Professional Documents
Culture Documents
ABG Easy As 123 PDF
ABG Easy As 123 PDF
Uploaded by
TaylorOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ABG Easy As 123 PDF
ABG Easy As 123 PDF
Uploaded by
TaylorCopyright:
Available Formats
ABG...EASY AS...
1,2,3
NORMAL VALUES & DEFINITIONS
NAME
pH
Acid
Base
DEFINITION
Refers to hydrogen ion (H+) levels, hence
the H in pH. H+ levels are important
because a lack of (deficit) or too much
(excess) will tell you if the patient is
acidotic or alkolotic. One confusing point
about pH is that it is an INVERSE ratio,
which means that the more H+ present, the
lower the pH and vice versa.
Can give away a H+ or can separate
(dissociate) hydrogen from its ion, so the
hydrogen is not positive and therefore no
longer an acid.
Acids are end products of metabolism and
must be buffered or excreted to achieve a
normal pH
Unlike Acids, bases can accept a H+ and
bond with hydrogen. They are all negative
and like to buffer body acids.
3 STEPS TO ABG INTERPRETATION
1. What is the pH? Is it alkolotic (57.45) or acidotic (67.35)?
VALUE
7.35 - 7.45
2. Whats happening with the respiratory system (CO2) and the metabolic systems (HCO3-)?
If the problem is in the lungs (respiratory) the CO2 will be heading in the opposite direction of the pH.
For example: respiratory acidosis: The pH will be low - 6pH 7.22 and the CO2 will be high - 5CO2 55mmhg
If the problem is metabolic the HCO3 will head in the same direction as the pH
For example: metabolic alkalosis: The pH will be high - 57.55 and the HCO3- will also be high HCO3- 535mmol/L.
Note: an easy way to remember for a Metabolic problem, think M as in the pH will head in the saMe direction as the
HCO3-. For respiratory the pH will head in the Opposite direction as the CO2.
3. Is there any (if any) compensation occurring?
No compensation:
pH remains abnormal, and the other value (where the problem isnt occurring, i.e. CO2 or HCO3-) will remain normal or has
made no attempt to help normalise the pH.
For example: in uncompensated metabolic acidosis:
pH 67.23, HCO3- 6 15mmol/L, and the CO2 will be normal at 40mmHg.
Partial compensation
pH is still abnormal, and the other value is abnormal in an attempt to help normalise the pH.
For example: in partially compensated respiratory alkolosis:
pH 57.62, CO2 627 and the HCO3- will be abnormal at 617mmol/L
Full compensation
The pH is normal, as the other value is abnormal and has been successful in normalising the pH.
For example: Fully compensated metabolic acidosis
pH 7.38, HCO3- 615mmol/L and the CO2 630mmHg 6
20 parts base /
1 part acid
WHAT CAUSES THESE CHANGES?
Base excess /
Base deficit
Represents an increase or decrease in the
-2 to +2mmol/L
amount of base compared with the amount
of acids present
HCO3-
Concentration of hydrogen carbonate in
blood. Used to determine along with pH
and CO2 source of acid base imbalance.
pCO2
Carbon dioxide partial pressure (tension). 35-45 mmhg
Reflects alveolar ventilation as it diffuses
across the alveolar capillary membrane and
blown off.
22-26mmol/L
METABOLIC ACIDOSIS
Can be caused by either an increase in circulating acids and or a loss of base (HCO3-). These include:
Renal failure (unable to excrete acids or H+)
Lactic acidosis (increase in circulating acids)
Keto - acidosis (increase in circulating acids)
Diarrhoea (HCO3- loss)
METABOLIC ALKOLOSIS
Can be caused by an increase in HCO3- or loss of metabolic acids. These include:
Prolonged vomiting (acid loss)
GI suctioning (acid loss)
Hypokalaemia (H+ (an acid) excreted to maintain electrolyte balance)
RESPIRATORY ACIDOSIS
Caused by increased CO2 levels which is then converted to an acid (H+) as the body tries compensate by excreting acids via the
kidneys. These include:
Hypoventilation:
- sedatives/sedation/opiates
Depression of respiratory centre in brain stem via trauma
Pneumonia
Pulmonary oedema
Asthma
RESPIRATORY ALKALOSIS
Caused by a hyperventilation, the body getting rid of too much CO2, for example:
Anxiety
Hypoxaemia (caused by heart failure)
paO2
Arterial oxygen tension. In other words
75-100mmhg
how well the lungs are able to pick up
oxygen, i.e. supply, but not demand (this
is shown in a mixed venous gas, discussed
later).
Lactate
When cells no longer have enough O2 for
0.5 - 2.0mmol/L
(Lactic Acid)
normal aerobic metabolism (cell hypoxia)
Anaerobic metabolism takes over resulting
in lactate production, leading to lactic acidosis
Hb
Amount of haemoglobin in blood possibly 135 - 180g/L 7
(Haemoglobin) capable of carrying oxygen.
MIXED VENOUS
BLOOD GAS VALUES
NAME
pH
HCO3pCO2
paO2
sO2
Superior
Vena Cava
VALUE
7.33 - 7.44
24-28mmol/L
41-57 mmhg
35-40mmhg
70 - 75%
Pulmonary
Artery
Mixed venous gases measures oxygen left in the
blood as it returns to the heart (right side) after
it has been pumped around the body supplying cells with oxygen.
The body normally extracts 25% of available
oxygen and leaves 75% in reserve in times of
stress or illness.4
TREATMENT GOALS/GUIDELINES
As with both acidosis and alkolosis, there needs to be a diagnosis of why the patients acid base status is such. Treatment is then
aimed at stabilising the initial/presenting problem as well the acid-base status. However, general guidelines include correcting the
fluid balance and electrolyte status. Continuous regular ABG monitoring and recording for trends. Possible dialysis to remove
excess lactate, manipulation of ventilation settings to adjust CO2 levels, but always maintaining tissue perfusion. Depending
on severity of HCO3- levels and pH - possible sodium bicarbonate infusion (this is controversial however). Of course any of the
adjustments made should only be done with an order from a medical officer, and must go through the intensive care team before
any changes to treatments are applied. 4
OXYHAEMOGLOBIN
DISSOCATION CURVE (ODC)
What is it and why is it important?
The ODC looks at the relationship between oxygen tension (pressure)
and oxygen saturation. It helps us better understand how our blood interacts
with oxygen, i.e. how and why it picks up and lets oxygen go.
The S shape tells us that after an amount of oxygen has accumulated in
the blood, there isnt room for any more, no matter how much oxygen you
throw at the haemoglobin molecule, it wont change
Affinity basically means how much we (or blood) are attracted to
someone (oxygen). The ODC shows us what changes the affinity of blood
for oxygen.
RIGHT SHIFT (ACIDOSIS)
Shift of the curve to right decreases affinity - meaning that the Hb isnt
very attracted to oxygen, and when it does pick up oxygen, it lets it go very
quickly.
Increased temperature and CO2 help this right shift.
LEFT SHIFT (ALKALOSIS)
Shift of the curve to the left increases affinity - meaning that the Hb is very
attracted (oh la la) to oxygen and when oxygen is picked up, the Hb has a
hard time letting it go at the cellular level. Decreased temperature and CO2
help the curve move to the left. 2
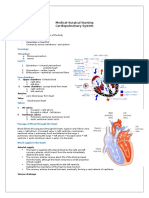
ALKALOSIS
The heart above shows where our subclavian central lines sits - in the superior vena cava, it is here we can take
venous blood gases. Samples from the pulmonary artery are however, more accurate and can only be performed
if the patient has a PA catheter.
ACIDOSIS
OTHER VALUES
OFTEN OVERLOOKED
Anion gap - This value is used in metablic acidosis to find the cause. It reflects unmeasured anions (negatively charged
ions) such as proteins, sulfates, phosphates etc. The equation to find the anion gap is (Na + K+) - (cl + [HCO3-])
a high value can be caused by keto or lactic acidosis, or renal failure. Low values may indicate hyponatremia or decreased
plasma protiens.
10-20mmol/L
OXYGEN STATUS
p50a - oxygen tension at 50% saturation on ODC. This is used to reflect affinity of Hb for oxygen.
25-29mmhg
FMetHb - This is the fraction of methaemoglobin. Think of methaemoglobin like haemoglobin, but we carry less than 1%
of it in our blood. As it is unable to combine with oxygen and it also decreases the oxygen carrying capacity of blood. Exposure to certain drugs and chemicals can dangerously elevate levels.
0.2-0.6%
FCOHb - Fraction of carboxyhaemoglobin. Much similar to FMetHb in its actions. Affinity of Hb for carbon monoxide is
200 times greater than that of oxygen and impairs oxygen transport and release (ODC shift to left, alkolosis). This level can
be high in heavy smokers.
0.0-8.0%
Fshunte - Relative physiological shunt. Basically the amount of venous (de-oxygenated) blood that did not receive oxygen
whilst travelling through the lungs. This can be caused by atelectasis, a pulmonary embolism (PE), mucous plugs and pulmonary oedema, all of which reduces oxygen transport into the blood.
1.0-10%
FO2Hb - fraction of oxyhaemoglobin. This measures just how much blood (Hb) is carrying oxygen compared to the total
amount of Hb capable of carrying. Similar to your SO2, however FCOHb and FMetHb are not included as they are not capable of carrying oxygen.
94-98%
Hct - Hematocrit. This shows us how much red blood cells there are in a sample of blood - i.e. how watered down (or not)
blood is. 36-44%7
REFERENCE LIST
1. Coleman, n. and Houston, L. 1998 Demystifying acid-base regulation Australian nursing Journal 5, 8, 23-26
2. Dickson, S. 1995 Understanding the Oxyhemoglobin Dissociation Curve. Critical care Nurse 15, 10, 54-58.
3. Minor, J. and George, E. 2008 Using mixed venous oxygen saturation to improve patient assessments. Nursing2008 56, 1, 1-3.
4. McCance, K. and Heuther, S. 1998 Pathophysiology, 3rd edn, Mosby, St. Louis, Missouri.
5. Pruitt, W. 2004 Interpreting Arterial blood gases: Easy as ABC. Nursing2004 34, 2, 50-53.
6. Tastota, F. 1994 Assessing A.B.G.s: Maintaining the delicate balance. Nursing94 24, 5, 34-44.
7. The Blood Gas Handbook, 2005. Radiometer Medical ApS RadiometerCopenhagen, Bronshoj.
8. Wikipedia contributors, 2008 Pulmonary artery. n.p. Available URL: www.wikipedia.org/w/index.php?title=Pulmonary artery&oldid=183841779 <Accessed 2008 February 2>
9. Wikipedia contributors, 2008 Oxygen-haemoglobin dissociation curve. n.p. Available URL: wikipedia.org/w/index.php?title=Oxygen-haemoglobin dissociation curve&oldid=187503438 <Accessed 2008 February 2>
Author: Amy ODonnell Date: 9th February, 2008
Hb
O2
O2
Hb
BICARBONATE/CARBONIC (HCO3-/H2CO3) BUFFERING SYSTEM
This is the most active buffering system.
Needs good renal function (HCO3- regulation)
Converts H+ (hydrogen) to H2CO3 (carbonic acid) which is then able to be blown off via the lungs as CO2.
H2O + CO2 n H2CO3 nH+ + HCO3 The H2O and CO2 is regulated via the lungs and HCO3- and H+ via the kidneys. An increase in one value can shift the
equation and helps maintain equilibrium.
A problem in either the lungs or kidneys or both can throw this equation out of whack, resulting in an acid base imbalance.
Other buffers include protein and phosphate buffers.
RESPIRATORY REGULATION OF ACID BASE BALANCE
Eliminates volatile acids (CO2)
Works within minutes
Dependent on HCO3- stores and adequate gas exchange
CO2 diffuses passively into the cerebrospinal fluid and the medulla controls rate and depth of respiration
RENAL REGULATION OF ACID BASE BALANCE
Eliminates fixed acids (harder to remove than volatile) H+
Regulates HCO3- depending on need
Controls phosphate and ammonia buffer systems
Bicarbonate/carbonic acid system can only work if bicarbonate stores are replaced by the kidneys.1
Works slowly, from hours to days
You might also like
- STUDENT-Sickle - Cell-FUNDAMENTAL ReasoningDocument7 pagesSTUDENT-Sickle - Cell-FUNDAMENTAL ReasoningSharon Tanveer0% (1)
- Lab ValuesDocument3 pagesLab Valuessurviving nursing school100% (1)
- Heart FailureDocument1 pageHeart FailureTrisha Vergara100% (1)
- Arterial Blood Gas Analysis - making it easyFrom EverandArterial Blood Gas Analysis - making it easyRating: 4.5 out of 5 stars4.5/5 (4)
- Cardiovascular SystemDocument10 pagesCardiovascular Systemsurviving nursing school100% (2)
- Cardiac Study GuideDocument11 pagesCardiac Study Guidejenwiley318096% (74)
- Practice StripsDocument9 pagesPractice StripsErica Yamamoto50% (4)
- Dysrhythmias Cheat SheetDocument2 pagesDysrhythmias Cheat SheetKatie Coughlan100% (2)
- ABG Practice Problems For N304Document4 pagesABG Practice Problems For N304dlneisha61100% (2)
- CCRN Synergy and Exam StartegiesDocument12 pagesCCRN Synergy and Exam StartegiesMarcus, RN100% (2)
- Shock Comparison ChartDocument2 pagesShock Comparison Chartlinnaete88% (8)
- Critical Care NursingDocument10 pagesCritical Care Nursingianecunar100% (10)
- CCRN-PCCN Review RenalDocument11 pagesCCRN-PCCN Review RenalGiovanni MictilNo ratings yet
- Cardiac Notes NursingDocument16 pagesCardiac Notes NursingYemaya8494% (17)
- Chart of Neuro DisordersDocument1 pageChart of Neuro DisordersNursingSchoolNotes100% (2)
- Respiratory FailureDocument7 pagesRespiratory FailuremuhammadridhwanNo ratings yet
- Neonatal Ventilation Made EasyDocument97 pagesNeonatal Ventilation Made EasyCảnh HoàngNo ratings yet
- ABG Interpretation 3.0Document73 pagesABG Interpretation 3.0Jesus Mario Lopez100% (1)
- Fluid and Electrolytes for Nursing StudentsFrom EverandFluid and Electrolytes for Nursing StudentsRating: 5 out of 5 stars5/5 (12)
- Respiratory Acidosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandRespiratory Acidosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Acid Base Who Is Your DaddyDocument66 pagesAcid Base Who Is Your Daddyjenn1722100% (14)
- ABGs Respiratory/MetabolicDocument3 pagesABGs Respiratory/MetabolicJoe B100% (1)
- Lower Respiratory Study SheetDocument13 pagesLower Respiratory Study SheetJune Rhoades100% (2)
- Blood Gas InterpretationDocument36 pagesBlood Gas InterpretationMary Charmaine DivinagraciaNo ratings yet
- Adult III Cardiac Study GuideDocument15 pagesAdult III Cardiac Study GuideNursingSchoolNotes100% (6)
- Cardiovascular Med Surg Memory Notebook of NursingDocument8 pagesCardiovascular Med Surg Memory Notebook of NursingdmsapostolNo ratings yet
- CCRNDocument17 pagesCCRNNílo Stárn100% (1)
- Cardiac GlycosidesDocument8 pagesCardiac GlycosidesShan Sicat100% (1)
- Simple Method of Acid Base Balance Interpretation: UsefulnessDocument2 pagesSimple Method of Acid Base Balance Interpretation: UsefulnessPeej ReyesNo ratings yet
- Nursing School EKGDocument43 pagesNursing School EKGRob Dickerson100% (1)
- ABG PracticeDocument16 pagesABG Practiceadni_wg100% (1)
- The Cardiovascular System ReviewDocument18 pagesThe Cardiovascular System ReviewDanisha Reeves100% (1)
- Shock Study Guide NursingDocument4 pagesShock Study Guide NursingKory Christina100% (2)
- Nursing Pharmacology Perfusion Study GuideDocument9 pagesNursing Pharmacology Perfusion Study GuideChelsea SmithNo ratings yet
- ABG InterpretationDocument11 pagesABG InterpretationertrggerNo ratings yet
- Mnemonics For NursesDocument52 pagesMnemonics For Nursessweetpearl27100% (3)
- Heart Blocks: "The Heart Block Poem"Document18 pagesHeart Blocks: "The Heart Block Poem"Bijay Kumar Mahato100% (1)
- Respiratory DrugsDocument58 pagesRespiratory DrugsMary Singleton100% (1)
- Med Surg Respiration-Cardiac NotesDocument11 pagesMed Surg Respiration-Cardiac Notesorganictallgirl50% (2)
- Arterial LinesDocument13 pagesArterial LinesberhanubedassaNo ratings yet
- Ccu Survival GuideDocument10 pagesCcu Survival Guideomegasauron0gmailcom100% (1)
- Stages of ShockDocument13 pagesStages of ShockA. P.No ratings yet
- Review Questions Fluid and ElectrolytesDocument2 pagesReview Questions Fluid and Electrolytesmarie100% (13)
- ICU Report SheetDocument1 pageICU Report SheetjejadayoNo ratings yet
- ABG Interpretation - ATSDocument5 pagesABG Interpretation - ATSHAMMYER ALROKHAMINo ratings yet
- Cardiac Study GuideDocument9 pagesCardiac Study GuideJane DiazNo ratings yet
- Dosage CalculationsDocument39 pagesDosage Calculationssalak9462900% (1)
- Med Surge 2 Mod 1 CardiacDocument13 pagesMed Surge 2 Mod 1 CardiacDirk Buckner100% (2)
- ECG StripsDocument5 pagesECG Stripssurviving nursing school100% (1)
- CCRN-PCCN Review GastrointestinalDocument23 pagesCCRN-PCCN Review GastrointestinalGiovanni MictilNo ratings yet
- Obstetrics - Cardiovascular Disease in PregnancyDocument3 pagesObstetrics - Cardiovascular Disease in PregnancyJonathanNo ratings yet
- Cardiac DisordersDocument15 pagesCardiac Disordersgold_enriquez100% (3)
- CCRN Cert Review Neuro IDocument16 pagesCCRN Cert Review Neuro IGiovanni MictilNo ratings yet
- Cardiac DrugsDocument35 pagesCardiac DrugsCristina Centurion100% (3)
- Community Health Study GuideDocument9 pagesCommunity Health Study GuideShae Thomas100% (3)
- Simple Method of ABGs InterpretationDocument9 pagesSimple Method of ABGs Interpretationjhorn_appleNo ratings yet
- Med Surg Final Exam MapDocument42 pagesMed Surg Final Exam MapAnais Hall-Garrison100% (1)
- Spotlight On Cardiac DrugsDocument2 pagesSpotlight On Cardiac Drugspauerish100% (2)
- Ecgs Made Easy 5th Edition Aehlert Test BankDocument14 pagesEcgs Made Easy 5th Edition Aehlert Test BankQuinn50% (2)
- Alterations in VentilationDocument10 pagesAlterations in VentilationCharisma Pastor100% (1)
- Acido Base NEJMDocument12 pagesAcido Base NEJMUlises de ÍtacaNo ratings yet
- Basic Chest X-Ray InterpretationDocument40 pagesBasic Chest X-Ray InterpretationEzekiel Arteta0% (1)
- Abg CaseAnswersDocument7 pagesAbg CaseAnswersDiana HyltonNo ratings yet
- Examination Content Guideline CDocument5 pagesExamination Content Guideline CNHNo ratings yet
- Miller 7th Ed Chapter I HistoryDocument35 pagesMiller 7th Ed Chapter I Historyjbahalkeh7570No ratings yet
- F&E ExamDocument3 pagesF&E ExamDino PringNo ratings yet
- Arterial Blood Gas Interpretation PDFDocument11 pagesArterial Blood Gas Interpretation PDFmail2mohsinaliNo ratings yet
- Questions - ABGDocument5 pagesQuestions - ABGHershey BarramedaNo ratings yet
- Annie - Acid Base BalanceDocument34 pagesAnnie - Acid Base BalanceAnnie GeorgeNo ratings yet
- Heart Failure Care Plan LippincottDocument62 pagesHeart Failure Care Plan LippincottDyllano100% (1)
- Arterial Blood Gas Cases: Case 1Document4 pagesArterial Blood Gas Cases: Case 1Diana HyltonNo ratings yet
- Market Review Blood Gas Analyser S 2010Document43 pagesMarket Review Blood Gas Analyser S 2010Fercalo AndreiNo ratings yet
- Basic Nursing Fundamentals EliminationDocument35 pagesBasic Nursing Fundamentals Eliminationlisa100% (1)
- Acid-Base Disorder 27 July 2017 PDFDocument48 pagesAcid-Base Disorder 27 July 2017 PDFPauline ChanNo ratings yet
- Ventilator Management: Introduction To Ventilator Management, Modes of Mechanical Ventilation, Methods of Ventilatory SupportDocument12 pagesVentilator Management: Introduction To Ventilator Management, Modes of Mechanical Ventilation, Methods of Ventilatory SupportDellNo ratings yet
- Acid Base Balance and Arterial Blood Gas AnalysisDocument29 pagesAcid Base Balance and Arterial Blood Gas AnalysisPaulus LukmanNo ratings yet
- Acid Base BalanceDocument56 pagesAcid Base BalanceVirendra Joshi100% (1)
- Abg Analysis NotesDocument32 pagesAbg Analysis Notesakheel ahammedNo ratings yet
- Respiratory Failure PDFDocument5 pagesRespiratory Failure PDFOxana Turcu100% (1)
- 86F Bancc Competency StatementsDocument82 pages86F Bancc Competency StatementsGrace SimarmataNo ratings yet
- Respiratory Disorder - NclexDocument27 pagesRespiratory Disorder - NclexDefensor Pison GringgoNo ratings yet
- Abg For PgsDocument39 pagesAbg For PgsMobin Ur Rehman Khan100% (3)
- Blood GasDocument9 pagesBlood GasNabila Souza NugrahaNo ratings yet
- Acute Hypercapnic Respiratory Failure Associated With HemodialysisDocument3 pagesAcute Hypercapnic Respiratory Failure Associated With HemodialysisMahmoud DiaaNo ratings yet
- Aspiration PneumoniaDocument16 pagesAspiration PneumoniaFeni DianiNo ratings yet
- Respiratory Anatomy & PhysiologyDocument28 pagesRespiratory Anatomy & PhysiologyKaye CorNo ratings yet
- Nclex 2Document147 pagesNclex 2Habet Fidem100% (6)