Professional Documents
Culture Documents
Matlab Code
Matlab Code
Uploaded by
Ranveer ChavdaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Matlab Code
Matlab Code
Uploaded by
Ranveer ChavdaCopyright:
Available Formats
10/13/16 12:34 PM
C:\Users\Ranvir...\Isotherm_Toluene3.m
1 of 1
clear all
close all
clc
% Toluene's critical Temperature and pressure
Tc = 591.79;
Pc = 4109000;
% Universal Gas Constant
R = 8.314;
% b and a for vdw EOS
b = (R*Tc)/(8*Pc);
a = (27*R^2*Tc^2)/(64*Pc);
% Declaring T as needed
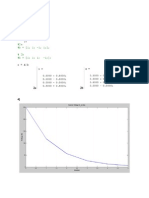
T = [550 560 570 580 590];
disp(a);
disp(b);
VPoints = 0.0003:0.00001:0.0009;
for i=1:1:numel(T)
PT=(R*T(i)./(VPoints-b)) - (a./(VPoints.^2));
u=-(R*T(i).*(log(VPoints-b)))+((R*T(i).*VPoints)./(VPoints-b))-((2*a).
/VPoints);
figure(1)
plot(VPoints,PT);
hold on
figure(2)
plot(u,PT);
hold on
end
% plotting isotherm for T =580K
%figure(1)
%plot(VPoints,PT);
%h=plot(VPoints,PT);
%set(h,'color',rand(1,3),'linewidth',1);
xlabel('u')
ylabel('pressure in N/m^2')
title('Isotherms for Toluene')
You might also like
- System Dynamics and Respinse Kelly SolutionsDocument447 pagesSystem Dynamics and Respinse Kelly SolutionsSteve Karkenny100% (6)
- Flutter Matlab Codes PDFDocument8 pagesFlutter Matlab Codes PDFcrimelife6No ratings yet
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Che 441 Washington State University Process Control Department of Chemical Engineering Fall, 2008 Richard L. ZollarsDocument13 pagesChe 441 Washington State University Process Control Department of Chemical Engineering Fall, 2008 Richard L. ZollarssophiaNo ratings yet
- !solution Manual Linear Systems and Signals B P LathiDocument154 pages!solution Manual Linear Systems and Signals B P Lathijustme123273% (11)
- Sol Ch6 Part2Document9 pagesSol Ch6 Part2mazharNo ratings yet
- MATLAB Commands For VDW Method For CO2Document3 pagesMATLAB Commands For VDW Method For CO2John Andrae MangloNo ratings yet
- 13 Problem Solving With MATLAB PDFDocument64 pages13 Problem Solving With MATLAB PDFAugusto De La Cruz CamayoNo ratings yet
- Final Transport ProjectDocument11 pagesFinal Transport Projectapi-457071846No ratings yet
- Deenbandhu Chhotu Ram University of Science and Technology, Murthal, SonepatDocument10 pagesDeenbandhu Chhotu Ram University of Science and Technology, Murthal, Sonepatdeepika vermaNo ratings yet
- Chapter 2Document41 pagesChapter 2Basel TahaNo ratings yet
- Matlab CodeDocument5 pagesMatlab CodeSri Varalakshmi MummidiNo ratings yet
- 10 Simulink PDFDocument32 pages10 Simulink PDFAugusto De La Cruz Camayo100% (1)
- Lab Assignment: NAME:-Ashutosh ChoureyDocument8 pagesLab Assignment: NAME:-Ashutosh ChoureyAshutosh ChoureyNo ratings yet
- DC To DC Two Quadrant ConverterDocument7 pagesDC To DC Two Quadrant Converterhamza abdo mohamoudNo ratings yet
- All All T NDocument9 pagesAll All T Nhr.rayhmanNo ratings yet
- Jatin Katiyar Signals and Systems LabDocument82 pagesJatin Katiyar Signals and Systems LabJatin katiyarNo ratings yet
- % Let Call Dbo/Dtr 'A'% %let Call B' As B %let Call DB'/DTR As A C%Document2 pages% Let Call Dbo/Dtr 'A'% %let Call B' As B %let Call DB'/DTR As A C%EceDirilNo ratings yet
- Chap3 4Document10 pagesChap3 4rpb109100% (1)
- EEE C364/INSTR C 364 Analog ElectronicsDocument15 pagesEEE C364/INSTR C 364 Analog ElectronicsAngad SehdevNo ratings yet
- Rland RCDocument33 pagesRland RCSyed Muhammad DanishNo ratings yet
- Lab 7Document8 pagesLab 7Noor UL AaienNo ratings yet
- MatLab AssignmentDocument2 pagesMatLab AssignmentHarsh DarjiNo ratings yet
- Visco MeterDocument5 pagesVisco MeterYasin KaradaşNo ratings yet
- Sampling in MatlabDocument3 pagesSampling in MatlabEngr. Naveed MazharNo ratings yet
- ProgramasDocument4 pagesProgramasJoel IngaNo ratings yet
- Bond PriceDocument2 pagesBond PriceTakeshi YamadaNo ratings yet
- Vector Processing MatlabDocument11 pagesVector Processing Matlabbero199450% (2)
- Matlab CodeDocument13 pagesMatlab CodeAvinash SinghNo ratings yet
- PFinal IME 8310-2020-30 - RemoteDocument6 pagesPFinal IME 8310-2020-30 - RemoteWilliam Alejandro Contreras MaestreNo ratings yet
- MATLABDocument2 pagesMATLABexia0012No ratings yet
- Gas PLDocument7 pagesGas PLAly Hassan WalyNo ratings yet
- Calculation of Receiver Sensitivity: T V V K T ° Where T V Calibrates Voltage As TemperatureDocument23 pagesCalculation of Receiver Sensitivity: T V V K T ° Where T V Calibrates Voltage As TemperaturevarunmrNo ratings yet
- Swing CurveDocument2 pagesSwing Curvechinmay pattnayakNo ratings yet
- Various Equations (We May Not Need Any of These!) : Fs T Ut FDocument8 pagesVarious Equations (We May Not Need Any of These!) : Fs T Ut FLaura AndrewsNo ratings yet
- Appendix Heat Exchanger Design 2 Okt 2014Document24 pagesAppendix Heat Exchanger Design 2 Okt 2014Alif Aizat AzmanNo ratings yet
- % Theoretical Design of A Required Transformer: WhileDocument4 pages% Theoretical Design of A Required Transformer: WhileZain AliNo ratings yet
- ch22m024 Q4Document5 pagesch22m024 Q4Prem ahujaNo ratings yet
- XasaDocument2 pagesXasaMuhammad AliNo ratings yet
- Aerodynamics For Engineering Students 6th Edition Houghton Solutions Manual 1Document16 pagesAerodynamics For Engineering Students 6th Edition Houghton Solutions Manual 1frederick100% (31)
- Cycle 1 DclabDocument30 pagesCycle 1 Dclabmd salmanNo ratings yet
- Excelente Arma MatlabDocument7 pagesExcelente Arma Matlabjaleusemia1111No ratings yet
- Allen J Wood Power Generation Operation OstDocument33 pagesAllen J Wood Power Generation Operation OstDhoviar M. AliNo ratings yet
- CN2116-HW7-Solution (XJP - 2011)Document12 pagesCN2116-HW7-Solution (XJP - 2011)Brian WatsonNo ratings yet
- Counter Current Heat ExchangerDocument10 pagesCounter Current Heat ExchangersanthoshreddyrebelnaniNo ratings yet
- Tugas1 PDFDocument1 pageTugas1 PDFMuhamad Fajar WicaksonoNo ratings yet
- New Text DocumentDocument5 pagesNew Text DocumentCristóbal KettererNo ratings yet
- Formulario de Q.A.III Celdas Electroquímicas: R D R M MDocument2 pagesFormulario de Q.A.III Celdas Electroquímicas: R D R M MresiduomortalNo ratings yet
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Fundamentals of Electronics 3: Discrete-time Signals and Systems, and Quantized Level SystemsFrom EverandFundamentals of Electronics 3: Discrete-time Signals and Systems, and Quantized Level SystemsNo ratings yet
- Combinatorial Algorithms: For Computers and CalculatorsFrom EverandCombinatorial Algorithms: For Computers and CalculatorsRating: 4 out of 5 stars4/5 (2)
- Computer Processing of Remotely-Sensed Images: An IntroductionFrom EverandComputer Processing of Remotely-Sensed Images: An IntroductionNo ratings yet
























































![Mathematical Tables: Tables of in G [z] for Complex Argument](https://imgv2-2-f.scribdassets.com/img/word_document/282615796/149x198/febb728e8d/1714993295?v=1)

