
r
s
t
r
a
t
i
g
r
a
p
h
i
c
i
n
t
e
r
v
a
l
Chondrichthyes
0
Figure 1. Estimates of completeness of the fossil record at
genus level based on two measures: (i) the proportion of
living families with a fossil record, and (ii) the probability of
genus preservation per stratigraphic interval based on a 107-
interval time-scale. (Modified from figs 1 and 3 in Foote &
Sepkoski (1999).)
represented genuinely new finds as opposed to the rein-
terpretation of earlier collected material and concluded
that only ca. 40% of new species were the result of new
collections. Foote & Sepkoski (1999) applied two inde-
pendent methods to estimate how complete a fossil record
major taxonomic groups had at the genus level. As
expected, some groups such as polychaetes have a very
poor record indeed, but taxa with a robust skeleton are
often well sampled (figure 1).
Finally, Kidwell & Flessa (1996) have investigated
details of how faithful are the death assemblages that
palaeontologists have to work with compared with those
seen in original communities today. Reassuringly, their
work suggests that most species with preservable hard
parts are in fact represented in local death assemblages
and commonly in the correct rank importance. Time aver-
aging on individual bedding planes is a problem but does
not obscure a good record of the natural range of com-
munity composition and structure.
Of course, the rock record that has been preserved and
is accessible to palaeontologists is only a small proportion
of what once existed. Thus, a major complicating factor
is that, although most organisms enter the geological rec-
ord, over time degradation of this record leads to the loss
of taxa. Furthermore, such degradation is not randomly
distributed through time, but is driven by cycles in Earth
history, particularly by sea-level cycles (Smith 2001). This
leads to a very heterogeneous distribution of well-sampled
and poorly sampled time-intervals as the proportion of
environments represented in the geological record varies
over time. Fossil preservation is also far from random over
time (e.g. Cherns & Wright 2000). An equally important
question therefore, is what proportion of the original fos-
silizable biota is captured by the geological record that we
have access to?
Following on from the pioneering work of Raup
(1976a,b), the recent finding that the diversity of fossils
Phil. Trans. R. Soc. Lond. B (2004)
through time is correlated with the amount of rock avail-
able for sampling (Smith 2001; Peters & Foote 2001)
raises the possibility that our view of species diversity over
time is, in large part, a reflection of a species/area effect.
Alroy et al. (2001) have started addressing this question
and found worrying evidence that raw taxon counts may
not be giving an accurate picture of biological diversity
over time, even for groups with a high preservation poten-
tial. A sizeable, and as yet unquantified, proportion of the
10% of taxa that begin with some reasonable potential of
being fossilized therefore are lost through destruction of
the geological record.
In summary, although only maybe 15% of species are
preserved in the geological record that survives today, the
specific palaeoenvironments that are represented have
generally been sampled efficiently, especially in the North-
ern Hemisphere. Recent studies suggest that more than
50% of the species in rocks available at outcrop are now
documented. Furthermore these biotas provide a generally
accurate snapshot of community structure and diversity.
Palaeontological taxonomists are not generally faced
with vast numbers of undescribed species waiting to be
plucked from the rock, except in the few parts of the world
that remain poorly explored palaeontologically. For mac-
rofossils, at least, it is more likely that new species will
come from the revision of existing material in museum
collections (Paul 1998). However, palaeontologists do
have to be aware of the large gaps that exist in coverage
that cannot simply be filled by better collecting.
4. NAMING SPECIES IN THE FOSSIL RECORD
In modern biodiversity studies it is common practice to
estimate both the amount of biodiversity that survives as
well as the probabilities of species extinction (Mace 1995;
May et al. 1995). These, in turn, are compared with
extinction rates and species longevity calculated from the
fossil record (e.g. Raup 1991; Jablonski 1995). In making
such comparisons there is the underlying assumption that
Recent and fossil species are, in some way
(phylogenetically, morphologically and geographically) the
same. In some cases this may be true. Coope (1995), in
documenting the extinction of Quaternary insects, specifi-
cally makes the point that such studies are precise because
the nature of the material is the same. Hence, the same
species concepts and recognition criteria can be used in
entomology and palaeoentomology. There are also other
cases of exceptional preservation where sufficient charac-
ters are preserved to enable the same criteria to be used
to distinguish fossil species as are used in the living fauna.
Amber, for example, preserves details of wing venation,
setae and genitalia in fossil insects in such detail that they
may be described with the same confidence as their living
relatives (e.g. bees; Engel 2001). In other cases the kinds
of characters used to define fossil species are commensur-
ate with those used in Recent taxonomy even though the
biological relationship may be distant. Among trilobites
with acute vision, for example, closely related species are
often distinguished by radical differences in surface orna-
ment, in a comparable manner to living decapod crus-
taceans.
However, from the broader palaeontological perspective
this may not be generally true. There are many species
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
Classication of extinct taxa P. L. Forey and others 643
concepts and recognition criteria used in palaeontology
and this leads to the named entities (species) representing
very different biological units. Such variability needs to
be taken into consideration when, for instance, tabulating
average species longevities in the fossil record (e.g. May
et al. 1995; table 1).
At one level, problems of species recognition are the
same as in the Recent world, even though they may be
more extreme in the fossil record. At another level there
are problems unique to recognizing fossil species.
Recent species concepts lead to different ways of
delimiting species (Wheeler & Meier 2000). The biologi-
cal species concept of Ernst Mayr (1942) is favoured by
most neontologists. However, most modern species are
not recognized on reproductive criteria. Instead, some
measure of morphological, behavioural or ecological dif-
ference is used as a surrogate for genetic incompatibility.
Thus, palaeontological species, recognized almost exclus-
ively on morphological differences, are in practice equally
as valid as modern species. However, there are several
problems and special cases.
(a) Recognition of species in the fossil record
(i) Fragmentary nature of fossils
Only a small part of the morphology is preserved, usu-
ally the skeleton, and this means that our view of total
morphological difference is both incomplete and distorted.
Extreme examples in the fossil record are belemnites,
conodonts and fish scales. Belemnites and conodonts rep-
resent a tiny fraction of the total morphology of the animal
and, as there are no Recent close relatives, we do not know
what range of skeletal morphology to expect in any one
palaeospecies. Belemnites show relatively little morpho-
logical variation and it is likely that we are severely under-
estimating the number of species at any one
stratigraphic level.
For conodonts, rare and fortuitously preserved assem-
blages demonstrate that very differently shaped cono-
donts, formally belonging to very different form taxa
belong to the same species (e.g. Klapper & Philip (1971)
were able to combine up to six nominal species to one
morphospecies). Although these assemblages are very use-
ful in associating some conodont species it is likely that
we have considerably overestimated the numbers of con-
odonts. Sweet (1988) estimated the number of named
conodont species to be almost 5000. However, after
adjusting for multi-element taxonomy this reduced to
1446 species (246 genera). Species erected on isolated fish
scales, or holothurian body-wall spiculation, suffer from
similar problems. Isolated conodonts and, at least, Palaeo-
zoic fish scales are also similar because they are used for
stratigraphic zonation and correlation and this leads to
inflation of species names, which can only be halted by
finding complete individuals. Indeed, some recently disco-
vered articulated thelodonts showed a body that included
scales previously identified on isolated scales as belonging
to two separate orders (Wilson & Caldwell 1998). Fortu-
nately, the practice of recognizing species on isolated
scales dramatically decreases for Mesozoic and Cenozoic
fishes.
The fragmentary and isolated nature of fossils some-
times leads to extreme complications where individual
parts have been described as separate species, placed in
Phil. Trans. R. Soc. Lond. B (2004)
completely different higher taxa. The Middle Cambrian
Burgess Shale of British Columbia has yielded several
enigmatic fossils. The giant arthropod predator Anomalo-
caris was known originally from its raptatory limbs. An
enigmatic jellyfish named Peytoia was described from the
same rock formation. Briggs (1979) finally proved that the
latter organism was actually the mouthparts of what was
probably the largest predatory animal in the Cambrian.
Perhaps the most extreme cases of fragmentary fossils
are seen in plants. Plants by their nature produce dispos-
able organs with a lifespan that is often much shorter than
the life of the individual. Organs such as leaves, cones and
flowers can be produced on an annual basis or continu-
ously throughout the year. In addition to these short-lived
parts, plants generate enormous amounts of pollen and
seeds or spores as part of their normal life cycle. Together
these organs and reproductive propagules are broadcast
into the environment in vast quantities providing by far
the greatest source of material for the plant fossil record.
The main difference therefore between species named for
living plants and those for fossils is that the former are
based on a connected set of organs that approximate to a
whole organism whereas the latter are, for the most part,
based on single organs and the nomenclature is designed
to deal with this. To this we must add an additional level
of complexity. Plant organs can be preserved in different
ways, and these can have quite different appearances. A
compressed specimen of a cone may look quite different
to one that is petrified, and more importantly it may pre-
serve a different but complimentary set of characters. With
fossil plants the mode of preservation is therefore also a
consideration, whereas this is irrelevant for their living
counterparts. These key distinctions have implications for
nomenclature (Chaloner 1986, 1999; Collinson 1986;
Meyen 1987; Greuter et al. 2000) as well as impacting on
how we interpret the meaning of named species of fossil
plants.
The problems of fragmentation and the vagaries of pres-
ervation inherent in the fossil record of plants have been
acknowledged by special provision through the many edi-
tions of the International Code of Botanical Nomenclature
(Botanical Code). In the current edition (Greuter et al.
2000) fossil plants may be treated as morphotaxaa fossil
taxon that, for nomenclatural purposes, comprises only
the parts, life-history stages or preservational states rep-
resented by the corresponding nomenclatural type
(Botanical Code article 1.2). In other words, plant parts
or different preservation states are recognized as distinct
taxa. For example, the genus Alethopteris is used as the
name for the foliage of certain Carboniferous seed ferns,
Laurocarpum is the name applied to a type of fossil endo-
carp (part of a seed) in the Eocene London Clay flora, and
Lagenicula is a genus of Carboniferous megaspore. One
consequence of morphotaxa is that different organs of the
same plant when found detached from one another are
assigned to different genera (Chaloner 1999). The roots
might receive one name, the bark another, cones, leaves,
wood, and spores or seeds yet more. The giant clubmosses
of the Carboniferous period provide a familiar example.
The bark and leafy branches may be called Lepidodendron
or Lepidoploios, the roots Stigmaria, the detached leaves
Cyperites or Lepidophylloides, and the fallen cones
Achlamydocarpon (female) or Lepidostrobus (male). Cones
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
644 P. L. Forey and others Classication of extinct taxa
Cystosporites
Lepidocarpon
Lepidostrobophyllum
female cone
Lepidostrobus
Lycospora
Knorria
Lepidophloios
Lepidophylloides
Stigmaria
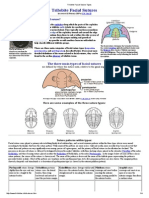
Figure 2. The naming of parts. Fossil plants exemplify well
the practice of naming different parts by different names.
Lepidodendron is a Carboniferous lycopod found as at least
nine kinds of morphotaxon, each given its own name
(Thomas 1981). (Reproduced, with permission, from Dr
Barry Thomas.)
may further fragment to give individual sporophylls (fertile
leaves) called Lepidostrobophyllum. The propagules come
in two types: megaspores might be called Cystosporites and
microspores Lycospora. Similarly, preservation state may
determine the genus to which a fossil can be assigned
(Chaloner 1999). The cones of the tree lycopod Sigillaria
are called Sigillariostrobus (compression) and Mazocarpon
(permineralization). The two states of preservation reveal
a different range of characters, and so they are defined in
different terms. The use of morphotaxa will inevitably
inflate the absolute number of plant species recorded in
fossil floras (figure 2).
The taxonomic status of a morphotaxon and its ability
to be placed within the hierarchy of plant life depend upon
the quality and quantity of information it displays. Some
morphotaxa are sufficiently well characterized to be placed
within families. These were formerly termed organ gen-
era (Chaloner 1999). Others are insufficiently well
characterized to be placed within a family, but it may be
possible to assign them to higher-level groups. Carpolithes
is a name used for certain types of dispersed seed. The
plants that produced Carpolithes can be placed in the class
Spermatophyta (seed plants), but the affinities within this
group are diverse. Previous codes of botanical nomencla-
ture named these form genera, a term that is now also
subsumed in the concept of morphotaxon (Greuter et al.
2000). Still other morphotaxa are clearly unnatural
groups. Dadoxylon is a morphogenus of fossil woods that
contains wood species from conifers in the Araucariaceae
as well as species from conifer-like plants in the extinct
Cordaitales. Based on wood anatomy alone, it may not be
Phil. Trans. R. Soc. Lond. B (2004)
possible to know to which family a species of Dadoxylon
belongs. Likewise, species of leafy shoots assigned to the
genus Brachyphyllum are attributable to conifers in the
Araucariaceae (extant) or Cheirolepidiaceae (extinct).
The specimens on which many species of Brachyphyllum
are based do not contain enough information to allow
them to be assigned to a family. Thus, some generic enti-
ties of fossil plants are not uniquely assignable to a family
either because they are insufficiently well characterized or
because they contain species from more than one family.
The latter are, for the most part, either polyphyletic or
paraphyletic groups.
The names of morphotaxa based on different organ
types can never be synonymized even when found to
belong to the same organism because morphotaxa can
only compete with those representing the same part, life-
history stage or preservational state (Botanical Code arti-
cle 11.7). The morphogenus Sigillaria, which was estab-
lished for bark fragments of a Carboniferous tree clubmoss
may, in part, represent the same biological taxon as the
morphogenus Mazocarpon, which was established for per-
mineralized cones. These generic entities can be used con-
currently. Furthermore, there is evidence that genera that
overlap conceptually have somewhat different ranges in
time, indicating that one suite of characters became
extinct or modified before the other. Lepidodendron acutum
bore cones of Flemingites russellianus containing mega-
spores called Lagenicula rugosa. These three genera do not,
however, have the same stratigraphic ranges (figure 3).
The first occurrence of Lagenicula predates significantly
that of Flemingites. We cannot assume that species of all
three genera were borne on the same type of plant and we
cannot simply treat them as synonyms (Chaloner 1986).
Following current practice therefore, synonymy cannot
come to the rescue, correcting for taxonomic inflation by
cutting out surplus species names. Furthermore, in most
instances, the plant that produced the partsthe concep-
tual whole organismdoes not have its own formal name.
In a further wrinkle to a complex nomenclature, not all
isolated plant parts are assigned to morphotaxa. Many leaf
fossils of the Tertiary Period can be placed in extant gen-
era. These genera are, of course, based on extant types
housed in herbaria. The practice of assigning fossil plants
to extant genera has had a chequered history that is plag-
ued by inconsistencies (Collinson 1986). Some authors
prefer not to place fossils in extant genera because the fos-
sil is only a part of the plant. How do we know that the
rest of the plant would conform in all its particulars to
the diagnosis? Carried to its logical conclusion, this would
mean that extant genera do not have a fossil record.
Others argue that one should use extant genera if the plant
part is consistent with this placement, even though most
of the organism remains unknown. The living genus
Equisetum is seen by many as recognizable in the Late
Mesozoic. Others assign Mesozoic species to the extinct
genus Equisetites, even though no morphological distinc-
tion is recognized between the two. This latter practice
might also lead to taxonomic inflation.
In palaeobotany, the nomenclature that has been
developed to deal with the fragmentary nature of fossil
plants and the different preservation states yields taxo-
nomic units that are mostly fundamentally different to
those of living plants. It is rare that the holotype of a fossil
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
Classication of extinct taxa P. L. Forey and others 645
Lagenicula
rugosa
Lepidostrobus
(Flemingites)
russellianus
Lepidodendron
acutum
spore cone tree
L. rugosa
L. agenicula
F. russellianus
Flemingites
L. acutum
Lepidodendron
P
e
r
m
.
C
a
r
b
o
n
i
f
e
r
o
u
s
D
e
v
o
n
i
a
n
Penn.
Miss.
Fam.
Fras.
Figure 3. The relationships among three morphotaxa (genera and species) of fossil plants and their stratigraphic ranges. The
species are believed to represent parts of a single species. Note that the three genera have different stratigraphic ranges. If
these ranges are correct, the genera cannot be regarded as synonyms. (Reproduced from Chaloner (1986) with the permission
of the author.)
resembles the holotype of a living species. For the most
part, fossil taxa are based on plant parts or organs. The
use of morphotaxa tends to inflate the absolute number
of plant species recorded in fossil floras. All things being
equal, one might expect similar levels of inflation through-
out the fossil record, so it is conceivable that the overall
shape of broad changes in plant diversity through time
might remain largely unaffected. However, in some cases
using separate parts may give rise to different diversity
curves as has been found, for instance, by Crane &
Lidgard (1990) when they compared the pattern of angio-
sperm diversification as measured by macroflora (leaves,
etc.) and by palynofloras (pollen). At the very least, one
can see how the diversity of large plants producing many
parts might be overestimated. Also, by comparison, spec-
ies diversity might be underweighted before periods of
exceptional innovation, such as the Devonian period when
many new organ systems first appeared.
(ii) Lack of spatial continuity
Fossils do not provide us with the same level of geo-
graphical continuity as Recent organisms. Therefore, it is
not possible to recognize clinal variation and, conse-
quently, geographically isolated finds are often interpreted
as different species, whereas in reality they may only rep-
resent geographical varieties of a single species. This is
also a problem in the modern world but here neontologists
do have the theoretical possibility of checking for this.
(iii) Lack of ontogenetic continuity
Palaeontologists, in general, are hampered by the lack
of knowledge of ontogenetic variation. Growth stages of
individual taxa have received separate names, which may
spuriously increase apparent diversity. Trilobites grow
from larvae a millimetre long to adults some centimetres
in length, during which ontogeny most of the features
Phil. Trans. R. Soc. Lond. B (2004)
change. The Ordovician trilobite genus Asaphoon Hutchi-
son & Ingham, 1975 was demonstrated to be no more
than a growth stage of a well-known form Asaphellus by
Fortey & Owens (1991). The Middle Cambrian trilobite
Sao acquired no less than seven synonyms in this manner.
Once again, this can, and indeed has, happened in the
modern world, particularly where there are morphologi-
cally and ecologically distinct larval stages, but the prob-
lem is far more acute in the fossil record and can only be
solved by fortuitous finds.
(iv) Parataxonomies
Other more specialized types of species taxonomy have
developed in the area of trace fossils including footprints,
burrows, borings, coprolites, enterospirae, etc.
(ichnotaxonomy). Currently the names of ichnotaxa are
binomials and are covered by the International Code of
Zoological Nomenclature (Zoological Code) (ICZN
1999) up to the genus level, although the species, genera,
etc. are not based on patterns of evolutionary descent and
sometimes can cross major biological boundaries. Never-
theless, some consider that Linnaean taxonomy should be
applied (Rindsberg 1998). It is universally recognized that
such taxonomy is artificial and such species are not used
in species counts or diversity indices. They are, however,
extremely useful in palaeoecological and sedimento-
logical studies.
(v) Virtual species
In very recent times a new kind of species has surfaced
in palaeontologythe virtual species. There are excep-
tional cases in which an imageand one readily available
on the Webhas become the description of a fossil taxon.
Reconstruction of superbly preserved three-dimensional
fossils from the Silurian of the Welsh borderland has been
based upon computer reconstructions from serial sections
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
646 P. L. Forey and others Classication of extinct taxa
Figure 4. The virtual fossil of a supposed aplacophoran
mollusc Acaenoplax hayae (Sutton et al. 2001), ca. 16 mm
long, from the Silurian of the Welsh Borderland. This image
was prepared from computer reassembly of serial sections.
(Courtesy of D. J. Siveter.)
that destroy the original specimen; the result is an image
that exists only virtually (Sutton et al. 2001). The intact
holotype specimen is retained for nomenclatural purposes
but the diagnosis is realized by the virtual image (figure 4).
(b) Species and time
The fact that the fossil record allows sampling through
time raises a whole set of taxonomic issues and problems
that are unique to palaeontology. These revolve around
how lineages are partitioned into taxonomic units and
what this means for extinction studies.
(i) Time and species recognition
The evolutionary species concept of Simpson (1961, p.
153) recognizes a species as an ancestordescendent
sequence of populations changing through time with its
own trends and tendencies. Here, a succession of sampled
populations each encompassing a slightly different range
in morphology follow one another. Such continua are
often divided into segments called species. Sometimes
these segments are delimited by comparison with the
amount of morphological variation expected in modern
representatives (Simpson 1961, p. 165) although clearly
this will only work with taxa having close Recent represen-
tatives. Sometimes they are delimited by arbitrary seg-
ments of time (e.g. every 5 million years)the
chronospecies (e.g. Gingerich 1979). More commonly,
the continuum is divided by stratigraphic occurrence and
this is particularly so in those organisms taken from strata
that accumulated in oceanic environments. Here, the
palaeontologist has an increased likelihood of complete
sampling of fossil populations through geological time.
Planktonic organisms are naturally preserved in such
Phil. Trans. R. Soc. Lond. B (2004)
environments, and the taxonomic treatment of their fossil
representatives has emphasized stratigraphical order and
statistical characteristics of successive populations in their
species concepts. Lineages of such species are routinely
and successfully used for stratigraphic correlation of
strata. Planktonic foraminiferans in Palaeogene and Neo-
gene strata and coccoliths are typically treated this way.
The planktonic, colonial graptolites typical of Ordovician
and Silurian deep-water strata exhibit similar chronostrati-
graphic lineages (figure 5; Cooper & Ni 1986).
In yet other instances the species are recognized because
of the breaks in stratigraphic sequence due to non-
deposition/erosion. All of these ways of recognizing species
are very different from species recognition in the modern
world. Thus the entities to which we give names may not
be comparable, either from one palaeontological study to
another or when comparing the Recent world and the fos-
sil record. At the very least, the different ways of recogniz-
ing species may have implications for our estimates of
species longevity.
(ii) Species extinction
Biologists are sometimes faced with the problem of
deciding whether an organism has truly gone extinct or
not, with uncertainty revolving around whether a remnant
population may be persisting unseen in some remote part
of the world. A similar problem of sampling also compli-
cates the interpretation of first and last occurrences of
species in the geological record, although it is possible to
calculate appropriate confidence intervals to range ends
(see Marshall 1998).
However, palaeontologists interested in extinction pat-
terns face a much more serious problem: not all last occur-
rences in the fossil record represent biological extinction.
This arises because of the way in which taxonomists have
subdivided lineages into chronospecies (see above). When
the fossil record is patchy, species are often known from
just a few specific horizons, and the gaps in their strati-
graphical distribution create convenient break points. A
good example of such a lineage is provided by the species
of the sea urchin Hagenowia from the Upper Cretaceous
Chalk (figure 6).
Here, a succession of species each slightly different in
morphology, follow one another through time. Because
each morphospecies is effectively confined to a short time-
horizon, it is likely that we are dealing here with samples
of a single, continuously evolving lineage. Consequently,
all but the terminal species end by taxonomic convention,
because genetic continuity is obviously maintained. Spe-
cies that disappear as a result of taxonomists partitioning
of a lineage are lost through pseudoextinction, which
should not be confused with genuine biological extinc-
tionthe loss of a genetic line.
Pseudoextinction is not confined to taxa at species level
but can occur wherever a monophyletic clade has been
abstracted from a larger clade so as to leave a plesio-
morphic basal remnant. Historically, paraphyletic groups
abound in the taxonomic literature, and where such
groups end stratigraphically earlier than the first appear-
ance of their derived sister group their termination marks
a pseudoextinction.
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
Classication of extinct taxa P. L. Forey and others 647
P. bellulus P jiangxiensis P. dumosus B
Apiograptus
harrisi
janus
manubriatus
texanus
P. tau
koi
P. gracilis
P. dumosus A
P. hastatus
?
P? geniculatus
P? aggestus
P? menaiensis
P? angel
victoriae
maximus
I
.
v
i
c
t
o
r
i
a
e
l
i
n
e
a
g
e
Pseudisograptus
manubriatus group
Y
a
p
e
e
n
i
a
n
C
a
s
t
l
e
m
a
i
n
i
a
n
m
a
x
i
m
o
-
d
i
v
e
r
g
e
n
s
m
a
x
i
m
u
s
v
i
c
t
o
r
i
a
e
Kalpinograptus
Glossograptina
Figure 5. Stratigraphic species. Collections of graptolites in successive strata showing gradual changes in modal morphology
give rise to phyletic lineages, which are named here as separate species according to the stratigraphic horizon in which they
occur. (Redrawn from Cooper & Ni (1986).)
Maastrichtian
Campanian
Santonian
Conacian
Turonian
Cenomanian
B. mucronata
G. quadrata
O. pilula
M. testudinarius
U. socialis
M. coranguinum
M. cortestudinarium
S. plana
T. lata
I. labiatus
U
p
p
e
r
C
r
e
t
a
c
e
o
u
s
Hagenowia
blackmorei
Hagenowia
anterior
Hagenowia
rostrata
Figure 6. The succession of species of the echinoid
Hagenowia in the chalk of western Europe. Fossil
populations come from only a small number of discrete
horizons and the two lower species end through
pseudoextinction.
Thus, simply counting last occurrences of taxonomic
names from the literature is likely to grossly overestimate
extinction levels, and an understanding of the phylogen-
etic relationships of taxa is required to estimate true levels
of biological extinction in the geological past.
Phil. Trans. R. Soc. Lond. B (2004)
5. THE USE AND MISUSE OF TAXONOMIC RANK
IN PALAEONTOLOGY
In this section we highlight some of the problems of
using supraspecific fossil taxa as data for diversity studies.
Most of the problems concern the use of ranks in classi-
fications and how those ranks may be affected by our
changing ideas of relationships between taxa.
(a) Comparison of ranks and species durations
The preamble to the Botanical Code is clear about the
purpose of naming groups: The purpose of giving a name
to a taxonomic group is not to indicate its characters or
history, but to supply a means of referring to it and to
indicate its taxonomic rank. (Greuter et al. 2000, pre-
amble, p. 1). Few would dispute the need for a name for
reference purposes, but the need to allocate taxonomic
rank is altogether a more controversial issue, particularly
at hierarchical levels above the genus (see de Queiroz &
Gauthier 1992; Mishler 2000). The taxonomic rank of a
group specifies its level in the Linnaean hierarchy accord-
ing to a rank order convention, which needs to be matched
to the underlying pattern of taxonomic groups as they are
discovered. Straightforward as this may seem, several
problems emerge. Taxonomic ranks within the Linnaean
hierarchy are arbitrary, meaning that they cannot be used
as comparable (objective) entities. A family of beetles and
a family of primates can represent very different concepts.
The rank given to a clade depends upon a complex set
of factors, including perceived morphological difference
from its close relatives, numbers of included species,
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
648 P. L. Forey and others Classication of extinct taxa
stratigraphical distance from closest relatives, extent of
work carried out on the group, etc. Assigning different
groups to similar Linnaean ranks may falsely imply histori-
cal equivalence. For instance, class Reptilia and class Aves
are very different entities, the former being paraphyletic
with some of its members (e.g. crocodiles) genealogically
more closely related to members of the class Aves than
they are to other members of the class Reptilia. Unfortu-
nately, in palaeontology the non-equivalence in morpho-
logical diversity, species numbers, stratigraphic duration
and genealogical history is often overlooked, and diversity
analyses that are based on counts of supraspecific taxa
make the unfounded assumption that taxa of similar rank
are directly equivalent units. Most of the compendia
include higher classifications that are of very variable con-
tent, with named monophyletic, paraphyletic, and even
polyphyletic groups, and these represent groupings of vari-
able scope in terms of both morphological and taxo-
nomic diversity.
Misuse of taxonomic rank data abounds in the
palaeontological literature. For example, counts of famil-
ies recorded per geological time-interval are often used as
proxies for species diversity, and the percentage drop of
recorded families is used as a measure of the proportion of
species loss at mass extinctions (Raup & Sepkoski 1982).
However, this assumes that 100 families in the Ordovi-
cian, on average, encompass the same number of species
as a 100 families in the Miocene. Yet we know that species
and family diversity curves follow different trajectories
over the Phanerozoic, with families in the Neogene con-
taining considerably more species, on average, than famil-
ies in the Palaeozoic (Flessa & Jablonski 1985). This
temporal bias means that family diversity at different times
in the geological record does not, on average, encompass
equivalent levels of basal (species) diversity.
It is important to realize that even at the species level
there are no grounds for considering taxonomic entities as
objectively equivalent in palaeontology. As discussed
above ( 4) morphospecies boundaries are, to a large
extent, dictated by the amount of morphological com-
plexity that is preserved, and a species of belemnite for
example represents a very different level in the biological
hierarchy than a species of amber-entombed insect. The
more complex the hard-part morphology a clade displays,
the more potential there is to create a more finely subdiv-
ided taxonomy. This is important because some people
(e.g. Stanley 1979) have used mean species duration in
the fossil record as a measure of biological species dur-
ation and evidence of a scala naturae. The fact that corals
and bivalves have a mean species duration of 1520 Myr
whereas trilobites, insects and mammals have a mean
species duration of less than 5 Myr, however, probably
tells us more about the complexity of the preservable hard-
part morphology and the limitations facing taxonomists
than about biological species duration.
(b) Problems of instability in naming taxa
The importance of using only monophyletic groups for
studies of patterns of past diversity has been demonstrated
by Patterson & Smith (1987) and Smith & Patterson
(1988). However, even acknowledging the desirability of
monophyletic groups there remain problems of naming
them as a result of changing ideas of phylogenetic
Phil. Trans. R. Soc. Lond. B (2004)
relationships and/or the introduction of newly discovered
fossils into an existing phylogeny. As sampling of diversity
improves, and as knowledge of the relationships among
both living and extinct organisms advances, the hierarchy
of relationships that one might need to express in a classi-
fication increases.
In comparing his two compendia of families of marine
organisms Sepkoski (1993, 2002) noted the substantial
differences in supraspecific classification that had
occurred during the intervening 10 years. In part, this was
due to changes in phylogenetic hypotheses, which were
reflected in the erection of new names of higher taxa as
well as the very different content of named groups. In
other cases, new names were erected to embrace knowl-
edge of newly discovered fossils. Such instability in classi-
fications causes difficulties when making comparisons
between different databases. Of course, this is a problem
associated with neontological as well as palaeontological
taxa. However, classifications involving fossils are parti-
cularly prone to instability. The inclusion of new fossils
often places them in stem positions; that is, as sister
groups to already existing named clades. To reflect this in
a standard Linnaean-ranked classification a new rank and
name may be required and can have a cascade effect,
demanding changes in the more inclusive ranks. Taxo-
nomic changes at one level may force a cascade of rank
changes further up or down the hierarchy. These ripples in
the nomenclature by themselves do not convey any useful
information. They are simply a necessary consequence of
the rank order convention. No new group is circum-
scribed; no information is added to the classification.
There have been a variety of suggestions to lessen the
impact of classificatory instability caused by relying on
Linnaean rank. Farris (1976) devised a system of prefixes
for the standard Linnaean ranks (family, order, etc.)
which, when used in combination (e.g Mega/hyper/
sub/super Family), could provide an almost limitless num-
ber of ranks. Comparable suggestions have been to switch
to a numbering system whereby numbers could be
inserted to signify additional ranks (Hennig 1966; Lvtrup
1977). Both of these systems, however, although logical,
really only exacerbate the problem of rank proliferation.
Another suggestion is to ignore the rank and use a sequen-
cing convention (Nelson 1972) in combination with the
rankless modifier plesion (Patterson & Rosen 1977). Ple-
sion can be associated with any Linnaean rank and simply
means that it is to be considered as the extinct primitive
sister taxon to the taxon listed below in the written classi-
fication. In other words, they considered the rank of the
fossil group to be decoupled from that of the rest of the
hierarchy. A third way is to abandon the Linnaean rank
order convention and simply give names to groups (nodes
on a phylogenetic tree) as and when required (for
example, if one wished to discuss the evolution of a
character complex or biogeographic history) (see also Fin-
lay 2004). Name endings would no longer have a mean-
ing. Classes, orders, families, etc. would disappear from
the taxonomists vocabulary and from the various codes.
Grouping would be expressed either by reference to a cla-
dogram or in a tabular form using indentation. This
approach was adopted by Crane & Kenrick (1997) in their
classification of fossil and Recent land plants. A fourth
way is to abandon Linnaean rank and to construct names
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
Classication of extinct taxa P. L. Forey and others 649
division Teleostei
superorder Elopocephala
superorder Osteoglossomorpha
order Hiodontiformes
family Hiodontidae
order Osteoglossiformes
suborder Osteoglossoidei
family Osteoglossidae
family Notopteridae
suborder Mormyroidei
division Teleostei
superorder Elopocephala
superorder Osteoglossomorpha
plesion genus Lycoptera
order Hiodontiformes
family Hiodontidae
suborder Mormyroidei
plesion genus Lycoptera
suborder Osteglossidae
family Osteoglossidae
family Notopteridae
division Teleostei
magnaorder Elopocephala
magnaorder Osteoglossomorpha
superorder Lycopteromorpha
family Lycopteridae
genus Lycoptera
superorder Osteoglossomorpha
grandorder Hiodontiformes
family Hiodontidae
grandorder Osteoglossiformae
order Osteoglossiformes
suborder Joffrichthyidei
family Joffrichthyidae
genus Joffrichthys
suborder Osteoglossoidei
family Osteoglossidae
family Notopteridae
order Mormyriformes
suborder Mormyroidei
division Teleostei [L]
superorder Elopocephala [L]
Osteoglossomorpha [P(stem-based)]
Lycoptera
order Hiodontiformes [L]
family Hiodontidae [L]
Osteoglossiformes [L]
Osteoglossoidei [P(stem-based)]
Joffrichthys
family Osteoglossidae [L]
family Notopteridae [L]
suborder Mormyroidei [L]
E
l
o
p
o
c
e
p
h
a
l
a
H
i
o
d
o
n
t
i
f
o
r
m
e
s
O
s
t
e
o
s
g
l
o
s
s
i
d
a
e
N
o
t
o
p
t
e
r
i
d
a
e
M
o
r
m
y
r
o
i
d
e
i
Joffrichthys
Lycoptera
E
l
o
p
o
c
e
p
h
a
l
a
H
i
o
d
o
n
t
i
f
o
r
m
e
s
O
s
t
e
o
s
g
l
o
s
s
i
d
a
e
N
o
t
o
p
t
e
r
i
d
a
e
M
o
r
m
y
r
o
i
d
e
i
(a) (c) (e)
(b) (d) ( f )
Figure 7. Effects on introducing fossils into classifications. Fossils often occupy stem positions in phylogenies leading to
difficulties in classifying the whole group. (a) Phylogeny of a primitive living group of teleost fishesthe Osteoglossomorpha
with the sister group, Elopocephala. (b) The introduction of two genera (in this case the fossil monospecific genera Lycoptera
and Joffrichthys) can cause difficulties if the classification is to reflect the phylogeny. (c) A standard ranked Linnaean
classification of the living taxa. (d) An altered Linnaean-ranked classification designed to reflect the phylogeny. This
incorporates intermediate ranks such as grandorder and magnaorder (ranks after McKenna 1975) as well as changing the rank
of already-established names. (e) An alternative method using the annotated Linnaean system where the phylogeny is read
from the classification as the sequence of branching is given by the order of listing, with the inclusion of the rankless category
plesion for the fossils as meaning plesiomorphic sistergroup. ( f ) An alternative using the PhyloCode system whereby taxa are
defined relative to a particular phylogeny. Here, the fossils can be incorporated by using a stem-based definition: for example,
Osteoglossomorpha could be defined as all those taxa more closely related to Hiodon alosoides (Hiodontiformes) than to Elops
saurus (Elopocephala). In the annotated Linnaean system the traditional Linnaean ranks need have no hierarchical relationship
to one another. Under PhyloCode nomenclature the ranks are meaningless and could be eliminated. Indeed, this is
recommended if the name is to be used in a PhyloCode sense. [L] and [P] refer to a notation to indicate under which system
(Linnaean or PhyloCode) the name is to be used.
with a specific phylogenetic definition tied to a particular
phylogenetic hypothesis. This last is the method suggested
by the PhyloCode (Cantino & de Queiroz 2000) and has
been much discussed in general (de Queiroz & Gauthier
1994; Bryant & Cantino 2002) and from a palaeonto-
logical perspective (Benton 2000; Brochu & Sumrall
2001; Dyke 2002). A rank-free classification does not
overcome the problem of competing classifications of the
same hierarchy. In either rank-free or rank-order usage a
taxonomic name cannot therefore stand unambiguously
alone. It needs to be accompanied by reference to a parti-
cular classification. Figure 7 provides an example of the
inclusion of fossil taxa with Recent organisms and the
Phil. Trans. R. Soc. Lond. B (2004)
classificatory consequences of following some of the differ-
ent strategies.
6. THE WAY FORWARD
In the preceding text we have highlighted many of the
problems in naming and classifying fossil taxa. In this con-
cluding section we offer some suggestions as to how we
may proceed. We do so in the context that fossil and
Recent taxa need to be classified together in a common
hierarchy that contains information about past as well as
Recent biodiversity (we therefore distance ourselves from
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
650 P. L. Forey and others Classication of extinct taxa
those who have suggested fossils be classified quite separ-
ately from Recent organisms; e.g. Crowson (1970)).
(a) The species
We have highlighted problems faced by palaeontologists
in trying to identify and name species. Many of these
problems, such as morphotaxa, different species rep-
resenting parts of the same organism or stages of life his-
tories will be difficult to overcome. One possibility is to
make the nature of these taxa more explicit at their point
of use (Collinson 1986). One could, for example, always
indicate the organ type or life cycle part along with the
binomial and its author on the first citation (e.g. Genus
species author [seed], Genus species author [larva]). For
chronospecies and stratigraphic species we suggest that
their use be discouraged and that species be established
on unique combinations of characters as advocated by
Wheeler & Platnick (2000). This will allow closer com-
parison with Recent species. We also recommend that
original descriptions should contain specific designation
of paratypes (recommended by the codes) along with the
holotype (demanded by the codes). This is because indi-
vidual fossils rarely display all of the features regarded as
diagnostic by the original author.
(b) Higher taxa
Most of the compendia on fossil taxa do contain some
form of higher classification to at least family level (often
more inclusive). Rarely, however, is there any indication
of whether the author(s) consider individual taxa to be
monospecific, monophyletic, paraphyletic or polyphyletic,
or indeed unknown. We recommend this information be
included as standard practice as this will avoid the many
deductions about diversity through time being based on
disparate and sometimes misleading data. To address the
problem of competing classifications of the same or similar
hierarchical structures, we recommend that the name of a
higher taxon always be accompanied on first citation by
reference to a classification. However, we stop short of
recommending adoption of the PhyloCode.
There is also a compelling case to be made for suggest-
ing that palaeontologists concentrate on compiling anno-
tated lists of genera rather than species (see below).
Genera are easier to recognize and free from many of the
species problems outlined above ( 4a). This, of course,
means that we support the maintenance of the binomial
(cf. Cantino et al. 1999).
(c) The case for Web-based data
Like many of our neontological colleagues we would
like to see the World Wide Web playing an increasingly
important role for the dissemination of taxonomic data on
fossils. Palaeontology would benefit from having a Web-
based taxonomy for exactly the same reasons that are cited
by neontological taxonomists (Scoble 2004), namely:
Web-based taxonomic databases can significantly reduce
the time lag between the acquisition and dissemination of
knowledge. For example, in the decade after Sepkoski first
published his family-level compilation of marine animals,
45% of the stratigraphic records alone were altered (owing
to reassignment, more refinement and addition of new
stratigraphic units; Sepkoski 1993). Thus, some of that
newly identified data were already up to 10 years out of
Phil. Trans. R. Soc. Lond. B (2004)
date when published in 1992. The ability to constantly
update taxonomic data is an obvious advantage of the
Web, but of course this assumes that there are dedicated
experts willing and able to undertake this task.
The pertinent primary literature for fossil species is scat-
tered through a multitude of books and journals, many of
them restricted to specialist libraries. If basic taxonomic
information can be placed on the Web it will help stan-
dardize the use of names by allowing easier access to criti-
cal data by many more people. Web-based lists make it
potentially easier to collate information (i.e. numbers of
genera/species from named horizons, etc.) with the possi-
bility of calculating rates of origination and extinctions
(e.g. the Web-based Fossil record 2, http://palaeo.gly.
bris.ac.uk/frwhole/FR2.html).
The description of fossil taxa can often involve reference
to many different partial specimens so as to capture the
complete morphology or the variation encompassed by
different preservation styles (see 4a). The unlimited
space for illustration on the Web is clearly an advantage.
There are, of course, some potential disadvantages of a
Web-based taxonomy but none is unique to palaeon-
tology. The host server has to be secure (Kaesler et al.
2001) and prepared to support the taxonomic site as a
long-term commitment, and Web sites need to be actively
maintained if they are to meet the aspirations of the scien-
tific community. The relative ease, flexibility and timeli-
ness of setting up Web sites encourages their birth. But,
this needs to be followed by nurturea continued invest-
ment in building, maintaining and updating. The cost
both in terms of commitment from individuals and finance
should not be underestimated. For example, the Plant
Fossil Record database (http://ibs.uel.ac.uk/ibs) was estab-
lished in 1992 with the aims of making available formal
descriptions of plant fossil genera and additional details
on their publication, their stratigraphic and geographical
distributions and their classification. Unfortunately,
10 years later this ambitious project appears to have
stalled, and there are still a great many gaps in the data
such that the aims of the database are by no means fully
met.
A related issue concerns archiving the data submitted
to Web sites. One of the advantages of hard paper copy
is the relative permanence of such a medium. We have a
continuous trail of taxonomic literature dating from before
Linnaeus. Improved techniques of paper conservation are
being developed with as much enthusiasm as there is will-
ingness to preserve paper records. We are less confident
about electronic copy, although the medium is too young
to be able to assess this critically. Archiving may be an
issue when the site is used as a repository for taxon distri-
butions. Different iterations of data applied to a species
or genus, etc. may vary (e.g. geographical and strati-
graphic data). It may be important to retain such data
(with dates) in a manner similar to the revisions of mono-
graphic treatments. There is, as far as we know, no stan-
dardized protocol for archiving electronic data.
The major outstanding administrative problem to be
solved is how Web-based taxonomy is to gain validation.
Taxonomic data can be posted on the Web without pass-
ing through any review process. To avoid a flood of poorly
construed taxa, it might be necessary to establish
accredited host sites and/or panels of experts who could
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
Classication of extinct taxa P. L. Forey and others 651
ensure quality control. None currently exists. In the
immediate future, the best way forward is to concentrate
on developing the Web for disseminating information
about established species rather than as an outlet for new
taxa. The best taxonomy has always come from individ-
uals with the experience and breadth of knowledge to pro-
vide an authoritative overview, and it is our belief that
data-rich and scientifically useful sites will become self-
evident to the wider community.
From our own experience the process of assembling a
database of images and accompanying text is really very
straightforward, but more than this is needed for a taxo-
nomic site to aid and direct the non-specialist. However,
all this requires care and attention to keys and cross-refer-
encing, and the design and construction of a user-friendly
framework to a taxonomic site takes considerably longer.
An important consideration of Web-based reference
taxonomy is therefore the feasibility of its goals. In our
experience taxonomic problems associated with defining
species are manifestly more complex and difficult than
those associated with monophyletic clades. Species-level
taxonomy usually requires data on large numbers of indi-
viduals to encompass ontogenetic, preservational, strati-
graphic and geographical variability, and is often based on
very subtle character assessments (see 4 for inherent
problems for species assessment). Consequently, species
boundaries are rarely unambiguous and obvious. By con-
trast, generic and higher taxonomic levels are usually
established on the basis of more major character traits that
are easier to define and illustrate (see also Boulter 2003).
Whereas we see a Web-based taxonomy at genus level and
above being relatively easily achieved, the goal of placing
all species on the Web seems massively over-ambitious
given present resources devoted to taxonomy.
(d) The need for specimen care
In general, fossil species are based upon type material
in recognized collections, and it is a stipulation for most
publications that material typifying a new species is
deposited in such a collection. We do not envisage a
change in this procedurefor example, to Web-based
typification. Fossil material is generally stable and perma-
nent, and frequently has a uniqueness that distinguishes
it from that forming the basis of Recent species. Re-collec-
tion of material from type localities is something that can-
not be taken for granted. Nor are synonymies produced by
revisions of fossil taxa ever objectively final. The material
needs to be available as the ground truth in perpetuity.
Some of the greatest biologists of the nineteenth century
left their legacy not merely in names, but in great collec-
tions which have been consulted periodically ever since
(Darwin on barnacles; Barrande on trilobites; Davidson
on brachiopods).
It is important that palaeontological collections are safe-
guarded for future revision. In some cases, taxonomic
research collections made by individuals and deposited in
university collections may be endangered when different
aspects of science rise to prominence in the same insti-
tution, and they are forgotten. The same applies to small
museums that do not know quite what they have. It would
be an advantage to gather such collections together under
a national umbrella where their future is guaranteed.
Phil. Trans. R. Soc. Lond. B (2004)
The authors thank their colleagues in the Department of Palae-
ontology for supplying information about fossil compendia and
databases in palaeontology. The value of an institution like the
Natural History Museum is that there is expertise on tap.
They also thank Mark Purnell (University of Leicester) for
information about conodont taxonomy, as well as comments
from two referees.
REFERENCES
Alroy, J. (and 24 others) 2001 Effects of sampling standardiz-
ation on estimates of Phanerozoic marine diversification.
Proc. Natl Acad. Sci. USA 98, 62616266.
Benton, M. J. 1993 The fossil record 2. London: Chapman &
Hall.
Benton, M. J. 1995 Diversification and extinction in the history
of life. Science 268, 5258.
Benton, M. J. 1998 The quality of the fossil record of ver-
tebrates. In The adequacy of the fossil record (ed. S. K. Dono-
van & C. R. C. Paul), pp. 269303. London: Wiley.
Benton, M. J. 2000 Stems, nodes, crown clades, and rank-free
lists: is Linnaeus dead? Biol. Rev. 75, 633648.
Boulter, M. C. 2003 Against the plant species. Cour. Forsch.-
Inst. Senckenberg 241, 319325.
Boureau, E
. 19641975 Traite de paleobotanique, vols 24.
Paris: Masson et Cie.
Briggs, D. E. G. 1979 Anomalocaris, the largest known Cambr-
ian arthropod. Palaeontology 22, 631664.
Brochu, C. A. & Sumrall, C. D. 2001 Phylogenetic nomencla-
ture and paleontology. J. Paleontol. 75, 754757.
Brodkorp, P. 19631978 Catalogue of fossil birds. Bull. Fla St
Mus. Biol. Sci. 723.
Bryant, H. N. & Cantino, P. D. 2002 A review of criticisms of
phylogenetic nomenclature: is taxonomic freedom the fun-
damental issue? Biol. Rev. 77, 3955.
Cantino, P. D. & de Queiroz, K. 2000 The PhyloCode. See
www.ohiou.edu/phylocode.
Cantino, P. D., Bryant, H. N., de Queiroz, K., Donoghue,
M. J., Eriksson, T., Hillis, D. M. & Lee, M. S. Y. 1999
Species names in phylogenetic nomenclature. Syst. Biol. 48,
790807.
Chaloner, W. G. 1986 Reassembling the whole fossil plant,
and naming it. In Systematic and taxonomic approaches in
palaeobotany (ed. R. A. Spicer & B. A. Thomas), pp. 6778.
Oxford: Clarendon.
Chaloner, W. G. 1999 Taxonomic and nomenclatural alterna-
tives. In Fossil plants and spores: modern techniques (ed. T. P.
Jones & N. P. Rowe), pp. 179183. London: The Geological
Society of London.
Cherns, L. & Wright, V. P. 2000 Missing molluscs as evidence
of large-scale, early skeletal aragonite dissolution in a Silur-
ian sea. Geology 28, 791794.
Collinson, M. E. 1986 The use of generic names for fossil
plants. In Systematic and taxonomic approaches in palaeobotany
(ed. R. A. Spicer & B. A. Thomas), pp. 91104. Oxford:
Clarendon.
Coope, G. R. 1995 Insect faunas in ice age environments; why
so little extinction? In Extinction rates (ed. J. H. Lawton &
R. M. May), pp. 5574. Oxford University Press.
Cooper, R. A. & Ni, Y.-N. 1986 Taxonomy, phylogeny and
variability of Pseudisograptus Beavis. Palaeontology 29, 313
363.
Cramer, F. H. & D ez, M. C. R. 1979 Katalog der fossilen Dino-
flagellaten, Hystrichspharen und verwandten Mikrofossilien.
Band 6, Acritatrcha, teil 3. Stuttgart: E. Schweizerbartsche
Verlagsbuchhandlung.
Crane, P. R. & Kenrick, P. 1997 Problems in cladistic classi-
fication: higher-level relationships in land plants. Aliso 15,
87104.
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
652 P. L. Forey and others Classication of extinct taxa
Crane, P. R. & Lidgard, S. 1990 Angiosperm radiation and
patterns of Cretaceous palynological diversity. In Major evol-
utionary radiations (ed. P. D. Taylor & G. P. Larwood), pp.
377407. Oxford: Clarendon.
Crowson, R. A. 1970 Classification and biology. Chicago, IL:
Aldine.
de Queiroz, K. & Gauthier, J. 1992 Phylogenetic taxonomy.
A. Rev. Ecol. Syst. 23, 449480.
de Queiroz, K. & Gauthier, J. 1994 Towards a phylogenetic
system of biological nomenclature. Trends Ecol. Evol. 9,
2731.
Dyke, G. J. 2002 Should paleontologists use phylogenetic
nomenclature? J. Paleontol. 76, 793796.
Engel, M. S. 2001 A monograph of Baltic amber bees and
evolution of the Apoidea (Hymenoptera). Bull. Am. Mus.
Nat. Hist. 259, 1192.
Farris, J. S. 1976 Phylogenetic classification of fossils with
Recent species. Syst. Zool. 25, 271282.
Fensome, R. A., Williams, G. L., Sedley Barss, M., Freeman,
J. M. & Hill, J. M. 1990 Acritarchs and fossil Prasinophytes:
and index to genera, species and infraspecific taxa. AASP
Foundation 25, 1771.
Finlay, B. J. 2004 Protist taxonomy: an ecological perspective.
Phil. Trans. R. Soc. Lond. B 359, 599610. (DOI 10.1098/
rstb.2003.1450.)
Flessa, K. W. & Jablonski, D. 1985 Declining Phanerozoic
background extinction rates: effects of taxonomic structure.
Nature 313, 216218.
Foote, M. & Raup, D. M. 1996 Fossil preservation and the
stratigraphic ranges of taxa. Paleobiology 22, 121140.
Foote, M. & Sepkoski, J. J. 1999 Absolute measures of the
completeness of the fossil record. Nature 398, 415417.
Fortey, R. A. & Owens, R. M. 1991 A trilobite fauna from the
highest Shineton Shales in Shropshire, and the correlation
of the latest Tremadoc. Geol. Mag. 122, 437464.
Gingerich, P. D. 1979 The stratophenetic approach to phy-
logeny reconstruction in vertebrate paleonotology. In Phylo-
genetic analysis and paleontology (ed. J. Cracraft & N.
Eldredge), pp. 4177. New York: Columbia University Press.
Greuter, W. E. A. (and 11 others) 2000 International Code of
Botanical Nomenclature (Saint Louis Code). Regnum vegetabile
138. Ko nigstein: Koeltz Scientific Books.
Harland, W. B., Holland, C. H. & House, M. R. 1967 The fos-
sil record: a symposium with documentation. London: Geologi-
cal Society of London.
Haynes, J. R. 1981 Foraminifera. London: Macmillan.
Hennig, W. 1966 Phylogenetic systematics. Urbana, IL: Univer-
sity of Illinois Press.
ICZN (International Commission on Zoological
Nomenclature) 1999 International Code of Zoological
Nomenclature, 4th edn. London: The International Trust for
Zoological Nomenclature.
Jablonski, D. 1995 Extinctions in the fossil record. In Extinc-
tion rates (ed. J. H. Lawton & R. M. May), pp. 2544.
Oxford University Press.
Jansonius, J. & Hills, L. V. 1978 Genera file of fossil spores.
Special publication, Department of Geology and Geophys-
ics, University of Calgary, Alberta, Canada. Issued on cards,
with many supplements.
Kaesler, R. L., Krebs, J. W. & Miller, D. L. 2001 The role and
design of databases in paleontology. In Fossils, phylogeny and
form: an analytical appraoch (ed. J. M. Adrain, G. D. Edge-
comb & B. S. Lieberman), pp. 377395. New York: Kluwer.
Kalgutkar, R. M. & Jansonius, J. 2000 Synopsis of fossil fungal
spores, mycelia and fructifications. AASP Foundation 39,
1429.
Kidwell, S. M. & Flessa, K. W. 1996 The quality of the fossil
record: populations, species and communities. A. Rev. Earth
Planet. Sci. 24, 433464.
Phil. Trans. R. Soc. Lond. B (2004)
Kier, P. M. & Lawson, M. H. 1978 Index of living and fossil
echinoids 19241970. Smithson. Contrib. Paleobiol. 34, 1
182.
Klapper, G. & Philip, G. M. 1971 Devonian conodont appar-
atuses and their vicarious skeletoal elements. Lethaia 4,
429452.
Kuhn, O. 1969 Handbuch der Palaoherpetologie. Munich: Dr
Freidrich Pfeil. (Formerly Stuttgart: Gustav Fischer Verlag.)
Lambert, J. & Thiery, P. 19091925 Essai de Nomenclature Rai-
sonnee des Echinides, vols 19, pp. iiii, 9607, pls 115.
Chaumont: Libraire Ferriere.
Little, C. T. S. 2001 Ancient hydrothermal vent and cold seep
faunas. In Palaeobiology II (ed. D. E. G. Briggs & P. R.
Crowther), pp. 447451. Oxford: Blackwell Science.
Loeblich, A. R. & Tappan, H. 1988 Foraminiferal genera and
their classification. New York: Van Nostrand Reinhold.
Lvtrup, S. 1977 The phylogeny of the Vertebrata. London:
Wiley.
Mace, G. N. 1995 Classification of threatened species and its
role in conservation planning. In Extinction rates (ed. J. H.
Lawton & R. M. May), pp. 197213. Oxford University
Press.
McKenna, M. C. 1975 Toward a phylogenetic classification of
the Mammalia. In Phylogeny of the primates (ed. W. P. Luck-
ett & F. S. Szalay), pp. 2146. New York: Plenum.
McKenna, M. C. & Bell, S. K. 1997 Classification of mammals
above the species level. New York: Columbia University Press.
Marshall, C. R. 1998 Determining stratigraphic ranges. In The
adequacy of the fossil record (ed. S. K. Donovan & C. R. C.
Paul), pp. 2353. Chichester: Wiley.
Maxwell, W. D. & Benton, M. J. 1990 Historical tests of the
absolute completeness of the fossil record of tetrapods.
Paleobiology 16, 322335.
May, R. M., Lawton, J. H. & Stork, N. E. 1995 Assessing
extinction rates. In Extinction rates (ed. J. H. Lawton &
R. M. May), pp. 124. Oxford University Press.
Mayr, E. 1942 Systematics and the origin of species. New York:
Columbia University Press.
Meyen, S. V. 1987 Fundamentals of palaeobotany. London:
Chapman & Hall.
Mishler, B. 2000 Deep phylogenetic relationships among
plants and their implications for classification. Taxon 49,
661683.
Moore, R. C. 1953 Treatise on invertebrate paleontology.
Lawrence, KS: Geological Society of America.
Nelson, G. J. 1972 Phylogenetic relationships and classi-
fication. Syst. Zool. 21, 227230.
Nichol, D. 1977 The number of living animals species likely
to be fossilized. Fla Sci. 40, 135139.
Patterson, C. & Rosen, D. E. 1977 Review of ichthyodecti-
form and other Mesozoic teleost fishes and the theory and
practice of classifying fossils. Bull. Am. Mus. Nat. Hist. 158,
81172.
Patterson, C. & Smith, A. B. 1987 Is periodicity of mass
extinctions a taxonomic artefact? Nature 330, 248251.
Paul, C. R. C. 1998 Adequacy, completeness and the fossil
record. In The adequacy of the fossil record (ed. S. K. Dono-
van & C. R. C. Paul), pp. 122. London: Wiley.
Peters, S. E. & Foote, M. 2001 Biodiversity in the Phanero-
zoic: a reinterpretation. Paleobiology 27, 583601.
Raup, D. M. 1976a Species diversity during the Phanerozoic:
a tabulation. Paleobiology 2, 279288.
Raup, D. M. 1976b Species diversity during the Phanerozoic:
an interpretation. Paleobiology 2, 289297.
Raup, D. M. 1991 A kill curve for Phanerozoic marine species.
Paleobiology 17, 3748.
Raup, D. M. & Sepkoski, J. J. 1982 Mass extinctions in the
fossil record. Science 219, 12391240.
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
Classication of extinct taxa P. L. Forey and others 653
Rindsberg, A. K. 1998 Workshop of Ichnotaxonomy, Bornholm,
Denmark, August 47 1998. See www.emery.edu/COLLEGE/
ENSV/research/ichnology/IN99-WITREP-1.HTM.
Romer, A. S. 1966 Vertebrate paleontology, 3rd edn. University
of Chicago Press.
Schultze, H.-P. 1978 Handbook of paleoichthyology. Munich: Dr
Freidrich Pfeil. (Formerly Stuttgart: Gustav Fischer Verlag.)
Scoble, M. J. 2004 Unitary or unified taxonomy? Phil. Trans.
R. Soc. Lond. B 359, 699710. (DOI 10.1098/rstb.
2003.1456.)
Sepkoski, J. J. 1982 A compendium of fossil marine families.
Milw. Public Mus. Contrib. Biol. Geol. 51, 1125.
Sepkoski, J. J. 1992 A compendium of fossil marine families,
2nd edition. Milw. Public Mus. Contrib. Biol. Geol. 83, 1156.
Sepkoski, J. J. 1993 Ten years in the library: how changes in
taxonomic databases affect perception of macroevolutionary
patterns. Paleobiology 19, 4351.
Sepkoski, J. J. 2002 A compendium of fossil marine genera.
Bull. Am. Paleontol. 363, 1560.
Simpson, G. G. 1961 Principles of animal taxonomy. New York:
Columbia University Press.
Smith, A. B. 2001 Large-scale heterogeneity of the fossil rec-
ord: implications for Phanerozoic biodiversity studies. Phil.
Trans. R. Soc. Lond. B 356, 351367. (DOI 10.1098/rstb.
2000.0768.)
Smith, A. B. & Patterson, C. 1988 The influence of taxonomic
method on the perception of patterns of evolution. Evol.
Biol. 23, 127216.
Stanley, S. M. 1979 Macroevolution: pattern and process. San
Francisco, CA: Freeman.
Phil. Trans. R. Soc. Lond. B (2004)
Stover, L. E. & Evitt, W. R. 1978 Analyses of pre-Pleistocene
organic-walled dinoflagellates. Stanford Univ. Publ. Geol. Sci.
15, 1300.
Stover, L. E. & Williams, G. L. 1987 Analyses of Mesozoic and
Cenozoic organic-walled dinoflagellates. AASP Foundation
18, 1243.
Sutton, M. J., Briggs, D. E. G., Siveter, D. J. & Siveter, D. J.
2001 An exceptionally preserved vermiform mollusc from
the Silurian of England. Nature 410, 461463.
Sweet, W. C. 1988 The Conodonta: morphology, taxonomy,
palaeoecology, and evolutionary history of a long-extinct animal
phylum. Oxford: Clarendon.
Thomas, B. 1981 The evolution of plants and flowers. Madrid:
Eurobook Ltd.
Wheeler, Q. D. & Meier, R. (eds) 2000 Species concepts and
phylogenetic theory. New York: Columbia University Press.
Wheeler, Q. D. & Platnick, N. I. 2000 The phylogenetic spec-
ies concept (sensu Wheeler and Platnick). In Species concepts
and phylogenetic theory (ed. Q. D. Wheeler & R. Meier), pp.
5569. New York: Columbia University Press.
Williams, G. L., Lentin, J. K. & Fensome, R. A. 1998 The
Lentin and Williams index of fossil dinoflagellates, 1998
edn. AASP Foundation 34, 1305.
Wilson, M. V. H. & Caldwell, M. W. 1998 The Furcaudi-
formes: a new order of jawless vertebrates with thelodont
scales, based on articulated Silurian and Devonian fossils
from northern Canada. J. Vertebr. Paleontol. 18, 1029.
Woodward, A. S. 18891901 Catalogue of the fossil fishes in the
British Museum (Natural History), parts 14. London: British
Museum (Natural History).
on October 11, 2014 rstb.royalsocietypublishing.org Downloaded from
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Ebook PDF Computer Accounting With Sage 50 2019 PDFDocument41 pagesEbook PDF Computer Accounting With Sage 50 2019 PDFelizabeth.leon666100% (33)
- Arthropod ClassificationDocument6 pagesArthropod ClassificationFarzeen Mehmood100% (1)
- Trilobite PDFDocument449 pagesTrilobite PDFTachipleus100% (3)
- Exercise 7: Historical Geology and Fossils: Earth and Beyond - An Introduction To Earth-Space Science Lab ManualDocument15 pagesExercise 7: Historical Geology and Fossils: Earth and Beyond - An Introduction To Earth-Space Science Lab ManualAtubrah PrinceNo ratings yet
- Identification of and ATPsensitive Potassium Channel in Mitochondria PDFDocument23 pagesIdentification of and ATPsensitive Potassium Channel in Mitochondria PDFCarolina RicárdezNo ratings yet
- Akopova mPTPdepolarizationCa2UBJ200577368-75 PDFDocument9 pagesAkopova mPTPdepolarizationCa2UBJ200577368-75 PDFCarolina RicárdezNo ratings yet
- Nteractions of Arsenate, Sulfate and Phosphate With Yeast Mitochondria PDFDocument10 pagesNteractions of Arsenate, Sulfate and Phosphate With Yeast Mitochondria PDFCarolina RicárdezNo ratings yet
- Mitochondrial Unselective Channels Throughout The Eukaryotic Domain PDFDocument9 pagesMitochondrial Unselective Channels Throughout The Eukaryotic Domain PDFCarolina RicárdezNo ratings yet
- 83 Pacheco-Marín, Vilma Maldonado 16 PDFDocument15 pages83 Pacheco-Marín, Vilma Maldonado 16 PDFCarolina RicárdezNo ratings yet
- Calcium Ionophoretic Activity of Chemically Synthesized Oligomeric Derivatives PDFDocument12 pagesCalcium Ionophoretic Activity of Chemically Synthesized Oligomeric Derivatives PDFCarolina RicárdezNo ratings yet
- Mitochondrial Permeability Transition Pore Sensitivity To Opening and Mechanistic Dependence On Substrate Availability PDFDocument13 pagesMitochondrial Permeability Transition Pore Sensitivity To Opening and Mechanistic Dependence On Substrate Availability PDFCarolina RicárdezNo ratings yet
- 54 DiazRuiz09 PDFDocument15 pages54 DiazRuiz09 PDFCarolina RicárdezNo ratings yet
- Diabetes Tipo 2 MexicoDocument15 pagesDiabetes Tipo 2 MexicoGuadalupe Gutierrez CelayaNo ratings yet
- Mitochondrial Free (Ca2+) Levels and The Permeability Transition PDFDocument8 pagesMitochondrial Free (Ca2+) Levels and The Permeability Transition PDFCarolina RicárdezNo ratings yet
- Closure of The Yeast Mitochondria Unspecific Channel PDFDocument8 pagesClosure of The Yeast Mitochondria Unspecific Channel PDFCarolina RicárdezNo ratings yet
- 50 DiazRuiz08 PDFDocument8 pages50 DiazRuiz08 PDFCarolina RicárdezNo ratings yet
- Comprehensive Analysis of Mitochondrial PDFDocument14 pagesComprehensive Analysis of Mitochondrial PDFCarolina RicárdezNo ratings yet
- Slow Activation of Fast Mitochondrial Ca2 Uptake by PDFDocument15 pagesSlow Activation of Fast Mitochondrial Ca2 Uptake by PDFCarolina RicárdezNo ratings yet
- The Mitochondrial Permeability Transition, Release of Cytochrome C and Cell Death PDFDocument6 pagesThe Mitochondrial Permeability Transition, Release of Cytochrome C and Cell Death PDFCarolina RicárdezNo ratings yet
- Physiological Uncoupling of Mitochondrial Oxidative PDFDocument9 pagesPhysiological Uncoupling of Mitochondrial Oxidative PDFCarolina RicárdezNo ratings yet
- Potassium Collapses The DP in Yeast Mitochondria While PDFDocument8 pagesPotassium Collapses The DP in Yeast Mitochondria While PDFCarolina RicárdezNo ratings yet
- Effect of Transient and Permanent Permeability Transition Pore Opening On NAD (P) H Localization in Intact Cells PDFDocument10 pagesEffect of Transient and Permanent Permeability Transition Pore Opening On NAD (P) H Localization in Intact Cells PDFCarolina RicárdezNo ratings yet
- Evidence For The Presence of A Reversible Ca2+-Dependent Pore PDFDocument4 pagesEvidence For The Presence of A Reversible Ca2+-Dependent Pore PDFCarolina RicárdezNo ratings yet
- The Ca2+-Induced Membrane Transition in Mitochondria PDFDocument8 pagesThe Ca2+-Induced Membrane Transition in Mitochondria PDFCarolina RicárdezNo ratings yet
- The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functional, 2017Document9 pagesThe Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functional, 2017Nora FitriNo ratings yet
- The Mitochondrial Permeability Transition From Yeast To Mammals PDFDocument6 pagesThe Mitochondrial Permeability Transition From Yeast To Mammals PDFCarolina RicárdezNo ratings yet
- Regulation of Mitochondrial ElectronTransport Chain Assembly PDFDocument25 pagesRegulation of Mitochondrial ElectronTransport Chain Assembly PDFCarolina RicárdezNo ratings yet
- The Mitochondrial Permeability Transition PDFDocument45 pagesThe Mitochondrial Permeability Transition PDFCarolina RicárdezNo ratings yet
- J. Biol. Chem.-1992-Bernardi-2934-9 PDFDocument6 pagesJ. Biol. Chem.-1992-Bernardi-2934-9 PDFCarolina RicárdezNo ratings yet
- Conventional Techniques Tomonitor Mitochondrial OxygenConsumption PDFDocument11 pagesConventional Techniques Tomonitor Mitochondrial OxygenConsumption PDFCarolina RicárdezNo ratings yet
- The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functional, 2017Document9 pagesThe Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functional, 2017Nora FitriNo ratings yet
- Effect of Magnesium On Calcium-Induced Depolarisation of Mitochondrial Transmembrane PotentialDocument10 pagesEffect of Magnesium On Calcium-Induced Depolarisation of Mitochondrial Transmembrane PotentialCarolina RicárdezNo ratings yet
- Calcium Binding and Translocation by The Voltage-Dependent Anion Channel: A Possible Regulatory Mechanism in Mitochondrial FunctionDocument9 pagesCalcium Binding and Translocation by The Voltage-Dependent Anion Channel: A Possible Regulatory Mechanism in Mitochondrial FunctionCarolina RicárdezNo ratings yet
- Jbacter00226 0379 PDFDocument6 pagesJbacter00226 0379 PDFCarolina RicárdezNo ratings yet
- Invertebrate Evolution and AdaptationDocument3 pagesInvertebrate Evolution and AdaptationMirko StambolićNo ratings yet
- Paleontology I I Fossil ClassificationDocument15 pagesPaleontology I I Fossil ClassificationkowsilaxNo ratings yet
- Articulo de Los Ojos de TrilobitesDocument14 pagesArticulo de Los Ojos de TrilobitesfeliNo ratings yet
- Topper2011Document17 pagesTopper2011Fandjy SandjayaNo ratings yet
- (Advances in Cell Aging and Gerontology 17) Mark P. Mattson (Eds.) - Sleep and Aging-Academic Press, Elsevier (2005)Document192 pages(Advances in Cell Aging and Gerontology 17) Mark P. Mattson (Eds.) - Sleep and Aging-Academic Press, Elsevier (2005)Marcos R Galvão BatistaNo ratings yet
- 911 EssaysDocument51 pages911 EssaysafabkedovNo ratings yet
- Crystal (Trilobite) Eyes by Richard ForteyDocument3 pagesCrystal (Trilobite) Eyes by Richard ForteyardeegeeNo ratings yet
- Late Ordovician Trilobites From TasmaniaDocument20 pagesLate Ordovician Trilobites From Tasmaniaardeegee100% (1)
- Foote CVDocument9 pagesFoote CVRavindr KumarNo ratings yet
- Phylum ArthopodaDocument205 pagesPhylum ArthopodaRubieNo ratings yet
- Evolution I: Multiple-Choice QuestionsDocument31 pagesEvolution I: Multiple-Choice Questionsdds uwuNo ratings yet
- Systematic ZoologyDocument10 pagesSystematic ZoologyZeenat MoambarNo ratings yet
- Arthropods Chelicerates: HexapodsDocument6 pagesArthropods Chelicerates: HexapodsnanaNo ratings yet
- Cuticular Microstructure of Some Silurian Homalonotid Trilobites From SwedenDocument3 pagesCuticular Microstructure of Some Silurian Homalonotid Trilobites From SwedenardeegeeNo ratings yet
- Early Silurian Trilobites AustraliaDocument41 pagesEarly Silurian Trilobites AustraliaardeegeeNo ratings yet
- Palaeogeography, Palaeoclimatology, Palaeoecology: Gregory J. RetallackDocument16 pagesPalaeogeography, Palaeoclimatology, Palaeoecology: Gregory J. RetallackSounak BiswasNo ratings yet
- Maddin Lecture 9A Cambrian ExplosionDocument73 pagesMaddin Lecture 9A Cambrian ExplosionSamuel AdewumiNo ratings yet
- Identificar Fosiles Por Su FormaDocument10 pagesIdentificar Fosiles Por Su FormaManuel ElescanoNo ratings yet
- 0613consmag4web PDFDocument36 pages0613consmag4web PDFSumatNo ratings yet
- Geology Notes For IFS (Statigraphy Concise)Document20 pagesGeology Notes For IFS (Statigraphy Concise)dun_aromatix50% (6)
- How Index Fossils Help Define Geologic TimeDocument2 pagesHow Index Fossils Help Define Geologic Timelasxdkasdas sdadwdacNo ratings yet
- A Systematic Revision of The Family Harpetidae Tri PDFDocument34 pagesA Systematic Revision of The Family Harpetidae Tri PDFBob KurdelmeyerNo ratings yet
- Fossil Environments in UtahDocument2 pagesFossil Environments in UtahMoab Utah100% (1)
- Trilobite Facial Suture Types PDFDocument4 pagesTrilobite Facial Suture Types PDFManuel ElescanoNo ratings yet
- 50-Million-Year-Old Sea Creatures Had A Leg Up On BreathingDocument3 pages50-Million-Year-Old Sea Creatures Had A Leg Up On BreathingFred PascuaNo ratings yet
- Walch's Trilobite ResearchDocument26 pagesWalch's Trilobite ResearchardeegeeNo ratings yet