Mass Transfer and Diffusion
Introduction to Mass Transfer
When a system contains two or more components whose concentrations
vary from point to point, there is a natural tendency for mass to be
transferred, minimizing the concentration differences within a system.
The transport of one constituent from a region of higher concentration to
a lower concentration is called mass transfer.
The transfer of mass within a fluid mixture or across a phase boundary is
a process that plays a major role in many industrial processes. Example of
such processes are:
Dispersion of gasses from stacks
Removal of pollutants from plant discharge by means of absorption
Stripping
St ippin of
f gases
s sf
from
m wastewater
st
t
Neurton diffusion within nuclear reactors
Air conditioning
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
�Objectives
Your objectives in studying this section are to be able to:
1. Understand mass transfer between phases.
2. Calculate interfacial mass-transfer rates in terms of local
mass-transfer coefficient for each phase.
3 D
3.
Define
fi
and
d use, where
h
appropriate,
i t overall
ll mass transfer
t
f
coefficients
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
�Mass Transfer and Diffusion
Steady-state ordinary molecular diffusion
Mass transfer by ordinary molecular diffusion occurs because of a
concentration difference or gradient; that is, a species diffuses
of
f decreasing
ng concentration.
n n
n.
The mass transfer rate is proportional to the area normal to the
direction of mass transfer and not to the volume of the mixture.
Thus the rate can be expressed as flux.
Thus,
flux
Mass transfer stops when the concentration is uniform.
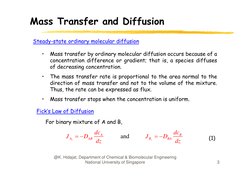
F k L
Ficks
Law of
f Diffusion
D ff
For binary mixture of A and B,
J Az DAB
dc A
dz
and
J Bz DBA
dcB
dz
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
(1)
�Mass Transfer and Diffusion
Many alternative forms of equations (1) are used, depending on the
choice of driving
g force or p
potential in the g
gradient. For example,
p ,
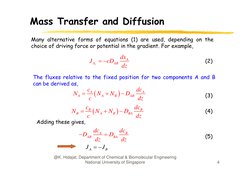
J Az cDAB
dx A
dz
(2)
The fluxes relative to the fixed position for two components A and B
can be derived as,
cA
dc A
N
D
A B AB
c
dz
c
dc
N B B N A N B DBA B
c
dz
NA
(3)
(4)
Adding these gives,
ddc A
ddc
DBA B
dz
dz
J A JB
DAB
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
(5)
�Mass Transfer and Diffusion
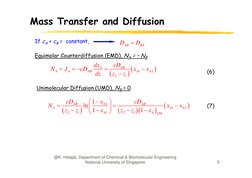
If cA + cB = constant,
DAB DBA
Equimolar Counterdiffusion (EMD), NA = NB
N A J A cDAB
dx A
cDAB
xA1 xA2
dz z2 z1
(6)
Unimolecular Diffusion (UMD),
(UMD) NB = 0
NA
1 xA2
cDAB
cDAB
ln
xA1 xA2
z2 z1 1 xA1 z2 z1 1 xA LM
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
(7)
�Convective Mass Transfer
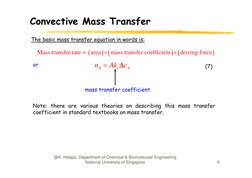
The basic mass transfer equation in words is:
Mass transfer rate = area mass transfer coefficient driving force
or
nA Akc cA
((7))
mass transfer coefficient
Note: there are various theories on describing
g this mass transfer
coefficient in standard textbooks on mass transfer.
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
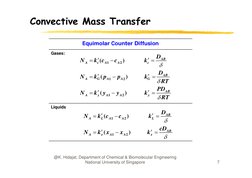
�Convective Mass Transfer
Equimolar Counter Diffusion
Gases:
N A kc (c A1 c A 2 )
kc
N A kG ( p A1 p A 2 )
kG
N A k y ( y A1 y A 2 )
DAB
DAB
RT
PDAB
k y
RT
Liquids
q
N A k L (c A1 c A 2 )
k L
DAB
N A k x ( x A1 x A 2 )
k x
cDAB
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
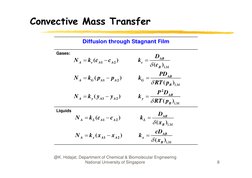
�Convective Mass Transfer
Diffusion through Stagnant Film
Gases:
N A k c ( c A1 c A 2 )
kc
DAB
(cB )LM
N A kG ( p A1 p A 2 )
kG
PDAB
RT ( pB )LM
N A k y ( y A1 y A 2 )
P 2 DAB
ky
RT ( pB )LM
Li id
Liquids
N A k L ( c A1 c A 2 )
kL
DAB
( x B )LM
N A k x ( x A1 x A 2 )
kx
cDAB
( x B )LM
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
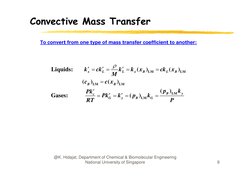
�Convective Mass Transfer
To convert from one type of mass transfer coefficient to another:
Liquids:
k x ck L
(cB )LM
Gases:
k L k x ( x B )LM ck L ( x B )LM
M
c( x B )LM
( pB )LM k y
Pkc
PkG k y ( pB )LM kG
RT
P
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
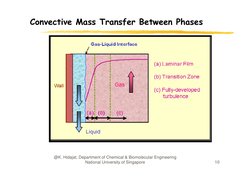
�Convective Mass Transfer Between Phases
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
10
�Convective Mass Transfer Between Phases
Two-Film Theory:
G phase
Gas
Liquid
i i phase
pAb
G phase
Gas
Liquid
i i phase
pAb
pAi
pAi
cAi
cAi
cAb
cAb
Interface
At the phase interface, cAi and pAi are in equilibrium,
c Ai f p Ai
N A kG ( p Ab p Ai ) kc (c Ai c Ab )
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
11
�Convective Mass Transfer Between Phases
Interfacial compositions:
kc
p Ab p Ai
kG c Ab c Ai
pA
pAb
Equilibrium
curve
pAi
cAb
cAi
cA
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
12
�Overall Mass Transfer Coefficients
Bulk gas phase
composition
pAb
Imaginary
composition
pointed to
measurable
variable
pAi
cAi
c*A
Bulk liquid phase
composition
cAb
Driving
force:
(c*A c Ab ) for liquid phase
p*A
( pAb p*A ) for gaseous phase
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
13
�Overall Mass Transfer Coefficients
Equilibrium
curve
pAb
N A K L c*A c Ab K G pAb p*A
pAi
mx
pAi p*A
mx
c Ai c Ab
p*A
cAb
Ab
*
*
Ab
A
Ab
Ai
Ai
A
cAi
c*A
p*A pAb pAi m x c Ai c Ab
N A N A mx N A
K G kG
kL
1
1 mx
K G kG k L
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
14
�Overall Mass Transfer Coefficients
Equilibrium
curve
pAb
In a similar manner, we can find
my
c c c
N A K L c*A c Ab K G pAb p*A
pAi
*
A
c Ab
my
p*A
cAb
*
A
cAi
*
A
Ai
Ai
c Ab
pAb pAi
c*A c Ai
c*A
c Ab p Ab pAi / m y c Ai c Ab
NA
NA
N
A
K L m y kG k L
1
1
1
K L m y kG k L
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
15
�Overall Mass Transfer Coefficients
Note:
for pAi = HAcAi
1
1 HA
K G kG k L
1
1
1
K L H A kG k L
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
16
�Mass Transfer Resistance
The resistance to mass transfer is defined as the reciprocal of the mass
transfer coefficient:
1
represents the resistance to mass transfer in the gas phase
KG
represents the resistance to mass transfer in the liquid phase
K
L
It is important to know if one of the 2 resistances is controlling the mass
transfer. If so, the rate of mass transfer can be increased by promoting
turbulence in and/or increasing the dispersion of the controlling phase.
phase
Recall the relationship between overall and film mass transfer coefficients, and
that the 1/K represents the mass transfer resistance.
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
17
�Mass Transfer Resistance
If mx is small (i.e. the equilibrium curve is very flat), the term mx/kL is not
significant, therefore:
KG
1
kG
and the major resistance to diffusional mass transfer lies in
the gas phase and the mass transfer is said to be gas-phase
controlled.
In this case, solute A can be interpreted as being very soluble in the liquid: at
equilibrium, a small concentration of A in the gas will bring about a very large
concentration in the liquid.
If my is large (i.e. the equilibrium curve is very steep), the term 1/mykG is not
significant, therefore:
1 1
K L kL
and
d the
h major
j resistance
i
to diffusional
diff i
l mass transfer
f lies
li in
i
the liquid phase and the mass transfer is said to be liquidphase controlled.
Solute A is relatively insoluble in the liquid: a very large concentration of A in the
gas phase is required to provide even a small change of concentration in the liquid.
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
18
�Mass Transfer Between Two Phases
Example:
In a dilute concentration region,
region equilibrium data for SO2 distributed
between air and water can be approximated by
pA = 25xA
where the partial pressure of SO2 is expressed in atmospheres. For an
absorption column operating at 10 atm, the bulk vapour and liquid
concentrations at one point in the column are yA = 0.01 and xA = 0.0. The
mass transfer coefficient for this process are
k x 10 kgmol/m 2 h mole fraction
k y 8 kgmol/m 2 h mole fraction
Assuming equimolar counter transfer, (a) find the overall liquid phase mass
transfer coefficient, (b) determine the interfacial compositions, xAi and yAi,
and (c) calculate the molar flux, NA
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
19
�Mass Transfer Between Two Phases
Solution:
(a)
pA = 25xA, but yA = pA/P yA = 2.5
2 5xA.
1
1
1
K x m y k y k x
Upon substituting my = 2.5 and the mass transfer coefficients into
the above equation, we obtain
1
1
1
K x (2.5)(8) 10
2
g
h mole fraction
K x 6.67kgmol/m
(b)
Using the rate ratio line,
k x y Ab y Ai
10
1.25
8
k y x Ab x Ai
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
20
�Mass Transfer Between Two Phases
yA
0.01
k x y Ab y Ai
1.25
k y x Ab x Ai
yA = 2.5xA
yAi
yA = 1.25xA + 0.01
0.0067
xAi
0.01
0.02
xA
0.00267
(c) The mass flux
2
N A k x ( x Ai x Ab ) 10(0.00267
(
0)) 0.0267 kgmol/m
g
h
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
21
�Mass Transfer Between Two Phases
Repeat part (b) of the previous example for bulk concentrations yA = 0.04
and xA = 0.01. Assuming transfer of component A through a stagnant film.
Solution
The determination of interfacial compositions for transfer through a
stagnant
t
t film
fil requires
i
th t a trial-and-error
that
t i l d
procedure
d
b used.
be
d
To begin we assume a counter diffusion to find the interfacial compositions.
k x y Ab y Ai
10
1.25
8
k y x Ab x Ai
y Ai 1.25 x Ai 0.0525
Equilibrium:
yAi = 2.5xAi
xAi = 0.014
yAi = 0.035
For diffusion through a stagnant film:
k x /(1 x A )LM y Ab y Ai
k y /(1 y A )LM x Ab x Ai
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
22
�Mass Transfer Between Two Phases
(1 x A )LM
(1 x Ai ) (1 x Ab ) (1 0.014)(1 0.01)
0.988
1 x Ai
1 0.014
ln
ln
1 0.01
Ab
(1 y Ai ) (1 y Ab ) (1 0.035)(1 0.04)
0.962
1 y Ai
1 0.035
ln
ln
1 0.04
Ab
k x /(1 x A )LM
10 / 0.988
0 988
Therefore,
1.217
8 / 0.962
k y /(1 y A )LM
(1 y A )LM
As before plot a line from the bulk concentrations with a slope equals to
1.217 to intersect with the equilibrium curve.
xAi = 0.01405
yAi = 0.0364
Use the new values for the log mean concentration differences.
Consequently, the interfacial conditions are xAi = 0.01405 and yAi =
0 0364
0.0364.
@K. Hidajat, Department of Chemical & Biomolecular Engineering
National University of Singapore
23