Professional Documents
Culture Documents
Methyl Tertiary Butyl Ether MTBE Full Report PDF
Methyl Tertiary Butyl Ether MTBE Full Report PDF
Uploaded by
Trà ĐáOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Methyl Tertiary Butyl Ether MTBE Full Report PDF
Methyl Tertiary Butyl Ether MTBE Full Report PDF
Uploaded by
Trà ĐáCopyright:
Available Formats
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
MEMBER OF GROUP AND SUPERVISORS
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
ACKNOWLEDGEMENT
First and foremost, thank you to Allah S.W.T for giving us the strength to finish up this
project report. Without Your Willingness we would not be able to complete this project.
It would be impossible to acknowledge adequately all the people who have been
influential, directly or indirectly in forming this project.
We would like to take this opportunity to express our deepest gratitude to our
supervisors, Encik Mohd Imran Bin Zainuddin and Puan Sunita Binti Jobli who has
given us his constant encouragement constructive advises and his patient in
monitoring our progress in this project.
Our appreciation and special thanks goes, Puan Hasnora Binti Jafri, Puan Junaidah
Binti Jai, Encik Aziz Bin Ishak for supplying the valuable information and guidance for
this project.
We greatly indebted to Encik Napis Bin Sudin for his cooperation and willingness to be
interviewed and for provide us with invaluable information and for his resourcefulness
in gathering material.
Special thanks owe to Puan Masni Bt Ahmad for her willingness to be interviewed and
for the painstaking care she has shown in assisting us throughout the project.
We also would like to express our appreciation to the Malaysia Industrial Development
Authority (MIDA), Pusat Informasi Sirim Berhad, Petronas Resource Center, Jabatan
Perangkaan Malaysia and Tiram Kimia Sdn.Bhd. (Kuala Lumpur) for their generous
supply of relevant documents and material needed research.
Last but not least to all my lecturers, family, friends and collegues for their
encouragement and kind support when we need it most.
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
ABSTRACT
The purpose for this MTBE or Methyl tertiary Butyl Ether plant is to produce 300,000
metric tonne/year. MTBE is the simplest and most cost effective oxygenate to produce,
transport and deliver to customers. The additive works by changing the oxygenate /
fuel ratio so that gasoline burns cleaner, reducing exhaust emissions of carbon
monoxide, hydrocarbons, oxides of nitrogen, fine particulates and toxic. Two units will
be considered which are the fluidizations, (Snamprogetti) Unit and the Etherification
Unit. The raw materials used are isobutane, methanol, and water as feedstock. In
addition, two types of catalysts are chromia alumina catalyzed compound in
Snamprogetti Unit, while sulphonic ion exchanged resin catalyzed is used in the MTBE
reactor. A good deal of catalyst has been devoted to improve the activity, selectivity,
and the lifetime of the catalysts.
In the Design Project 2, we emphasize in the individual chemical and mechanical
designs for selected equipments in the plant. The chosen equipments are Catalytic
Cracking Reactor, Multitubular Fixed Bed Reactor, MTBE Distillation Column, LiquidLiquid Extraction Column and Heat Exchanger.
Design Project 2 also includes Process Control, Safety, Economic Evaluation, Process
Integration and as well as Waste Treatment, which are considered as group works.
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CONTENTS
TITLE
PAGE
DECLARATION
II
CERTIFICATION
III
ACKNOWLEDGEMENT
ABSTRACT
VI
LIST OF TABLES
LIST OF FIGURES
LIST OF NOMENCLATURES
REPORT 1
CHAPTER 1 PROCESS BACKGROUND AND INTRODUCTION
1.1 Introduction
1.2 Historical Review of MTBE Production Process
1.2.1 UOP Oleflex Process
1.2.2 Philips Star Process
1.2.3 ABB Lummus Catofin Process
1.2.4 Snmprogetti Yartsingtez FBD Process
1
2
3
3
3
4
CHAPTER 2 PROCESS SELECTION
2.1
2.2
Method Consioderation
Detailed Process Description
2.2.1 Snaprogetti Yarsingtez fbd Process
2.2.2 MTBE Unit
2.2.3 Distillation Column Unit
2.2.4 Liquid-Liquid Extraction Unit
5
7
7
8
8
9
CHAPTER 3 ECONOMIC SURVEY
3.1
3.2
3.3
3.4
Market Survey
3.1.1 World Market
Asia Market
Demand
Production Capacity
10
10
11
11
14
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.5
3.6
3.7
Supply
Market Price
3.6.1 Methanol
3.6.2 Isobutane
3.6.3 Catalyst
3.6.4 Conclusion
Economic Analysis
3.7.1 Break Even Analysis
3.7.2 Data Calculation1
14
15
15
16
16
16
17
17
20
CHAPTER 4 PLANT LOCATIONS & SITE SELECTION
4.1
4.2
4.3
4.4
Plant Location
24
General Consideration On the site Selection
24
4.2.1 Location with Respect To Marketing Area 25
4.2.2 Raw Material supply
25
4.2.3 Transport Facilities
25
4.2.4 Availability Of Labor
25
4.2.5 Availability Of Utilities
26
4.2.6 Environmental Impact and Effluent Disposal 26
4.2.7 Local Community Considerations
26
4.2.8 Land (Site Consideration)
26
4.2.9 Political and Strategic Consideration
27
Overview on Prospective Locations
27
4.3.1 Teluk Kalong
28
4.3.2 Tanjung Langsat
28
4.3.3 Bintulu
29
Conclusion
33
CHAPTER 5 ENVIRONMENTAL CONSIDERATION
5.1
5.2
5.3
Introduction
Stack gas
5.2.1 Gas Emission treatment
Wastewater Treatment
5.3.1 Wastewater characteristic
5.3.1a) Priority pollutants
5.3.1b) Organic
5.3.1c) Inorganic
5.3.1d) pH and Alkalinity
5.3.1e) Temperature
5.3.2 Liquid waste treatment
5.3.2a) Equalization treatment
5.3.2b) Solid waste treatment
5.3.3 Waste Minimization
34
35
35
35
35
36
36
37
37
38
38
38
39
41
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CHAPTER 6 SAFETY CONSIDERATION
6.1
6.2
6.3
Introduction
42
Material Safety Data Sheet
43
6.2.1 Isobutane
43
6.2.1.1 Product Information
43
Physical & Chemical Properties
43
6.2.1.2 Immediate Health Effects
44
6.2.1.3 First Aid Measure
44
6.2.2 N-Butane
44
6.2.2.1 Handling and Storage
45
6.2.3 Methanol
45
6.2.4 MTBE
46
6.2.4.1 Physical State and Appearance46
6.2.4.2 Physical Dangers
46
6.2.4.3 Chemical Dangers
47
6.2.4.4 Inhalation Risks
47
6.2.5 TBA
47
6.2.5.1 Recognition
48
6.2.5.2 Evaluation
48
6.2.5.3 Controls
48
Hazard Identification & Emergency Safety & Health Risk 49
CHAPTER 7 MASS BALANCE
7.1
7.2
7.3
7.4
7.5
7.6
7.7
7.8
7.9
Snamprogetti -Yarsingtez FBD Unit
Separator
Mixer
MTBE Reactor
7.4.1 1st Reaction in rector
7.4.2 2nd Reaction in reactor
7.4.3 3rd Reaction in reactor
7.4.4 Overall reaction
Distillation Column
Liquid Extraction Column
Distillation Column
Overall reaction system; flow diagram
Scales Up Factor
51
53
53
54
55
56
57
58
59
60
61
62
63
CHAPTER 8 ENERGY BALANCES
8.1
8.2
Energy Equation
Energy balance: Sample of calculation
8.2.1 Pump 1
8.2.2 Cooler 1
8.2.3 Separator
8.2.4 MTBE Reactor
8.2.5 Pump 2
64
65
73
75
76
78
79
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
8.2.6
8.2.7
8.2.8
8.2.9
8.2.10
8.2.11
8.2.12
8.2.13
8.2.14
8.2.15
8.2.16
Mixer
Expander 1
Cooler 1
Distillation Column 1
Cooler 2
Pump 3
Extraction Column
Pump 4
Pump 5
Distillation Column 2
Cooler 3
CHAPTER 9 HYSYS
80
81
82
84
86
87
88
89
91
92
93
95
APPENDICES
REPORT 2
CONTENTS
PAGE
CHAPTER 1 CHEMICAL DESIGN AND MECHANICAL DESIGN
SECTION 1 CATALYTIC CRACKING DESIGN
2.2
1.1
Introduction
1.2
Estimation of Cost Diameter of Reactor
1.3
Calculation of TDH Height
1.4
Minimum Fluidization Velocity
1.5
Calculation for Terminal Velocity
1.6
Find the Value Kih
1.7
Find the value Eo
1.8
Calculation of Solid Loading
1.9
Calculation for Holding Time
1.10 Calculation for Pressure Drop
1.11 Determine the Direction and Flowrate
1.12 Design of Cyclone
1.13 Calculation for Mechanical Design
Mechanical Design
2.2.1
Introduction
2.2.2
Design stress
2.2.3
Welded Joint Efficiency
1
3
4
4
5
8
9
10
12
14
15
17
21
58
59
59
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
2.2.4
2.2.5
2.2.6
2.2.7
2.2.7.1
2.2.8
2.2.9
2.2.10
2.2.11
2.2.12
2.2.13
2.2.14
2.2.15
2.2.16
2.2.17
2.2.18
2.2.19
2.2.20
2.2.21
Corrosion allowance
Minimum thickness of cylindrical section of shell
Minimum thickness of domed head
Loading stress
Dead weight load
1.2.7.1
Dead Weight of Vessel
1.2.7.2
Weight of the Tubes
1.2.7.3
Weight of Insulation
1.2.7.4
Weight of Catalyst
1.2.7.5
Total Weight
1.2.7.6
Wind Loading
1.2.7.7
Analysis of Stresses
Dead Weight Stress
Bending Stress
Radial Stress
Check Elastic Stability
Vessel Support
Skirt Thickness
Height of the Skirt
Bending Stress at Base of the Skirt
Bending Stress in the Skirt
Base Ring and Anchor Bolt Design
Compensation for Opening and Branches
Compensation for Other Nozzles
Bolted Flange Joint
2.2.20.1
Type of Flanges Selected
2.2.20.2
Gasket
Flange face
SECTION 3
3.1
3.2
3.3
3.4
59
59
60
61
61
61
62
62
63
63
63
64
65
65
66
67
68
68
69
70
70
71
73
74
74
74
75
75
MTBE DISTILLATION COLUMN
Introduction
Selection f Construction Material
Chemical Design
3.3.1 Determine the Number of Plate
3.3.2 Determination of Number of Plate
3.3.3 Physical Properties
3.3.4 Determination of Column Diameter
3.3.5 Liquid Flow Arrangements
3.3.7 Plate Layout
3.3.8 Entrainment Evaluation
3.3.9 Weeping Rate Evaluation
3.3.13 Number of Holes
3.3.14 Column size
Mechanical Design
3.4.1 Material construction
3.4.2 Vessel Thickness
3.4.3 Heads and closure
3.4.4 Total Column Weight
78
79
79
81
88
89
89
90
91
91
94
95
96
98
98
99
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.5
3.4.5 Wind Loads
3.4.6 Stiffness Ring
Vessel Support Design
SECTION 4
4.1
4.2
4.3
5.1
5.2
5.3
DESIGN OF LIQUID-LIQUID EXTRACTION COLUMN
Introduction
Chemical Design
4.2.1 Choice of Solvent
4.2.2 Estimation the Physical Properties
4.2.3 Determination the Number of Stage
4.2.4 Sizing of Sieve Tray
4.2.5 Number of Holes
4.2.6 Column Parameter
4.2.7 Weeping Evaluation
Mechanical Design
4.3.1 Material Construction
4.3.2 Vessel Thickness
4.3.3 Design of Domed Ends
4.3.4 Column Weight
4.3.4.1 Dead Weight of Vessel
4.3.4.2 Weight of Plate
4.3.4.3 Weight of Insulation
4.3.4.4 Total weight
4.3.4.5 Wind Loading
4.3.5 Analysis of Stress
4.3.5. 1 Longitudinal & Circumferential Pressure Stress
4.3.5.2 Dead weight
4.3.5.3 Bending Stress
4.3.5.4 Buckling
4.3.6 Vessel Support Design
4.3.6.1 Skirt Support
4.3.6.2 Base Ring and Anchor
4.3.7 Piping Sizing
SECTION 5
100
100
100
103
104
104
104
105
107
107
107
108
110
111
111
112
112
113
113
113
114
114
115
115
115
115
116
117
117
119
122
HEAT EXCHANGER DESIGN
Introduction
5.1.1 Designing the heater
Chemical Design
5.2.1 Physical Properties of the Stream
5.2.2 The Calculation
5.2.3 Number of Tubes Calculation
5.2.4 Bundle and Shell Diameter
5.2.5 Tube Side Coefficient
5.2.6 Shell Side Coefficient
5.2.7 Overall Heat Transfer Coefficient
5.2.8 Tube Side Pressure Drop
5.2.9 Shell Side pressure Drop
Mechanical Design
5.3.1 Design Pressure
127
129
130
130
131
133
134
135
137
139
140
140
142
142
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
5.3.2
5.3.3
5.3.4
5.3.5
5.3.6
5.3.7
5.3.8
5.39
5.3.10
5.3.11
5.3.12
5.3.13
5.3.14
5.3.15
Design Temperature
Material of Construction
Exchanger Type
Minimum Thickness
Longitudinal Stress
Circumferential Stress
Minimum Thickness of Tube wall
Minimum Thickness of Head and Closure
Minimum Thickness of the Channel Cover
Design Load
Pipe Size Selection for the Nozzle
Standard Flanges
Design Of Saddles
Baffles
142
142
143
143
144
144
144
145
146
147
150
150
152
152
CHAPTER 2 PROCESS CONTROL AND INSTRUMENTATION
2.1
2.2
2.3
Introduction
Objective of control
Control system design sheet
2.3.1 Heat Exchanger
2.3.2 Catalytic cracking fluidized bed reactor
2.3.3 Compressor
2.3.4 Condenser
2.3.5 Separator
2.3.6 Fixed bed reactor
2.3.7 Distillation Column
2.3.8 Liquid -liquid extraction Column
2.3.9 Distillation Column
2.3.10 Mixer
2.3.11 Expander
154
155
156
156
157
158
159
160
161
162
163
164
165
166
CHAPTER 3 SAFETY CONSIDERATION
3.1
3.2
3.3
3.4
Introduction
Hazard and Operability Study
Plant Start Up and Shut Down Procedure
3.3.1 Normal Start Up and Shut Down the Plant
3.3.1.1 Operating Limits
3.3.1.2 Transient Operating and Process Dynamic
3.3.1.3 Added Materials
3.3.1.4 Hot Standby
3.3.1.5 Emergency Shut Down
3.3.2 Start up and Shut down Procedure for the main
Equipment
3.3.2.1 Reactor
3.3.2.2 Distillation Column
3.3.2.3 Liquid-Liquid Extraction Column
3.3.2.4 Heat Exchanger
Emergency Response Plan (ERP)
167
168
170
171
171
172
172
172
172
172
172
173
174
175
175
10
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.5
3.4.1 Emergency Response Procedures
3.4.2 Evacuation Procedures
3.4.3 Fires
3.4.4 Explosion, Line Rupture or Serious Leak
3.4.5 Other Emergencies
Plant Layout
176
176
177
177
177
178
CHAPTER 4 ECONOMIC EVALUATION
4.1
4.2
4.3
4.4
Introduction
Cost Estimation
Profitability Analysis
4.3.1 Discounted Cash flow
4.3.2 Net Present Value
4.3.3 Cumulative Cash flow Diagram
4.3.4 Rate of Return
4.3.5 Sensitivity Analysis
4.3.6 Payback Period
Conclusion
184
187
199
199
202
203
204
205
206
208
CHAPTER 5 PROCESS INTEGRATION AND PINCH TECHNOLOGY
5.1
5.2
5.3
5.4
5.5
Introduction
Pinch Technology
The Problem Table Method
The Heat Exchanger Network
Minimum number of exchangers
209
209
210
214
216
CHAPTER 6 WASTE TREATMENT
6.1
6.2
6.3
6.4
6.5
6.6
Introduction
Wastewater Treatment
Wastewater Treatment Plant Design
Sludge Treatment
Waste Treatment Plant Layout
Absorption tank using granular activated carbon
6.6.1 Analysis of the absorption process
6.6.2 Breakthrough Absorption capacity
220
221
224
229
230
231
232
233
APPENDICES
11
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
LIST OF TABLES OF DESIGN I
TABLE
TITLE
1.1
The Physical and Chemical Properties of MTBE
2.1
The Comparison of the UOP Oleflex, Philips Star
PAGE
2
SP-Isoether FBD Process
3.1
Trade Balance of MTBE in Asia and Pacific
12
3.2
MTBE Balances for Asia and Pacific
13
3.3
Production, Import, Export & Consumption in Europe in
Year 2000
14
3.4
Supplies MTBE Plant in Asia & Pacific
15
3.5
Standard Price for Isobutane
16
3.6
Cost of Producing MTBE 500000 tonne/year
18
3.7
Value in US Dollar Converted to RM
20
3.8
Value in US Dollar Converted to RM per tonne
20
3.9
Data Calculation by using Microsoft Excel in RM
23
4.1
The Comparison of the Potential Site Location
30
4.2
The Comparison of Location in term of Weightage Study
31
4.3
The Electricity Tariffs (Industrial Tariff) for Peninsular
Malaysia and Sarawak
33
12
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
LIST OF TABLES OF DESIGN II
TABLE
TITLE
PAGE
Chapter 1
Section 1
1.1
Calculation for Terminal Velocity in Different Size of dp.
1.2
Correlation of Three Investigators
10
1.3
Data Calculation to Find Solid Loading
12
1.4
Summary of Mechanical Design
40
3.1
The Composition in Feed Stream
80
3.2
The Composition in Top Stream
80
3.3
The Composition in Bottom Stream
80
3.4
The Average Relative Volatility,
3.5
The Non-key Flow of the Top Stream
82
3.6
The Non-key Flow of the Bottom Stream
83
3.7
MTBE Equilibrium Curve
85
3.8
Provisional Plate Design Specification
97
3.9
Summarized Results of Mechanical Design
101
3.10
Design Specification of the Support Skirt
102
4.1
Provisional Plate Design Specification
106
4.2
Summary of the Mechanical Design
118
4.3
Stress Analysis for Liquid-Liquid Extraction Column
119
4.4
Design Specification of the Support Skirt
119
4.5
Piping Sizing for Liquid-liquid Extraction Column
120
Section 3
82
Section 4
Section 5
5.1
Properties of Raw Material (Isobutane and N-butane)
and Steam for (E100)
5.2
5.3
130
Summary of Chemical Design For
Heat Exchanger In Series
141
By taking D = 100 mm, the selected tube nozzle
149
13
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
TABLE
TITLE
PAGE
5.4
By taking D = 500 mm, the selected tube nozzle is:
149
5.5
Standard Flange for Inlet isobutene
150
5.6
Standard Flange for Outlet isobutene
151
5.7
Standard Flange for Inlet Steam
151
5.8
Standard Flange for Outlet Steam
151
5.9
Using Ds = 600mm, the Standard Steel Saddles
for Vessels up to 1.2m
5.10
152
Summary of Mechanical Design For
Heat Exchanger in Series
153
2.1
Parameter at Heat Exchanger
151
2.2
Parameter at Catalytic Cracking Fluidized Bed Reactor
152
2.3
Parameter at Compressor
153
2.4
Parameter at Condenser
154
2.5
Parameter at Separator
154
2.6
Parameter at Fixed Bed Reactor
155
2.7
Parameter at MTBE Distillation Column
156
2.8
Parameter at Liquid-liquid Extraction Column
157
2.9
Parameter at Distillation Column
158
2.10
Parameter at Mixer
159
2.11
Parameter for Expander
160
Important Features in a HAZOP Study
170
4.1
Labor Cost
189
4.2
Estimation Cost of Purchase Equipment
197-198
4.3
Annual Cash flow Before Tax
200
4.4
Annual Cash flow After Tax
201
4.5
Present Worth Value
202
Chapter 2
Chapter 3
3.1
Chapter 4
14
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.6
After Tax Cumulative Cash Flow
TABLE
203
TITLE
PAGE
4.7
Present Value (RM) When i = 30% & i = 40%
204
4.8
Future Value (RM) When MARR = 15%
205
4.9
Simple Payback Period
206
4.10
The Interpolation Simple Payback Period
206
4.11
Discounted Payback Period
207
4.12
The Interpolation Discounted Payback Period
207
5.1
Shows the process data for each stream.
210
5.2
Interval Temperature for Tmin = 10oC
211
5.3
Ranked order of interval temperature
212
5.4
Problem Table
213
Chapter 5
Chapter 6
6.1
Parameter Limits for Wastewater and Effluent under the Environmental Quality
Act 1974
6.2
208
Functions of Pumps in the Waste Treatment Plant
215
15
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
LIST OF FIGURES OF DESIGN I
FIGURE
3.1
TITLE
PAGE
MTBEs Role in US Gasoline grew rapidly
Through 1995
10
3.2
World MTBE Demand (1998-2010) Mod Scenario
11
3.3
MTBE supply & Demand Asia and Pacific
13
3.4
Breakeven Analysis Chart Calculated by using Excel
19
5.1
.
Functional Elements in a Solid-Waste Treatment System
40
16
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
LIST OF FIGURES OF DESIGN II
FIGURE
TITLE
PAGE
Chapter 1
Section 1
1.1
Illustration Diagram of the Reactor
1.2
CDRe2 and CD/Re vs. Reynolds Number
Analysis of Stresses
67
3.1
MTBE Distillation Column
78
3.2
McCabe-Thiele Diagram
86
5.1
Heat Exchanger in Series for the Heating Process
129
5.2
Steel Pipe Nozzle
149
5.3
Standard Flange
150
2.1
Control Scheme for the Heat Exchanger
156
2.2
Control Scheme for Catalytic Cracking
Section 2
2.1
Section 3
Section 5
Chapter 2
Fluidized Bed Reactor
157
2.3
Control Scheme for the Compressor
158
2.4
Control Scheme for the Condenser
159
2.5
Control Scheme for the Separator
160
2.6
Control Scheme for the Fixed Bed Reactor
161
2.7
Control Scheme for the MTBE Distillation Column
162
2.8
Control Scheme for the Liquid-liquid Extraction Column
163
2.9
Control Scheme for the Distillation Column
164
2.10
Control Scheme for the Mixer
165
2.11
Control Scheme for the Expander
166
17
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
FIGURE
TITLE
PAGE
Chapter 3
3.1
Methyl tert-Butyl Ether (MTBE) Plant Layout
180
3.2
Methyl tert-Butyl Ether (MTBE) Plant Evacuation Routes
181
3.3
PID before HAZOP
182
3.4
PID after HAZOP
183
Cumulative Cash Flow (RM) Versus Year
203
5.1
Diagrammatically representation of process stream
210
5.2
Intervals and streams
211
5.3
Heat Cascade
212
5.4
Grid for 4 stream problem
213
5.5
Grid for 4 Stream Problem
214
5.6
Proposed Heat Exchanger Network
216
6.1
The Sludge Treatment System
229
6.2
Waste Treatment Plant Layout
231
Chapter 4
4.1
Chapter 5
Chapter6
18
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Ar
Archimedes number
acceleration
settling chamber longitudinal cross-sectional area
dimension
constant
CD
drag coefficient
concentration
system diameter
particle diameter
de
effective fiber diameter
E,
field intensity
cross-sectional area
Pr
Fronde number
gravitational acceleration
height
precipitation constant ,
Cross sectional area of catalytic reactor
Aor
Area of orifice
C Ag
Concentration of gas reactant
CD
Drag coefficient
d Bv
Diameter of bubble in the bed
dp
Particle diameter
Diffusivity
Dt
Diameter of catalytic reactor
Thickness
Activation energy
19
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
FBo
Mass flow of coal to the catalytic reactor
FC
Fixed carbon mass fraction
Hbed
Height of bed
Hh
Height of Catalytic reactor
Joint factor
Reaction rate constant
Reaction rate constant
K eq
Equilibrium constant
Height above the bed
Total no of orifice
No of holes in 1 m2 area
Nor
No of orifice in 1 m2 area
PCO , PH 2 O
Pi
Design stress
Partial pressure
rC , rS -
Rate of reaction
Ideal gas constant
Ret
Reynolds number
Rp
Radius of particle
Total holding time
Temperature
Uo
Superficial gas velocity
Umf
Minimum fluidization velocity
Ut
Terminal velocity
VBed
Volume of bed
WBed
Weight of coal in bed
WC
Total mass of carbon
Conversion factor
Fitting parameter (for this design is 0.21)
Fitting parameter (for this design is 0.66)
Gas density
Molar density
Bulk density of catalyst
Particle density
20
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Gas viscosity
Time for complete conversion of reactant particle
Pressure drop
total elutriation rate of particles
Ef
frictional force of particles
Ei
entrainment rate of panicle size i
Ei
elutriation rate of particle size i
Eo
total entrainment rate at bed surface
total elutriation rate of particles
gravitational acceleration constant
gc
gravitational conversion constant, m kg/s2 kg -force
Gi
solids flow rate
height above dense bed surface
Rep
particle Reynolds Number = g (U o U ts ) d p /
Ret
dpU g /
time
Umf
minimum fluidization velocity
Uo
superficial gas velocity
Usi
solid velocity (upward)
Us
single particle terminal velocity of particle size i
weight fraction of bed
Ws
weight of solid particles in verlical pipe having length h
Xi
weight fraction of particle size i in bed
Greek Symbols
voidage in freeboard
21
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
voidage in freeboard for system having only particle size i
solid friciion coefficient
gas density
particle density
22
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CHAPTER 1
PROCESS BACKGROUND AND INTRODUCTION
1.1
INTRODUCTION
Methyl tertiary butyl ether (MTBE) is produced by reacting isobutene with methanol
over a catalyst bed in the liquid phase under mild temperature and pressure. Isobutene
can be obtained from stream cracker raffinate or by the dehydrogenation of isobutane
from refineries. Ether in general is a compound containing an oxygen atom bonded to
two carbon atoms.
In MTBE one carbon atom is that of a methyl group CH3 and the other is the central
atom of a tertiary butyl group, -C (CH3)). At room temperature, MTBE is a volatile,
flammable, colorless liquid with a distinctive odour. It is miscible with water but at high
concentrations it will form an air-vapour explosive mixture above the water, which can
ignite by sparks or contact with hot surfaces.
MTBE has good blending properties and about 95% of its output is used in gasoline as
an octane booster and an oxygenate (providing oxygen for cleaner combustion and
reduced carbon monoxide emissions). It is also used to produce pure isobutene from
C4 streams by reversing its formation reaction. It is a good solvent and extractant.
Table 1.1: The Physical and chemical properties of MTBE
23
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Chemical formula
Molecular structure
Oxygen content
Physical state (at normal
C5H12O
(CH3)4CO
18.2 wt%
Colorless liquid
temperature and pressure)
Boiling point
Melting point
Flash point
Autoignition temperature
Flammable limits in air
Relative density
Vapour pressure
Reactive index
Color
Water solubility
55.2oC
-108.6 oC
30 oC
425 oC
1.5 8.5%
0.7405g/ml at 20 oC
245 mm Hg at 25 oC
1.3690 at oC
Colorless
42000mg/l at 25 oC (<10% in
water, miscible with ethanol and
1.2
Partition coefficient n-
diethyl ether)
1.06
octanol/water (log10)
Henrys Law Constant
65.4 Pa/m3/mol
HISTORICAL REVIEW OF MTBE PRODUCTION PROCESS
The MTBE plants actually consist of six units: Isomerization Unit (including
deisobutanizer), Dehydrogenation Unit, MTBE Unit, Methanol Recovery Unit,
Oxygenate Removal Unit and Olefin Saturation Unit. A common offsite utility system
will be incorporated to distribute the required utilities to each unit. There are four
method of producing MTBE implemented under license as the following:
1. UOP-Oleflex Process
2. Phillips STAR Process
3. ABB Lummus Catofin Process
4. Snamprogetti-Yarsingtez FBD (SP-Isoether) Process.
1.2.1
UOP-Oleflex Process
24
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The UOP-Oleflex process uses multiple side-by-side, radial flow, moving-bed reactors
connected in series. Preheated feed and interstage heaters supply the heat of reaction.
The reaction is carried out over platinum supported on alumina, under near isothermal
conditions. The catalyst system employs UOP's Continuous Catalyst Regeneration
(CCR) technology. The bed of catalyst slowly flows concurrently with the reactants and
is removed from the last reactor and regenerated in a separate section. The
reconditioned catalyst is then returned to the top of the first reactor.
processes
involved
are
the
deisobutenization,
the
isomerisation
The typical
and
the
dehydrogenation process that has been commercial in Malaysia.
1.2.2
Philips Star Process
The second one is the Philips Steam Active Reforming (STAR) Process. The Phillips
Steam Active Reforming (STAR) Process uses a noble metal-promoted zinc aluminate
spinel catalyst in a fixed-bed reactor. The reaction is carried out with steam in tubes
that are packed with catalyst and located in a furnace. The catalyst is a solid,
particulate noble metal. Steam is added to the hydrocarbon feed to provide heat to the
endothermic reaction, to suppress coke formation, and to increase the equilibrium
conversion by lowering partial pressures of hydrogen and propane.
1.2.3
ABB Lummus Catofin Process
The ABB Lummus Catofin Process uses a relatively inexpensive and durable
chromium oxidealumina as catalyst. This catalyst can be easily and rapidly
regenerated under severe conditions without loss in activity. Dehydrogenation is
carried out in the gas phase over fixed beds. Because the catalyst cokes up rapidly,
five reactors are typically used. Two are on stream, while two are being regenerated
and one is being purged. The reactors are cycled between the reaction and the
reheat/regeneration modes, and the thermal inertia of the catalyst controls the cycle
time, which is typically less than 10 minutes. The chromium catalyst is reduced from
Cr6+ to Cr3+ during the dehydrogenation cycle. The raw materials used to produce
MTBE by using this method are butanes, hydrogen and as well as recycled isobutene
from the system itself. In this process, there is an isostripper column, which separates
the heavies, and the light ends from which then could produce MTBE.
25
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
1.2.4
Snamprogetti-Yartsingtez FBD (SP-Isoether)
The Snamprogetti-Yarsingtez SP-Isoether (FBD) Process uses a chromium catalyst in
equipment, which is the fluidized bed that resembles conventional fluidized catalytic
cracking technology used in the oil refinery. The catalyst is recirculated from the
reactor to the regeneration section on a 3060-min cycle. The process operates under
low pressure and has a low-pressure drop and uniform temperature profile.
Snamprogetti has been presenting and marketing their hydrogenation technology,
ISOETHER 100, since 1997. This process is to be used to convert MTBE units by
utilizing Snamprogettis MTBE Water Cooled Tubular Reactor Technology. In this SPIsoether Process, the products are MTBE and isooctagenas (iso octane gas). In this
SP-Isoether Process the catalyst used in the isoetherification reactor is the same as
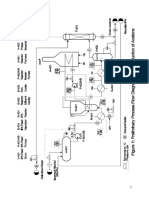
those other typical processes, which is Platinum. (Please refer Appendix A Figure
1.3).
Four method processes of the MTBE above are favorable among the
petrochemical firms.
CHAPTER 2
PROCESS SELECTION
26
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Suitable process, which is gives a lot of profit and less problem is an important in order
to determinant for the success of a plant. This chapter will briefly discuss the best
process selected based on a few criteria. It covers general consideration, detailed
consideration for process selection and conclusion on the process selection.
2.1
METHOD CONSIDERATION.
From the processes mentioned earlier, there are many ways to produce MTBE. It is
essential to choose the best method that will be used to produce MTBE. The selection
of the method must consider the safety of the plant, minimum waste or by product
generated, efficient and economical. Snamprogetti-Yarsingtez SP- Isoether FBD
process will be chosen as the method to produce MTBE. More detailed reasons for the
selection of this process are: High conversion (greater than 98 %) with few by-products
compared to other process. From the economy aspect,Snamprogetti-Yarsingtez FBD
Process can reduce the cost of setting up the plant as it can be implied in any of typical
MTBE-produced plant, known as Financial Safety Net.(When an MTBE plant faces an
oversupplied MTBE market, Isoether makes it possible to switch production from
MTBE to a superior Alkylate.). As for the safety aspects of the plant, as the
Snamprogetti-Yarsingtez FBD is a safe process as it just use the fluidize bed to the
process of producing MTBE. The process operates under low pressure and has a lowpressure drop and this means that the fluidized bed is physically not harmful to anyone.
As for the temperature, it operates under uniform temperature profile. As the
temperature is not high, this means that the process is not as dangerous as other hightemperature-operated process. But, precautions should be taken seriously all the time,
as we do not know when an accident could happen even in the safest place. As for the
waste by using the Snamprogetti-Yarsingtez FBD Process, the product of the process
is only MTBE and other effluent and as well as flue gas which are not harmful to the
environment.
Table 1.1 The comparison of the UOP-Oleflex, Philips Star, ABB Lummus Catofin and
Snamprogetti- Yartsingtez SP-Isoether FBD process.
27
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Method and
UOP-Oleflex
STAR Philip
ABB Lummus
Snamprogetti-
Consideration
Process
process
Process
Yarsingtez FBD
Investment cost
Investment cost
Lower capital
process
Reduce the cost
is very modest
were evaluated
investment
of setting up the
Economic
for 700 BPSD
plant as it can be
Consideration
(650tonne/day)
implied in any of
feed capacity
typical MTBE-
Efficiency
97-99%
98%
99 .99%
produced
Greater than 98%
1. Higher per
1. The Stabilized
1.CD Tech
1.Environmental
pass
Product Is Near
Efficiently Uses
Friendly
conversion and
Equilibrium
The Heat
2.Financial
at least 1-2%
Mixture Of
Released By An
Safety Net.
higher catalyst
Isobutane.
Exothermic
(When an MTBE
selectivity as a
2.The Light-End
Reaction.
plant faces an
result of lowest
Yield Fr. Cracker
2.Conducting 2
oversupplied
operating
Is Less Than 1
Unit Operations
MTBE market,
pressure and
Wt% Butane
In 1 Equipment
Isoether makes it
temperature.
Feed
(Isobutylene
Selectivity)
Advantages
possible to switch
2. No catalyst
production from
losses.
MTBE to a
superior Alkylate.)
Disadvantages
1. Less
1. Much heat is
1. The Reaction
1. Not widely
efficiencies
needed as
Must Take Place
practiced in
furnace is used.
In The Liquid
industry, as it
Phase Catalyst
needs thorough
Must Remain
research to
Completely
implement it.
Wetted.
2.The Reaction
Cannot Be Overly
28
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Endothermic
2.2.1
DETAILED PROCESS DESCRIPTION
2.2.2
Snamprogetti-Yartsingtez SP-Isoether (FBD) Process
The Snamprogetti-Yarsingtez SP-Isoether (FBD) Process uses a chromium catalyst in
equipment, which is the fluidized bed that resembles conventional fluidized catalytic
cracking technology used in the oil refinery with 65% isobutane (i-C4H10) conversion to
produce isobutene.
Dehydrogenation reaction that occur in this process:
iC4H10
iC4H8 + H2
The main feature of this process is that the catalyst filled annuli are connected in such a way
that small, discrete amounts of catalyst can be withdrawn from the bottom of a reactor,
and sent to the top of the reactor. Catalyst withdrawn from the bottom of the reactor is
sent to a separate regeneration section for regeneration prior to being sent to the top of
the reactor. The catalyst is recirculated from the reactor to the regeneration section on
a 3060-min cycle. The reactor and regeneration sections are totally independent of
each other. The regeneration section can be stopped, even for several days, without
interrupting the dehydrogenation process in the reactor section. The vaporized
isobutane is fed along with fresh catalyst to the first, called reactor, and the spent
catalyst is separated from the products and sent to the regenerator, where air (O 2) is
added to oxidize the carbon. The reactor cracks the isobutane and forms coke on the
catalyst. Then in the regenerator the coke is burned off and the catalyst is sent back
into the reactor. The magic of this process is that the reactor-regenerator combination
solves both the heat management and coking problems simultaneously. Burning off the
coke is strongly exothermic, and this reaction in the regenerator supplies the heat
(carried with the hot regenerated catalyst particles) for the endothermic cracking
reactions in the reactor.
The process operates under low pressure and has a low-pressure drop and
uniform temperature profile. Products that have been produced from this unit are
29
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
isobutene. Isobutene available in the C4 stream from the Snamprogetti-Yarsintez FBD
unit will be combining with methanol, which is sourced from the Sabah Gas Industries
methanol plant in Labuan to produce, fuel-grade MTBE with a high-octane value in the
MTBE unit.
2.2.3
MTBE Unit
The MTBE unit includes two sections such as the main reaction section and the
finishing reaction. In the main reaction section, 98% conversions of isobutene occurs
mainly in the main reactor which are designed to provide the mechanical ands thermal
conditions required by the expanded catalyst bid technology.
Reactions occur in this unit are:
1.
iC4H8 (isobutene) + CH3OH (methanol)
2.
CH3OH + CH3OH
(CH3)2O + H2O (DME)
3.
iC4H8 + H2O
C4H10O (TBA)
C5H12O (MTBE)
The reactor is operated in an up-flow direction with an external liquid recycle to
remove the heat of reaction and to control the expansion of the catalyst bed. This
selective reaction of methanol with isobutene is conducted in liquid phase at moderate
temperature on an ion exchange resin type catalyst. The expansion of the catalyst bed
in the reactor is ensured by pump around circulation loop with a cooling water cooler to
control the reactor feed temperature to remove the heat of reaction. Resin traps on top
of each reactor to trap resin in case of carryover with the liquid. In the finishing reactor
section, isobutene final conversion is achieved in a catalytic column where reaction
and distillation are performed simultaneously.
2.2.4
Distillation Column Unit
30
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
This column includes a separation column yielding MTBE product at the bottom and
(isobutene, isobutene, normal butane, water and DME) with methanol entrained by
azeotropy at the top. The reaction section bed is contained in the upper part of this
column. An excess of methanol is maintained corresponding to the amount leaving the
tower in the azeotrope. The required methanol is passed through guard beds and
filtered prior to being charged to the catalytic column to achieve final conversion.
Bottom MTBE product and the other by-product such as TBA, DME is sent to rundown
tanks under level control after cooling in feed/bottom exchanger and trim cooler.
The overhead of the column is condensed in the air-cooled condenser under
pressure control. One part of the liquid is sent to the column as reflux and the other
part to the liquid-liquid extraction unit after cooling.
2.2.5
Liquid-Liquid Extraction Unit
In this unit methanol will extract from the isobutene, isobutene, normal butane to
produce C4 raffinate from the overhead of the column and at the bottom, methanol and
water are produced. C4 raffinate from this unit we decided to sell to the Korea.
CHAPTER 3
ECONOMIC SURVEY
31
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.1 MARKET SURVEY
3.1.1
World Market
The MTBE market has been in strong continuous growth since 1992. For instance, the
1998 world consumption was approximately 19.5 million tonnes, about double that of
1992, representing an annual growth rate of about 12%. Present trends indicate a mild
growth in 2000, up to 20 million tonnes, with US consumption slightly declining and
other parts of the world growing (EEA 2000). The MTBEs role in U.S. gasoline grew
rapidly through 1995 given away in figure 3.1.
Figure 3.1 MTBEs role in U.S. gasoline grew rapidly through 1995
(Sources: Local Issues, Global Implications)
3.2 ASIA MARKET
Most Asia countries such as South Korea, Japan, Hong Kong, Taiwan, China, Malaysia,
Singapore, Philippines and Thailand, have already phased lead out of their gasoline pool
and are replacing it with oxygenates such as MTBE. Due to MTBEs relative ease in
blending into gasoline, easy transportation and storage, as well as relatively cheap and
abundant supply, MTBE is the most widly use oxygenate in Asia.
However, the use of MTBE in gasoline blending is not mandatory for countries
like South Korea and Thailand. South Korea, for instance, requires a 1.3% - 2.3%
32
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
oxygenate content in gasoline during the winter, compared to a minimum of 0.5% for
the summer. In other Asian Countries, MTBE is mainly use as an octane booster to
replace lead. (source: features mtbe asias.html).
3.3 DEMAND
World demand of MTBE mod scenario is about 4.1 mil ton per annum consumption in
US West Coast at stake due to the legislation from 1998 to 2010. It has as an impact
on 80% of PETRONAS MTBE exports to the US. This mod scenario is representing in
figure 3.2.
Figure 3.2: World MTBE demand (1998-2010) mod scenario
(Sources: Petronass Library Kuala Lumpur City Center (KLCC)
U.S. demand is about 250,000b/d, dominates MTBE consumption. Most MTBE
is used to comply with mandated oxygen content rules for gasoline supplied to either
RFG or wintertime carbon monoxide areas. A small amount may be utilized for octane
enhancement.
In Europe, MTBE demand is estimated about 60,000 b/d. MTBE use in Europe
is essentially confined to Octane enhancement, and about 6,000 b/d is exported to the
United States. Eastern Europe currently consumed about 10,000 b/d of MTBE.
In Asia, demand for MTBE in this region is expected to grow at much more
rapid rate than elsewhere in the world. The rate will taper off late in decade from about
12% per year to about 8% by the turn of the century, since the early rapid growth has
33
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
been fed by the lead phase down which should be nearly complete by 2000. Throughout
the period, the region will be a net importer of MTBE, mostly obtained from the Middle
East. The trade balance of MTBE in Asia and Pacific is expected to be in table 3.1.
(Sources: MTBE annual Report)
Table 3.1 Trade Balance of MTBE in Asia and Pacific
(Sources: MTBE annual Report)
Capacities listed are the average available during the year. Details for 1995 and 1999
of MTBE Balance for Asia and Pacific are shown in table 3.2. These data are also
shown graphically in figure 3.3 which indicate for MTBE supply and demand Asia and
Pacific. (Sources: MTBE annual Report)
Table 3.2: MTBE Balance for Asia and Pacific
(Sources: MTBE annual Report)
34
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
MTBE supply and demand
Asia and Pacific
Figure 3.3: MTBE supply and demand Asia and Pacific
(Sources: MTBE annual Report)
Demand for MTBE expected to be marginally firmer in the near future as Asian
Countries such as Indonesia and India are working totally phase out lead from their
gasoline pool. Supply on other hand is expected to remain abundant, as Asia is able to
produce about 3 million Mt/yr of MTBE for its Captive consumption. In addition to this,
Asia attracts a regular supply of about 500,000 ton/yr of MTBE from Middle Eastern
and Europe sources.(Reference: features mtbe asias.html).
35
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.4 PRODUCTION CAPACITY
Commercial production of MTBE started in Europe in 1973 and in the US in 1979. Total
worldwide production capacity in 1998 was 23.5 million tones and the actual production
was 18 million tones
The annual production volume of MTBE in the year 2000 in the Europe was
2,844,000 tons. About 129,000 tonnes was imported and about 479 000 tonnes were
exported outside the Europe in the year 2000 ((Dewitt & Company Inc. 2002). The
majority of the exported volume (> 83%) was exported to USA and Canada. The
majority of exported volume (> 80%) was transported as non-blended MTBE and
minority as a component of petrol (blended). The annual consumption of MTBE within
the Europe was hence 2,495,000 tons in the year 2000 (see table below). For the future
no substantial increase in MTBE usage is expected. (Dewitt & Company Inc. 2002).
Table 3.3: Production, import, export and consumption in Europe in year 2000
(tonnes/year) souces: (Dewitt & Company Inc. 2002).
Production
2 844 000
Import into Europe
129 000
Export outside Europe
479 000
Consumption
2 495 000
The world's MTBE industry today is operating at about 80% of capacity. The US
is by far the largest market, having about 43% of the production capacity but
consuming 63% of total global output. On stability, the Middle East is the swing
producer, exporting more than 50,000 bbl/day to the US and elsewhere.
3.5
SUPPLY
DeWitts Company estimates for local production of MTBE a summarized in table 3.4.
Most of plants unit are refinery-based units taking isobutylene from FCCU units, or as
Raffinate I from olefins plants. Since olefin plants in the region a mostly naphthabased, they produce significant quantities of C4 olefins for this purpose. There is one
butane-based plant in Malaysia. Table 3.4 also shown for MTBE plants suppliers to
Asia and Pacific.
36
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 3.4 Suppliers MTBE plant in Asia and Pacific
(Sources: MTBE annual Report)
3.6 MARKET PRICE
3.6.1
Methanol
Price of methanol, as feedstock in Asia is $240 - $280 /ton. While in Europe, the prices
is $265 - $270 / ton free on board (fob) Rotterdam. In U.S. the price of methanol is 76
cts 77cts/ gal in fob.
Global Methanol demand is expected to increase to 3.5 % per year over the
next 5 years, compared to 1.0% - 1.5% growth in 2002 and 2003. Those lower growth
rates are attributable to the phase-out of Methyl tert-butyl ether (MTBE) as oxygenate
in gasoline in California, and slower economic growth in China caused by SARS.
Methanol growth in China is forecast at 7% - 8.5% per year, fueled by formaldehyde
and acetic acid demand. (Chemicals Week)
3.6.2
Isobutane
Standard price for isobutene is stated by followed:
37
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 3.5 : Standard price for isobutane
Grade
Purity
Grade 4.0
99.99%
Grade 3.0
99.9%
Instrument
99.5%
3.6.3
Cylinder Size
LP30
LP15
LP05
LP01
1/2 Ton
LP30
LP15
LP05
LP01
1 Ton
LP30
LP15
LP05
LP01
Volume
lbs
117
60
23
6
490
117
60
23
6
490
117
60
23
6
Price per Cylinder
RM900.00
RM600.00
RM370.00
RM200.00
RM1225.00
RM380.00
RM240.00
RM170.00
RM100.00
RM890.00
RM293.00
RM185.00
RM100.00
RM75.00
Catalyst
Price of Chromia catalyst Compound USD60 000/Rottedam (Rdam) from the existing
plant. (En Mohd. Napis, from MTBE plant, Gebeng )
3.6.4
Conclusion
Our company will import the methanol and isobutane as feedstock, from Petronas
Malaysia and United State (US) respectively. Methanol feedstock will be supplied from
Gurun, Kedah production capacity of 66,000 ton/year. For the second feedstock,
isobutane (instrument grade) will be supplied by Chevron Phillips Chemical Company
LP, 10001 Six Pines Drive, The Woodlands, Texas, US by shipping method.
MTBE is suitable as a gasoline additive which simultaneously increases the
octane rating of the fuel and adds oxygen which promotes cleaner burning. When used
in place of lead-based octane enhancers, dual environmental benefits are realized, a
reduction in atmospheric lead concentrations and reduced emissions of carbon
monoxide and other smog forming chemicals. Since the 1970s, the worldwide
38
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
consumption of MTBE has increased significantly and many new facilities have been
constructed to support the growing market (Kirschner, 1996; Riddle, 1996).
MTBE production will increase in future in Asia, Asia Pacific, Middle East and
Europe even though MTBE is banned in California but not in the entire nation of the
United States.
3.7
ECONOMIC ANALYSIS
An economic analysis used to smooth the progress of based on existing plant. This
analysis is important to ensure that the chemical plants converge and the economics is
satisfactory before the plant operate. All the data taken from MTBE Annual 1994,
DeWitt & Company
Incorporated, 16800 Greenpoint Park, Suite 120 N, Houston,
Texas, that given by Petronas Library, KLCC.
3.7.1
Break-Even Analysis
When chemical engineers determine outlay for any type commercial process, they
want these costs to be enough accuracy to provide reliable decision. To accomplish
this, they must have a complete understanding of the many factors that can affect
costs. Break-even analysis is important to ensure that the plant can give profit before
the plant can run.
The objective of break even analysis is to find the point, in dollars or in ringgits
and units, at which costs equal revenues. This point is the break even point. Break
even analysis requires an estimation of fixed costs, variable costs and revenue.
Fixed costs are costs that continue even if no units are produced. Examples
include depreciation, taxes, debt, and mortgage payments. Variable costs are those
that vary with the volume units produced. The major components of variable costs are
labor and materials. However, others cost, such as the portion of the utilities that varies
with volume, are also variable cost. The different between selling price and variable
cost is contribution. Only when total contribution exceeds total fixed cost will there be
profit.
39
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Another element in break-even analysis is the revenue function. From the
graph, revenue begins at the origin and proceeds upward to the right, increasing by
selling price of each unit. Where the revenue function crosses the total cost line (the
sum of fixed and variable costs), is the break even point, with a profit corridor to the
right and a loss corridors to the left.
Table 3.6: Cost of producing MTBE 500,000 ton/year
(Sources: DeWitt & Company Incorporated, Annual Report)
Table 3.6 showed that the cost of production of MTBE based on existing plant
producing 500,000 ton/year. From table 3.6, given data, break-even analysis can be
calculated to know the break-even point figure. Figure below indicate that break-even
chart, where it has been calculated by using excel that shown in table 3.8 and based
on the data given from table 3.6.
40
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 3.4 : Break even analysis chart calculated by using excel.
From the break even chart figure above, the value of break-even point at the
existing capacity of 500,000 ton/year is 185,629.85 tons in units and RM
244,679,817.14 in Ringgit Malaysia (RM). This value indicates the minimum units and
values needed to be sold. The given capacity of 500,000 tons/year can give profit to
the company. The margin of safety (MOS) calculated from the graph, which is
314,370.15 tons and RM414,373,182.86. Margin of safety (MOS) in percentage of
sales is 62.87%. The sale is allowed to drop about 62.87% before the company will
incurred a loss.
In other word, at selling 300,000 tons/year capacity will also give profit to our
company. The margin of safety from the graph for 300,000 ton/year calculated is
114,370.15 tons and RM150,751,982.86. The margin of safety (MOS) as percentage of
sales is 38.12%. The sale is allowed to drop about 38.12% before the company will
incurred a loss. All the data calculation is shown in the next section.
41
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.7.2
Data Calculation
All the data based on 500,000 tons/year producing MTBE from existing plant.
Table 3.7 Values in USD converted to RM
(Sources: Data collected from table 3.6)
Total revenue, TR
Total variable cost, TVC
Total fixed cost, TFC
RM 659,053,000.00
RM 504,754,000.00
RM 57,285,000.00
Total Revenue (TR), MTBE (500,000 ton),
TR
= Quantity of MTBE X Price of MTBE
= QMTBE X PMTBE
= 500,000 tons X USD346.87 X 3.8
= RM 659,053,000
Total cost
TC
= total fixed cost + total variable cost
= TFC + TVC
Where,
Total fixed cost
= 500,000 ton X USD30.13 X 3.8
= RM 57,285,000.00
Total variable cost
= 500,000 ton X USD (226.4 + 39.26) X 3.8
= RM 504,754,000.00
Total cost, TC
= RM57,285,000.00 + RM 504,754,000.00
= RM 562,039,000.00
Tables 3.7 represent cost per unit ton converted into Ringgit Malaysia (RM), taking
datas directly from the table 3.6.
Table 3.8 Values in USD converted to RM per ton
(Sources: Data collected from table 3.6)
Revenue (RM) per ton
RM1,318.00
Variable cost (RM) per ton
RM 1,009.51
Fixed cost (RM) per ton
RM114.57
Break-even point in ton can be calculated based on formula equation, which given by
follow:
42
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Break-even point, BEP (tons)
= Total Fixed cost
Contribution/ton
where,
Contribution/ton
= revenue / ton - variable cost / ton
BEP (tons) =
______RM 57,285,000.00_____
(RM1, 318.00 - RM 1,009.51)
185,629.85 tons (the minimum capacity)
Next, Break-even point in RM can be calculated based on formula equation, which
given by follow:
BEP (RM)
Break-even point, BEP (tons) X revenue / ton
185,629.85 tons X RM1, 318.00
RM 244,679,817.14
Beside that, margin of safety and percentage of sale can be calculated as follows:
For 500,000 ton/year production,
Margin of safety (MOS) in units = Budgeted sale (units) - BEP (units)
= 500,000 tons - 185,629.85 tons
= 314,370.15 tons
Margin of safety (MOS) in RM = Budgeted sale (RM) - BEP (RM)
= RM 659,053,000.00 - RM 244,679,817.14
= RM 414,373,182.86
Margin of safety (MOS) as percentage of sales = MOS (RM) x 100%
Sales(RM)
= RM 414,373,182.86 x 100%
RM 659,053,000
= 62.87%
43
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
For 300,000 ton/year production,
Margin of safety (MOS) in units = Budgeted sale (units) - BEP (units)
= 300,000 tons - 185,629.85 tons
=
114,370.15 tons
Margin of safety (MOS) in RM = Budgeted sale (RM) - BEP (RM)
= RM 395,431,800 - RM 244,679,817.14
= RM 150,751,982.86
Margin of safety (MOS) as percentage of sales = MOS (RM) x 100%
Sales(RM)
= RM150,751,982.86 x 100%
RM 395,431,800
= 38.12%
Table 3.9 shown that the calculation of break-even point by using excel.
Table 3.9: Data calculation by using excel in RM
(Sources: Data taking from table 3.7)
44
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CAPACITY
TFC1
57,285,000.00
10000
57,285,000.00
20000
TVC1
0
TR1
TC1
57,285,000.00
10,095,080.00
13,181,060
67,380,080.00
57,285,000.00
20,190,160.00
26,362,120
77,475,160.00
40000
57,285,000.00
40,380,320.00
52,724,240
97,665,320.00
60000
57,285,000.00
60,570,480.00
79,086,360
117,855,480.00
80000
57,285,000.00
80,760,640.00
105,448,480
138,045,640.00
100000
57,285,000.00
100,950,800.00
131,810,600
158,235,800.00
120000
57,285,000.00
121,140,960.00
158,172,720
178,425,960.00
140000
57,285,000.00
141,331,120.00
184,534,840
198,616,120.00
160000
57,285,000.00
161,521,280.00
210,896,960
218,806,280.00
180000
57,285,000.00
181,711,440.00
237,259,080
238,996,440.00
200000
57,285,000.00
201,901,600.00
263,621,200
259,186,600.00
220000
57,285,000.00
222,091,760.00
289,983,320
279,376,760.00
240000
57,285,000.00
242,281,920.00
316,345,440
299,566,920.00
260000
57,285,000.00
262,472,080.00
342,707,560
319,757,080.00
280000
57,285,000.00
282,662,240.00
369,069,680
339,947,240.00
300000
57,285,000.00
302,852,400.00
395,431,800
360,137,400.00
320000
57,285,000.00
323,042,560.00
421,793,920
380,327,560.00
340000
57,285,000.00
343,232,720.00
448,156,040
400,517,720.00
360000
57,285,000.00
363,422,880.00
474,518,160
420,707,880.00
380000
57,285,000.00
383,613,040.00
500,880,280
440,898,040.00
400000
57,285,000.00
403,803,200.00
527,242,400
461,088,200.00
420000
57,285,000.00
423,993,360.00
553,604,520
481,278,360.00
440000
57,285,000.00
444,183,520.00
579,966,640
501,468,520.00
460000
57,285,000.00
464,373,680.00
606,328,760
521,658,680.00
480000
57,285,000.00
484,563,840.00
632,690,880
541,848,840.00
500000
57,285,000.00
504,754,000.00
659,053,000
562,039,000.00
CHAPTER 4
45
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
PLANT LOCATIONS AND SITE SELECTION
4.1
PLANT LOCATION
The location of the plant can have a crucial effect on the profitability of a project and the
scope for future expansion. Many factors must be considered when selecting a suitable
site. A good location is required to optimise the production of the plant. It is important to
know that, not all Malaysian industrial park caters the need of a chemical plant. Also not
all industrial park allows the building of chemical plants. Our industrial parks are divided
into categories such as: 1. Light industrial
2. Medium industrial
3. Heavy industrial
4. General industrial
5. Hi-tech industrial
4.2
GENERAL CONSIDERATION ON THE SITE SELECTION
All the information about plant locations are based on the data gathered from the
Malaysian Industrial Development Authority (MIDA). And we refer detail information on
important factors that need to be considered in the site selection. In the process of
selecting the location, we did some evaluation. Among the principle factors considered
are:
4.2.1
Location With Respect To Marketing Area
For materil that are produced in bulk quantities, such as cement, fertilizer, raw material
of petrochemical product, where the cost of product per tone is relatively low and the
cost of transport a significant fraction of the sales price, the plant must located close to
the primary market. This consideration will be less important for low volume production,
high priced products; such as pharmaceuticals, plastisizer and etc. in an international
46
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
market, there may be an advantage to be gained by locating the plant within an area
with preferential tariff agreement.
4.2.2
Raw Material Supply
The availability and price of suitable raw materials will often determine the site location.
Plant producing bulk chemicals are best located close to the source of the major raw
material, where this is also close to the marketing area.
4.2.3
Transport Facilities
The transport of materials and products to and from the plant will be an overriding
consideration in site selection. If practicable, a site that we are consider that close to at
least two major forms of transport: road, rail, waterway or a sea port. Road transport
being increasing used, and is suitable for local distribution from central warehouse. Rail
transport will be cheaper for the long distance transport of bulk chemicals
.
Air transport is convenient and efficient for the movement of personnel and
essential equipment and supplies and the proximity of the site to a major airport also
considered.
4.2.4
Availability of Labour
Labour that will be needed for construction of the plant and its operation. Skilled
construction workers will usually be brought in from outside the site area, but there
should be an adequate pool of unskilled labour available locally and labour suitable for
training to operate the plant. Skill tradesman will be needed for plant maintenance.
Local trade union customs and restrictive practices will have to be considered when
assessing the availability and suitability of the local labour for requirement and training
.
4.2.5
Availability of Utilities
47
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Chemical processes invariably require large quantities of water for cooling and general
process used and the plant must be located near a source of water of suitable quality.
Process water may be drawn from a river, wells or purchased from a local authority.
At some site, the cooling water required can be taken from a river or lake or
from the sea; at other locations cooling towers will be needed.
Electrical power will be needed at all sites. Electrochemical processes that
required large quantities of power: for example, aluminium smelters need to be located
close to a cheap source of power. A competitively priced fuel must be available onsite
for steam and power generation.
4.2.6
Environmental Impact and Effluent Disposal
All industrial processes produce waste products and full consideration must be given to
the difficulties and cost of their disposal. The disposal of toxic and harmful effluents will
be covered by local regulations and the appropriate authorities must be consulted
during the initial site survey to determine the standards that must be met.
An environmental impact assessment should be made for each new project or
major modification of addition to an existing process.
4.2.7
Local Community Considerations
The proposed plant must fit in with and be acceptable to the local community. Full
consideration must be given to the safe location of the plant so that it does not impose
a significant additional risk to the community.
On a new side, the local community must be able to provide adequate facilities
for the plant personnel: schools, banks, housing and recreational and cultural facilities.
4.2.8
Land (site consideration)
48
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Sufficient suitable land must be available for the proposed plant for future expansion.
The land should ideally be flat, well drained suitable load-bearing characteristics. A full
site evaluation should be made to determine the need for piling or other special
foundations.
4.2.9
Political and Strategic Considerations
Capital grants, tax concessions and other inducements are often given by government
to direct new investment to preferred locations such as areas of high unemployment.
The availability of such grants can be the overriding factor in site selection.
4.3 OVERVIEW ON PROSPECTIVE LOCATIONS
Our process is a petrochemical base process; therefore we choose to locate our plant in a
petrochemical complex. The reason is quite simple; a petrochemical complex could
simplify the formation and the maintenance of a chemical plant. It could also cut the daily
operation cost and saving us the hassle of transportation.
In Malaysia there are only three such places, known as the Integrated
Petrochemical Complexes. These complexes are situated in each of the site below:
1. Telok Kalong Industrial Park.
2. Tanjung Langsat Industrial Park.
3. Bintulu Industrial Park.
Other than the above factors, the capacity of plant was also taken into consideration in
determining the suitability of site. Plant capacity will determine how big the space required
to build the plant and the storage area and also the mode of transportation to be use.
The manufacture of MTBE is classified as a petrochemical project. Several
locations of industrial area particular at Teluk Kalong Industrial Area in Terengganu,
Tanjung Langsat Industrial Area in Johor and Bintulu Industrial Area, Sarawak that we are
refer for location.
49
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.3.1
Teluk Kalong
Teluk Kalong Industrial Estate located 9.6 km from Kemaman. Total area available
167.46 hectares. The price of land in ranges RM 0.46 to RM 4.18 per Feet Square.
This area is proposed for petrochemical and heavy industry petrochemical.
The Electricity is generated at the following station. Total generation capacity is
900 MW. Local consumption is less than 1/3. No major breakdown, low frequency of
interruption. Water most plentiful with surplus capacity. Water supply capacity at
various treatment plants total 331000-meter cube per day, with planned upgrading for
additional requirement. Kenyir Lake with 39000 hectares of water with 134 metre
average depth, make Terengganu a potential export of water middle East. Water
supply is in Bukit Shah. Water tariffs (industrial) are RM1.15 metre cube. The raw
materials supplier of isobutene is availability from Chevron Philips Chemical Company
LP, United State and methanol is availability from Petronas Malaysia, Labuan.
1.
Airport facilities
Terengganu major industrial locations are serve by 3 airports
- Kuantan
- Kerteh
- Kuala Teregganu
2.
4.3.2
Kuala Teregganu
Port Facilities
Kemaman Port, Kerteh Port and Kuantan Port
Tanjung Langsat Industrial Park
Tanjung Langsat is designed as hub for heavy/medium industries with all the
necessary infrastructure and service facilities. 91.43 km distance from Johor Baharu.
The infrastructure works such as the Pasir Gudang Segamat Highway. Sungai
Johore Bridge and dedicated Port in Tanjung Langsat. Tanjung Langsat Industrial
Complex is a sprawling area just a stones through from Pasir Gudang Industrial Area.
A total hectare still available is 1,085.95. Selling price is RM8 to RM22 square feet. In
term of seaport two seaports are currently being constructed at Tanjung Pelepas,
50
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
located 40 km west of Johore Baharu city and Tanjung Langsat located 10 km east of
the Johore Port. Tenaga Nasianal Berhad (TNB) provides electricity.
Two airports in the 50km radius. There is the Sultan Ismail International Airport
(common known locally as Senai Airport) in Johore Baharu and the Changi
International Airport in Singapore. The Sultan Ismail International Airport, which is
located about 30km to the north west of JB city, is currently being expended and
upgrades to become the regional airport for southern peninsular Malaysia.
4.3.3
Bintulu
The distance from nearest town is 224.29 km from Sibu. Type of industries is light and
medium petrochemical. Area available is 77 hectares. Selling price RM2.5 to RM10 per
feet square. Electricity supplies by Sarawak Electricity Supply Cooperation (SESCO).
Airport facilities - Bintulu Airport
Port Facilities - Bintulu Port
51
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 4.1 The Comparison of The Potential Site Location:
Teluk Kalong
Industrial Park
Tanjung Langsat
Industrial Park
Bintulu Industrial
Park
9.6 km from Kemaman
91.43 km from Johor
Baharu
224.29 km from Sibu
Types of
Industry
Isobutane from US and
methanol from Labuan
Petrochemical and heavy
industry
Isobutane from US and
methanol from Labuan
Petrochemical light and
medium
Isobutane from US and
methanol from Labuan
Petrochemical light and
medium
Area Available
167.46 hectares
1085.98 hectares
77 hectares
RM 0.46 - 4.18
RM 8.00 - 22.00
RM 2.50 - 10.00
Electricity
Supply
Tenaga National Berhad
Tenaga National Berhad
Sarawak Electrycity
Supply Cooperation
(SESCO)
Water Supply
Bukit Shah Water
Treatment
Road Facilities
Kuala TerengganuKuantan-Kuala LumpurKuala Terengganu-KertehTeluk Kalong-KuantanKuala Lumpur
Distance from
the nearest town
Raw Material
Land Price
2
(RM/ft )
Airport Facilities
Port Facilities
Water Tariffs
3
(RM/m )
Kuala Terengganu Airport
Kerteh Airport
Kemaman Port,
Kerteh Port
Kuantan Port
RM 1.15
Syarikat Air Johor and
Logi Air Sg. Layang
Syarikat Air Sarawak
North-South Highway
from Bukit Kayu Hitam to
Singapore-
Major Road : Bintulu Sibu and Bintulu - Miri
Senai International
Airport
Bintulu Airport
Pasir Gudang Port
Bintulu Port
RM 1.68 (0-20 m 3)
RM2.24 (more than
20 m 3)
RM 0.95 (0 -25 m 3)
RM1.20 (more than
25 m 3)
(Source: MIDA)
A few proposed plant sites were narrowed down based on the above factors (table 4.2).
Table 4.2 is a summary of location and factors being considered. After detailed study of
52
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
the factors, each was given weightage and was estimated. The result tabulated in table
4.2 for the purpose of comparison.
Table 4.2 The Comparison of Location in term of Weightage Study
Weightage
Telok Kalong
Industrial
Area
Tanjung
Langsat
Industrial
Area
Bintulu
Industrial Area
Marketing Area
10
Raw Material
10
Transport
10
Availabillity of
Labour
10
Utilities
10
Total Land
Available
10
Climate
10
Price of Land
10
Local
Community
Consideration
10
Incentives
10
TOTAL
100
80
79
77
0 to 10 with 10 is the best
Table 4.3 The Electricity Tariffs (Industrial Tariff) for Peninsular Malaysia and Sarawak
53
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Tariff
Peninsular
Malaysia
Tariff D (Low Voltage, and less than 6.6
supply) for all consumptions
Cost per kWh
kV
Tariff E1 (Medium Voltage General, 6.6 kV - 66 kV
supply) for all consumptions. For each kW of maximum
demand per month: RM 17.30
25.8
19.8.
Tariff E2 (Medium Voltage Peak/Off-Peak, 6.6 kV 66kV supply)
Peak period (0800-2200 hours),
20.8
Off-Peak period (2200-0800 hours).
12.8
For each kW of maximum demand per month during
peak period: RM21.70
Tariff E3 (High Voltage Peak/Off-Peak, more than 132
kV supply)
Peak period (0800 -2200 hours),
Off-Peak period (2200 - 0800) hours).
For each kW of maximum demand per month during
peak period: RM 20.80
Sarawak
Tariff 11
1st 100kWh
In excess of 100kWh to 3000 kWh
In excess of 3000 kWh
Minimum charge per month: RM 10.00
Tariff 12
All units
17.8
10.8
40
30
21
17
For each kW of maximum demand per month: RM12.00
Minimum charge : RM 12.00 per kW x billing demand
Tariff 13 (Peak/Off-Peak)
Peak period ( 0700 - 2400 hours)
Off-Peak period ( 0000 - 0700 hours)
For each kW of maximum demand per month during
peak period: RM20.00
Minimum charge: RM 20.00 per kW x billing demand.
17
10
(Source: MIDA)
54
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.4
CONCLUSION
Based on the factor weightage studied, it can be concluded that Telok Kalong Industrial
Estate is the most suitable and practical location to choose as a site for MTBE plant.
The philosophy of in situ consumption of much of the production MTBE,
together with remaining product aimed directly at the export market and also makes the
need for port facilities of paramount importance. The Tanjung Langsat and Bintulu
Industrial Area are not impressive for MTBE plant. There are many other reasons
influences our decision including:
Nearest of the Kuantan Port, Kemaman Port and Kerteh Port facilities is more
convenient and economically for export and import purposes.
Excellent and consistent support from bulky oil, gas and chemical supplier from
Kerteh.
Constantly upgrading existing and developing new infrastructure, facilities and supporting
industries. These include the construction of roads; to increase accessibility to and from
the estates are scheduled.
55
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CHAPTER 5
ENVIRONMENTAL CONSIDERATION
5.1
INTRODUCTION
Nowadays, environmental issues become very important. Besides this, a good waste
treatment system is also important in order to reduce and minimize
environmental pollutants. The chemical waste in the form of solid, liquid and
gases must be treated before being discharged into sewage, drain and
atmospheres.
Any chemical plant to be set up in Malaysia must follow the rules and
regulations under the Department of Environment (DOE) Malaysia, which includes the
Environmental Quality Act 1974. Under Environmental Quality Act (Sewage and
Industrial Effluents) Regulation 1979 and Environment Quality Act (Clean Air) 1978.
The plant owner or waste generator must ensure that waste generated disposed
appropriately to prevent environmental pollution. The proper and suitable methods
should be implemented in dealing with the waste disposal. Kualiti Alam Sdn. Bhd is
one of the licensed contractors specialized in the industrial waste disposal in Malaysia.
MTBE plant is not excluded from these regulations. As our plant produces
MTBE and other byproducts like raffinate but generally they are not hazardous to the
environment and human if safety measures are taken into consideration. These
environmental considerations depend on the location of our plant. The plant will follow
the Standard B of water quality measurement and also need some waste treatment
facilities to minimize the pollution from our plant.
56
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
STACK GASES
Gas Emission Treatment
Direct flame combustion was used to burn the excess gas. Flare is usually open ended
combustion unit. Therefore, the combustion process will be controlled by flow
rate of gases mixture to prevent incomplete combustion.
Another treatment is thermal combustion. It is an incinerator used in the cases
where the concentration of combustible gases is too low to make direct flame
incineration insufficient condition. The temperature of operation depends upon the type
of pollutant in waste gas. Thermal combustion must be carefully designed to provide
safe, efficient operation and to prevent incomplete combustion. Time, temperature, and
oxygen must be carefully monitored. (Howard et. al 1985)
Stack gas means the product of combustion process usually occur at machine or
generator. It is usually the fuels used occurred in the complete combustion
process, but it produced unwanted gas such as carbon monoxide, sulphur oxide
and other gases.
In our MTBE plant, the stack gases is only Hydrogen and it is stored in a
special tank before being sold to interested company at market price.
5.3
5.3.1
WASTEWATER TREATMENT
Wastewater Characteristics
Wastewater characteristics vary widely from industry to industry. Obviously, the
specific characteristics will affect the treatment techniques chosen for use in meeting
discharge requirements. Because of the large number of pollutant substances,
wastewater characteristics are not usually considered on a substance-by-substance
basis. Rather, substances of similar pollution effects are grouped together into classes
of pollutants or characteristics are indicated below.
5.3.1(a) Priority Pollutants
57
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Recently, greatest concern has been for this class of substances for the reasons given
previously. These materials are treated on an individual-substance basis for regulatory
control. Thus each industry could receive a discharge permit that lists an acceptable
level for each priority pollutant.
5.3.1(b) Organics
The organic composition of industrial wastes varies widely, primarily due to the
different raw materials used by each specific industry. These organics include proteins,
carbohydrates, fats and oils, petrochemicals, solvents, pharmaceutical, small and large
molecules, solids, and liquids. Another compilation is that a typical industry produces
many diverse waste streams. Good practice is to conduct a material balance
throughout an entire production facility. This survey should include a flow diagram,
location and sizes of piping, tanks, and flow volumes, as well as an analysis of each
stream.
An important measure of the waste organic strength is the 5-day biochemical
oxygen demand (BOD5). As this test measures the demand for oxygen in the water
environment caused by organics released by industry and municipalities, it has been
the primary parameter in determining the strength and effects of a pollutant. This test
determines the oxygen demand of a waste exposed to biological organisms (controlled
seed) for an incubation period of five days. Usually this demand is caused by
degradation of organics according to the following simplified equation, but reduced
inorganics in some industries may also cause demand (i.e., Fe2+, S2- and SO32-).
Organic waste + O2
CO2 +H2O
In general, low-molecular-weight water-soluble organics are biodegraded
readily. As organic complexity increases, solubility and biodegrability decrease. Soluble
organics are metabolized more easily than insoluble organics. Complex carbohydrates,
proteins and fats and oils must be hydrolyzed to simple sugars, aminos, and other
organics acids prior to metabolism. Petrochemicals, pulp and paper, slaughterhouse,
58
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
brewery, and numerous other industrial wastes containing complex organics have been
satisfactorily treated biologically, but proper testing and evaluation is necessary.
5.3.1(c) Inorganics
The inorganics is most industrial wastes are the direct result or inorganic compounds in
the carriage water. Soft-water sources will have lower inorganics than hard-water or
saltwater sources. However, some industrial wastewaters can contain significant
quantities of inorganics which result from chemical additions during plant operation.
Many food processing wastewaters are high in sodium.
While domestic wastewaters have a balance in organics and inorganics, many
process wastewaters from industry are deficient in specific inorganic compounds.
Biodegration of organic compounds requires adequate nitrogen, phosphorus, iron, and
trace salts. Ammonium salts or nitrate salts can provide the nitrogen, while phosphates
supply the phosphorus.
5.3.1(d) pH and Alkalinity
Wastewaters should have pH values between 6 and 9 for minimum impact on the
environment. Wastewaters with pH values less than 6 will tend to be corrosive as a
result of the excess hydrogen ions. On the other hand, raising the pH above 9 will
cause some of the metal ions to precipitate as carbonates or as hydroxides at higher
pH levels. Alkalinity is important in keeping Ph values at the right levels. Bicarbonate
alkalinity is the primary buffer in wastewaters. It is important to have adequate alkalinity
to neutralize the acid waste components as well as those formed by partial metabolism
or organics.
Many neutral organics such as carbohydrates, aldehydes, ketones, and
alcohols are biodegraded through organics acids which must be neutralized by the
available alkalinity. If alkalinity is inadequate, sodium carbonate is a better form to add
than lime. Lime tends to be hard to control accurately and results in high pH levels and
precipitation of the calcium which forms part of the alkalinity. In a few instances,
sodium bicarbonate may be the best source of alkalinity.
59
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
5.3.1(e) Temperature
Most industrial wastes tend to be on the warm side. For the most part, temperature is
not a critical issue below 37oC if wastewaters are to receive biological treatment. It is
possible to operate thermophilic biological wastewater-treatment systems up to 65oC
with acclimated microbes. Low-temperature operations in northern climates can result
in very low winter temperatures and slow reaction rates for both biological treatment
systems and chemical treatment systems.
Increased viscosity of wastewaters at low temperatures makes solid separation
more difficult. Increased viscosity of wastewaters at low temperatures makes solid
separation more difficult. Efforts are generally made to keep operating temperatures
between 10 and 30oC if possible.
5.3.2
Liquid Waste Treatment
5.3.2(a) Equalization Treatment
Liquid treatment generally is necessary in any plant. In our plant, we also have liquid
treatment but in general, we only state the general method, as our plant does not
produce any significant liquid waste. In any liquid waste treatment, we need
equalization treatment. The equalization treatment is an initial procedure in liquid waste
treatment. The purpose of equalization is to minimize and control the fluctuation in
liquid waste characteristic. Besides it provides the suitable and optimum condition for
biological and chemical treatment. It also provides adequate damping to minimize the
chemical consumption. The procedure will occur in the equalization tank. The size of
tank and time of equalization process depend on the liquid waste amount.
The Activated Sludge process will be used for this treatment. It is carried out in
Aerobic condition. The main purpose of activated sludge process is to remove soluble
and insoluble organic matter that converted into flocculants microbial suspension and
settable microbial. It also permits the use of gravitational solid liquid separation
technique for the above requirement.
The organic matter where measured in the form of BOD and COD serves as
food and energy source for microbial growth. It converts the pollutant into microbial cell
60
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
and oxidized end product such as CO2 and H2O by microbial activities. Therefore,
Submersible Aerator as mixing device will supply the oxygen and nutrient into aeration
tank and therefore improves the quality of the liquid. (Howard et. al, 1985)
5.3.2(b)
Solid Waste Treatment
The solid waste treatment will be minimized by regenerating the catalyst. Regeneration
processes depend on the characteristic of catalyst after whole reaction. Licensed
contractor will dispose the solid waste to follow the DOE regulation. By the way,
the scheduled maintenance activities will be implemented.
Dewatering system will be used to solidify and extract the catalyst. Therefore,
clarifier and filter press were used in these treatments. Clarifier is used to clarify any
impurities before going through the filters. The size of equipment depends on the flow
rate and holding time of these processes. Maintenance activities will be scheduled
based on the availability of workers and machines. Skilled and experienced workers
will do the maintenance activities, (Bailed, 1995).
61
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Industrial
Process
Waste
Reduction
Waste
Generation
Re-use
Storage
Transfer/
Transport
Processing/
Recovery
Collection
Disposal
Recycling/
Reuse
Figure 5.1 Functional Elements in a Solid-waste Treatment System.
62
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
5.3.3 Waste Minimization
Waste minimization means the optimization process to minimize the waste come out of
the plant. It will be done by source reduction and recovery of the sources. The source
reduction refers to preventative measured taken to reduce the amount of waste, which
produced in this process. Recovery of the sources is aimed to reuse the excess
methanol to produce the MTBE.
Waste production from the plant could be reduced by implementing these
procedures:
-
Raw material modification,
Product reformulation,
Process modification,
Improvement in operating practices.
The most important is by improving the product yield and this means
minimization of waste generation. It will be accomplished through improvement in
catalyst efficiency and proper maintenance activities.
63
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CHAPTER 6
SAFETY CONSIDERATION
6.1
INTRODUCTION
For years, those employed in the chemical industry have known that the safe operation of
chemical plant is essential to the industrys continued ability to survive. The human,
political and financial costs of having accidents are just too high for the chemical
industry to not exhibit excellence in their efforts to operate plants in safe and
environmentally responsible ways. The chemical industry has an outstanding record in
both transportation safety and the safe operations of its processes. That effort has
resulted in a dramatic and steady decline in releases and waste produced at chemical
sites.
Actions that should be taken to avoid serious chemical plant accidents are as follows:
1. In most cases involving large volumes of highly hazardous chemicals, excess
flow valves are in place that would stop a rapid flow of the chemicals
2. When highly hazardous chemicals are involved, processes have fixed
protection, as well as trained emergency response teams that could handle the
incident.
3.
Appropriate reaction control or inhibiting systems are in place to interrupt
runaway reactions if cooling, heating and pressure relief are not considered
adequate.
4. Control systems are designed to detect heat or pressure of a chemical reaction
and to control that reaction.
5. Work more closely with local and state law enforcement groups.
64
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
6.2
MATERIAL SAFETY DATA SHEET
6.2.1
Isobutane (Instrument Grade)
Product Number(S): 0001020533, 0001020534, 0001020535, 0001020536
Synonyms: Methylpropane; Iso
Company Identification:
Chevron Phillips Chemical Company Lp
10001 Six Pines Drive
The Woodlands, Tx 77380
6.2.1.1 Product Information:
Msds Requests: (800) 852-5530
Technical Information: (800) 852-5531
Colorless liquefied gas, odorless.
- Flammable gas. May cause flash fire
- Contents under pressure
- Detection of leak via sense of smell may not be possible if odorant has degraded
- Contact with liquefied gas can cause frostbite
- Liquid can cause eye and skin injury
- Reduces oxygen available for breathing
6.2.1.2 Physical And Chemical Properties
Appearance and odor: colorless liquefied gas, odorless.
Ph: na
Vapor pressure: 72 psia @ 37.8 c
Vapor density (air=1): 2.1
Boiling point: -12c (10.4f)
Solubility: negligible
Percent volatile: 100 % volume
Specific gravity: 0.564 @ 15.6 c
Evaporation rate: >1
65
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
6.2.1.3 Immediate Health Effects:
Eye: Because the liquid product evaporates quickly, it can have a severe chilling effect
on eyes and can cause local freezing of tissues (frostbite). Symptoms may include
pain, tearing, reddening, swelling and impaired vision.
Skin: Because the liquid product evaporates quickly, it can have a severe chilling
effect on skin and can cause local freezing of tissues (frostbite). Symptoms may
include pain, itching, discoloration, swelling, and blistering. Not expected to be harmful
to internal organs if absorbed through the skin.
Ingestion: Material is a gas and cannot usually be swallowed.
Inhalation: This material can act as a simple asphyxiant by displacement of air.
Symptoms of asphyxiation may include rapid breathing, in coordination, rapid fatigue,
excessive salivation, disorientation, headache, nausea, and vomiting.
Convulsions, loss of consciousness, coma, and/or death may occur if exposure to high
concentrations continues.
6.2.1.4 First Aid Measures
Eye: Flush eyes with water immediately while holding the eyelids open. Remove
contact lenses, if worn, after initial flushing, and continue flushing for at least 15
minutes. Get immediate medical attention.
Skin: Skin contact with the liquid may result in frostbite and burns. Soak contact area
in tepid water to alleviate the immediate effects and get medical attention.
Ingestion: No specific first aid measures are required because this material is a gas
and cannot usually be swallowed.
Inhalation: For emergencies, wear a niosh approved air-supplying respirator. Move
the exposed person to fresh air. If not breathing, give artificial respiration. If breathing is
difficult, give oxygen. Get immediate medical attention.
6.2.2
N-Butane
N-Butane synonym with I-Butane, Butane, and Normal Butane is a flammable gas. NButane is heavier than air and may travel considerable distance to an ignition source.
66
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
N-Butane is listed under the accident prevention provisions of section 112(r) of the
Clean Air Act (CAA) with threshold quantity (TQ) of 10000 pounds.
Physical and Chemical Properties
Parameter
value
Physical state
units
: Gas
o
Vapor pressure at 70 F
: 31
psia
Vapor density at STP
: 2.07
Evaporation point
: not available
Boiling point
: 31.1
Freezing point
: -0.5
pH
: not available
Solubility
: insoluble
Odor and appearance
: a colourless and odourless gas
Stability
: stable
Condition to avoid
: high temperature
6.2.2.1 Handling and storage
Protect cylinders from physical damage. Store in cool, dry, well- ventilated area away
from heavily trafficked areas and emergency exits. Do not allow the temperature where
cylinders are stored to exceed 130oF. Cylinders should be stored upright and firmly
secured to prevent falling or being knocked over. Full and empty cylinders should be
segregated. Use a first in first out inventory systems to prevent full cylinders from
being stored for excessive periods of time. Never carry a compressed gas cylinder or a
container of a gas in cryogenic liquid form in an enclosed space such as a car trunk,
van or station wagon. A leak cans re4sult in a fire, explosion, asphyxiation or a toxic
exposure.
6.2.3
Methanol
Methanol synonyms with Methyl alcohol and in chemical family alcohol with the
formula CH3OH. Methanol is a clear, colourless, mobile, volatile, flammable liquid and
its soluble in water, alcohol and ether.
67
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Physical and Chemical properties:
Parameter
value
Physical state
: liquid
Boiling Point
: 64.7oc
Vapor Pressure (20oc)
: 128 mb
Vapor Density (air=1)
: 1.11
Solubility in water ,%wt
: full
Specific Gravity
: 0.792 g/cm3
Appearance and odor
: liquid-colorless-odor specific
Fire and Explosion Hazard data:
Flash point
: closed cup: 12oc
Flammable limits, % vol
: Lel: 6, Uel : 36.5
Extinguishing media
: Foam CO2 halogenated agents
Special fire fighting
: Avoid contact with oxidizing materials
Unusual fire and explosion : Moderate
Reactivity Data:
Stability
: Medium
Conditions to avoid
: Oxidizing materials
Incompatibility
: Sulfo-chromic mixtures
Special Precautions
Precaution to be taken in handling and storing Methanol: store in iron or steel
containers or tanks. Small quantities can be stored in reinforced glass containers.
6.2.4
MTBE
6.2.4.1 Physical state, appearance
MTBE is chemically stable; it does not polymerize, nor will decompose under normal
conditions of temperature and pressure. Unlike most ether, MTBE does not tend to
form peroxides (auto-oxidize). The physical state of MTBE is that MTBE is in the form
of liquid at room temperature (25oC). It is a colourless liquid with the billing point at
68
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
55.2oC 131.4oF. The freezing point of MTBE is 108.6oC 163.5oC. The density of
MTBE at 25oC is 735g/cm3.
6.2.4.2 Physical dangers
MTBE is non-reactive. It does not react with air, water, or common materials of
construction. The reactivity of MTBE with oxidizing materials is probably low. However,
without definitive information, it should be assumed that MTBE reacts with strong
oxidizers, including peroxides.
6.2.4.3 Chemical dangers
MTBE is highly flammable and combustible when exposed to heat or flame or spark,
and it is a moderate fire risk. Vapours may form explosive mixtures with air. It is
unstable in acid solutions. Fire may produce irritating, corrosive or toxic gases. Runoff
from fire control may contain MTBE and its combustion products.
Occupational exposure limits (OELs)
Routes of Exposure
6.2.4.4 Inhalation risk
Like other ethers, inhalation of high levels of MTBE by animals or humans results in the
depression of the central nervous system. Symptoms observe red in rats exposed to
4000 or 8000 ppm in air included labored respiration, ataxia, decreased muscle tone,
abnormal gait, impaired treadmill performance, and decreased grip strength. These
symptoms were no longer evident 6 hours after exposure ceased. A lower level of
MTBE, 800ppm did not produce apparent effects (Daughtrey et al., 1997).
A number of investigations have been conducted to examine the self-reported
acute MTBE in gasoline vapors during use by consumers. This research includes both
epidemiological studies and studies involving controlled exposure of volunteers to
MTBE at concentrations similar to those encountered in refueling an automobile
(Reviewed in USEPA, 1997, and California EPA, 1998). In general, the studies
involving controlled human exposures in chambers to levels of MTBE similar to those
experienced during refueling and driving an automobile have not shown effects of
69
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
MTBE on physical symptoms (e.g. irritation), mood, or performance based tests of
neurobehavioral function.
6.2.5
TBA (TERT - BUTYL ACOHOL)
CAS Number: 75-65-0
Synonyms:
tert-Butanol
2-methyl-2-propanol
TBA
t-butylhydroxide
1,1-dimethylethanol
trimethylmethanol
trimethylcarbinol
6.2.5.1 Recognition
NIOSH/OSHA Health Guideline. Summarizes pertinent information about for workers
and employers as well as for physicians, industrial hygienists,and other occupational
safety and health professionals who may need such information to conduct effective
occupational safety and health programs.
6.2.5.2 Evaluation
1. Health Hazards. Routes of exposure, summary of toxicology, signs and
symptoms, emergency procedure.
2. Workplace Monitoring and Measurement.
3. Medical Surveillance. Workers who may be exposed to chemical hazards
should be monitored in a systematic program of medical surveillance that is
intended to prevent occupational injury and disease. The program should
include education of employers and workers about work-related hazards,
placement of workers in jobs that do not jeopardize their safety or health, early
detection of adverse health effects, and referral of workers for diagnosis and
treatment.
70
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
6.2.5.3 Controls
1. Exposure Sources and Control Methods.
2. Personal Hygiene Procedures.
3. Respiratory Protection. Conditions for respirator use, respiratory protection
program.
4. Personal Protective Equipment. Protective clothing should be worn to prevent
any possibility of skin contact. Chemical protective clothing should be selected
on the basis of available performance data, manufacturers' recommendations,
and evaluation of the clothing under actual conditions of use.
5. Emergency Medical Procedures. Material Safety Data Sheets (MSDS's) include
chemical specific information on emergency medical and first aid procedures as
referenced under the OSHA Hazard Communication standard, 29 CFR
1910.1200, (g)(2)(X). This standard requires chemical manufacturers and
importers to obtain or develop an MSDS for each hazardous chemical they
produce or import. Employers shall have an MSDS in the workplace for each
hazardous chemical, which they use.
6. Storage.
7. Spills and Leaks. In the event of a spill or leak, persons not wearing protective
equipment and clothing should be restricted from contaminated areas until
cleanup has been completed.
6.3
HAZARD IDENTIFICATION & EMERGENCY SAFETY & HEALTH RISK
ASSESSMENT
Safety & Health Risks vary with the type of industry & the magnitude of the emergency.
The severity of the risk too will vary with especially where there are chemicals,
combustible gases, potential for fire & explosion etc. These hazards may not only pose
a danger to the health of working in a particular plant but also the adjacent community.
In the event of a major disaster property both within and outside the plant will be
damaged. The real and potential hazards at the work place must be identified and the
Safety & Health Risks that they pose assessed. This will require a close scrutiny of all
71
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
work place buildings, their design, electrical wiring, transport and storage facilities, the
work processes, workstation design, safe operating procedures, list of chemicals
substances used, their quantity, storage, daily transfer, safe usage and disposal.
MSDSs of the chemical too have to be studied as regards their toxicity, volatility, and
their potential for a fire and/or explosion and adverse health affects both short term and
long term.
The possible emergencies/disaster in a industry could be:
Fire/ explosion
Chemical spill
Radioactive material spill
Biological material spill
Personal injury
The best action plan is prevention from an emergency. This is where one has to
work closely with operation personnel to make sure that all operations are safe and
comply with OSH Legislations. All persons at work are aware of the safe procedure
and also follow those procedures. Unfortunately in the real world, mostly human
factors- accident & emergency do occur. This is why emergency response plans have
to be written up, communicated to all concerned and tested for effectiveness.
Depending on the gravity the workplace emergency can be categorized in to Level 1,
Level 2, or Lever 3 emergency.
Level 1 Emergency- the first responder without having to call the disaster
response team or outside help can effectively manage such incident. Examples; a
small fire easily smothered, chemical spill easily contained and cleaned, injury minor
and treated at site by rendering first aid.
Level 2 Emergency - an incident that requires technical assistance from the
disaster response team and may need outside help. Examples; fire that need technical
from trained personnel and specialized equipment spill that can only be properly
contained by specialized equipment.
72
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Level 3 Emergency- these are major disaster that are difficult to contain even
with trained personnel and outside help. Examples, spill that cannot be properly
contained or abated even by highly trained team and the use of sophisticated special
equipment. Fire involving toxic material that is too large to control and are to burn. This
may require the evacuation of civilians across jurisdictional boundaries
73
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CHAPTER 7
MASS BALANCE
7.1
SNAMPROGETTI UNIT (REACTOR AND REGENERATOR)
Stream S5 = 164.74 kgmole/hr
0.393 C4H8
0.393 H2
0.212 iC4H10
0.002 nC4H10
100 kgmole/hr
0.996 iC4H10
0.004 nC4H10
S2
Given from
MSDS
Assume steady-state system,
Basis = 100 kgmole/hr of S2
74
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The fraction at stream S2 acquired from isobutane instrument grade, MSDS.
Reaction occurred in the reactor,
iC4H10
C4H8
H2
Flowrate in kgmole/hr of iC4H10 in the feed stream of S2
= 0.996 (100)
= 99.6 kgmole/hr iC4H10
Balanced Based upon the stoichoimetric ratio with 65% conversion of iC4H10 to obtain
C4H8.
Since, 65% conversion in the reactor,
kgmole/hr of C4H8 obtained
= 0.65 (99.6)
= 64.74 kgmole/hr
35% of iC4H10 unreacted
= 99.6
64.74
= 34.86 kgmole/hr
Based upon stoichiometric ratio
(inert)
(unreacted)
n C4H10
+ iC4H10
0.4
C4H8
99.6
H2
64.74
(kgmole/hr)
iC4H10
64.74
+ n C4H10
34.86
0.4
(kgmole/hr)
Input
S2
Stream
Component
(inert)
MW
Molar flow
Output
S5
Mass flow kg/hr
Molar flow
Mass flow kg/hr
kg/kgmole
kgmole/hr
C4H8
56
kgmole/hr
64.74
3625.44
H2
64.74
129.4
iC4H10
58
99.6
5776.8
34.86
2021.88
n C4H10
Total
58
0.4
23.2
5800
0.4
23.4
5800
75
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
7.2
SEPARATOR
Stream S10 = 64.74 kgmole/hr
1 H2
Stream S9 = 164.74 kgmole/hr
0.393 C4H8
0.393 H2
0.212 iC4H10
Stream S11 = 100 kgmole/hr
0.002 nC4H10
0.6474 C4H8
0.3486 iC4H10
0.0040 nC4H10
Input
S9
Stream
Component
Output
S10
S11
MW
Molar flow
Mass flow
Molar flow
Mass flow
Molar flow
Mass flow
kg/kgmole
kgmole/hr
kg/hr
kgmole/hr
kg/hr
kgmole/hr
kg/hr
C4H8
56
64.74
3625.44
H2
64.74
129.4
64.74
129.4
iC4H10
58
99.6
5776.8
34.86
2021.88
n C4H10
Total
58
0.4
23.2
5800
129.4
0.4
23.4
5670.6
7.3
MIXER
S13 = 64.74kgmole/hr
S14 = 71.62 kgmole/hr
1 CH3OH
0.996 CH3OH
0.004 H2O
S27 = 0.406 kgmole/hr
0.3596 CH3OH
0.6404 H2O
76
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Input
Stream
Component
S13
MW
Molar flow
Output
S14
S27
Mass flow
Molar flow
Mass flow
Molar flow
Mass flow
kg/kgmole
kgmole/hr
kg/hr
kgmole/hr
kg/hr
kgmole/hr
kg/hr
CH3OH
32
71.214
2278.848
0.146
4.67
71.36
2283.52
H2O
Total
18
2278.848
0.26
4.68
9.356
0.26
4.685
2288.205
7.4
MTBE REACTOR
Assumption : 98% conversion of C4H8 (2% remains unconverted)
Reactions involve in the reactor,
1. C4H8
CH3OH
2. 2CH3OH
3. C4H8
C5H12O
C2H6O
H2O
H2O
C4H10O
Stream S11 = 100 kgmole/hr
0.6474 C4H8
0.3486 iC4H10
0.0040 nC4H10
R
e
ac
to
r
S15
kgmole/hr
C4H8
iC4H10
nC4H10
S14 = 71. 214 kgmole/hr
CH3OH
CH3OH
H2O
C5H12O
C4H10O
C2H6O
H2O
7.4.1
1st REACTION IN REACTOR
C4H8
CH3OH
C5H12O
77
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
kgmole/hr of C4H8 in the stream S11 = 100(0.6474)
=
64.74 kgmole/hr C4H8
Balance based upon stoichiometric ratio with 98% conversion.
CH3OH is classified an excess.
The unreacted of CH3OH (excess) = (71.36 - 64.74)
=
6.62 kgmole/hr
Since 98% conversion in the reactor,
kgmole/hr of C5H12O obtained = 0.98 (64.74)
= 63.44 kgmole/hr C5H12O obtained
From the stoichiometric ratio,
98%
C4H8
64.74
CH3OH
conv.
71.214
C5H12O +
C4H8
63.44
1.3
CH3OH
7.92
unconverted
kgmole/hr
kgmole/hr
64.74 kgmole/hr
1 C4H8
R
e
ac
to
r
kgmole/hr
C4H8
CH3OH
C5H12O
64.74 kgmole/hr
1 CH3OH
Component
MW
Input
Molar flow
Mass flow
Output
Molar flow
Mass flow
78
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
C4H8
(kg/kgmole)
56
(kgmole/hr)
64.74
(kg/hr)
3625.44
(kgmole/hr)
1.3
(kg/hr)
72.8
CH3OH
32
71.36
2283.52
7.92
253.44
C5H12O
Total
88
5908.96
63.44
5582.72
5908.96
7.4.2
2nd REACTION IN REACTOR
From 2nd reaction, stoichiometric ratio shown below:
Since the ratio between methanol and dimethylether is 2CH3OH : 1C2H6O ,
98% conversion methanol (CH3OH) into dimethylether (C2H6O) = 1.3 (0.98)
2
=
0.637 kgmole/hr
98%
2CH3OH
conv.
7.92
C2H6O +
3.88
H2O
2CH3OH
3.88
0.16
unconverted
kgmole/hr
7.92 kgmole/hr
1 CH3OH
kgmole/hr
R
e
ac
to
r
kgmole/hr
CH3OH
C2H6O
H2O
Input
Output
79
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Component
MW
Molar flow
Mass flow
Molar flow
Mol
Mass flow
kg/kgmole
kgmole/hr
(kg/hr)
(kgmole/hr)
Fraction
(kg/hr)
CH3OH
32
7.92
253.44
0.16
0.02
5.12
C2H6O
46
3.88
0.49
178.48
H2O
18
3.88
0.49
69.84
1.0
253.44
Total
7.4.3
253.44
3rd REACTION IN REACTOR
The 3rd reaction and its stoichiometric below,
From 1st reaction, kgmole/hr of C4H8 remain is 1.3 and 3.88 kgmole/hr of H2O is
obtained in 2nd reaction.
Since C4H8 is limiting reactant to react with H2O, only 1.3 kgmole/hr of H2O needed to
react with C4H8
H2O is classified an excess.
The unreacted of H2O (excess) = (4.14 - 1.3)
=
C4H8
1.3
H2O
4.14
2.84 kgmole/hr
C4H10O +
1.274
C4H8
0.026
H2O
2.866
unconverted
kgmole/hr
kgmole/hr
1.3 kgmole/hr
1
C4H8
80
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
R
e
ac
to
r
4.166 kgmole/hr
0.3058C4H8
0.3058C4H10O
0.688H2O
4.14 kgmole/hr
1 H2O
Input
Component
MW
Molar flow
Mass
Molar
Mass
Molar
Mass
kg/kgmole
kgmole/hr
flow
flow
flow
flow
flow
kgmole/hr
-
(kg/hr)
-
kgmole/hr
0.026
(kg/hr)
1.456
4.14
74.52
2.866
51.588
1.274
94.276
4.166
147.32
C4H8
56
1.3
(kg/hr)
72.8
H2O
18
C4H10O
74
Total
7.4.4
Output
1.3
72.8
4.14
74.52
OVERALL MASS BALANCE ON MTBE REACTOR
S11 = 100 kgmole/hr
0.6474 C4H8
0.3486 iC4H10
0.0040 nC4H10
81
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
R
e
ac
to
r
S15 = 100.676 kgmole/hr
0.0002 C4H8
0.3261 iC4H10
0.0037 nC4H10
0.0015CH3OH
0.5934 C5H12O
S14 = 71.36 kgmole/hr
0.0119 C4H10O
1 CH3OH
0.0363 C2H6O
0.0268 H2O
Input
Input
Output
S11
S14
S15
MW
Molar flow
Mass
Molar flow
Mass
Molar flow
Mass
(kg/kg
(kgmole/hr)
flow
(kgmole
flow
(kgmole/h
flow
C4H8
mole)
56
64.74
(kg/hr)
3625.44
/hr)
-
(kg/hr)
-
r)
0.026
(kg/hr)
1.456
iC4H10
58
34.86
2021.88
34.860
2021.88
n C4H10
58
0.4
23.2
0.4
23.2
CH3OH
32
71.36
2283.52
0.16
5.12
C5H12O
88
63.44
5582.72
C2H6O
46
3.88
178.48
C4H10O
74
1.274
94.276
H2O
18
0.26
2.866
51.588
106.906
7958.72
Component
Total
7.5
100
5670.52
71.62
4.68
2288.2
DISTILLATION COLUMN
Assume that 90% of methanol in bottom.
82
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
S17 = 40.321 kgmole/hr
0.0006 C4H8
S15 = 100.906 kgmole/hr
0.0002 C4H8
0.8646 iC4H10
0.3261 iC4H10
0.0099 nC4H10
0.0037 nC4H10
0.0037 CH3OH
0.0015 CH3OH
0.0249 H2O
0.5934 C5H12O
0.0962 C2H6O
0.0119 C4H10O
S16 = 66.585 kgmole/hr
0.0363 C2H6O
0.9528 C5H12O
0.0268 H2O
0.0191 C4H10O
0.0279 H2O
0.0002 CH3OH
S15
Component
MW
kg/kgmole
Molar
Mass
S16
Molar flow
Mass
S17
Molar flow
Mass
flow
flow
(kgmole/hr)
flow
(kgmole/hr)
flow
C4H8
56
kgmole/hr
0.026
(kg/hr)
1.456
(kg/hr)
-
0.026
(kg/hr)
1.456
iC4H10
58
34.86
2021.88
34.86
2021.88
n C4H10
58
0.4
23.2
0.4
23.2
CH3OH
32
0.16
5.12
0.011
0.352
0.003
4.768
C5H12O
88
63.44
5582.72
63.44
5582.72
C2H6O
46
3.88
178.48
3.88
178.48
C4H10O
74
1.274
94..276
1.274
94..276
H2O
Total
18
2.866
106.906
51.588
7958.72
1.860
66.585
33.48
5710.828
0.013
40.321
18.108
2247.892
7.6
LIQIUD LIQUID EXTRACTION
S 20 = 12.029 kgmole/hr
1 H2O
S 21 = 39.166 kgmole/hr
0.0007 C4H8
0.8901 iC4H10
0.0102 nC4H10
0.0991 C2H6O
83
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
S18 = 40.321 kgmole/hr
S23 = 13.184 kgmole/hr
0.0006 C4H8
0.0113 CH3OH
0.8646 iC4H10
0.9887 H2O
0.0099 nC4H10
0.0037 CH3OH
0.0962C2H6O
0.0249 H2O
Input
Output
S18
S20
Mass
MW
Molar flow
kg/kgmole
(kgmole/hr)
C4 H8
56
0.026
(kg/hr)
1.456
Component
flow
Molar flow
(kgmole/hr)
S21
Mass
flow
(kg/hr)
-
Molar flow
(kgmole/hr)
S23
Mass
flow
0.026
(kg/hr)
1.456
Mass
Molar flow
flow
(kgmole/hr)
-
(kg/hr)
-
iC4H10
58
34.86
2021.88
34.86
2021.88
n C4H10
58
0.4
23.2
0.4
23.2
CH3OH
32
0.149
4.768
0.149
4.768
C5H12O
88
C2H6O
46
3.88
178.48
3.88
178.48
C4H10O
74
H2O
Total
18
1.006
40.321
18.108
2247.892
12.029
12.029
216.522
216.522
39.166
2225.016
13.035
13.184
234.63
239.398
7.7
DISTILLATION COLUMN
S26 = 0.407 kgmole/hr
S24 = 13.185 kgmole/hr
0.0113 CH3OH
0.9887 H2O
0.146 CH3OH
0.260 H2O
84
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
S25 = 12.778 kgmole/hr
1 H2O
S24
Component
MW
kg/kgmole
Molar
Mass
S25
Molar flow
Mass
S26
Molar flow
Mass
flow
flow
(kgmole/hr)
flow
(kgmole/hr)
flow
C4H8
56
kgmole/hr
-
(kg/hr)
-
(kg/hr)
-
(kg/hr)
-
iC4H10
58
n C4H10
58
CH3OH
32
0.149
4.776
0.003
0.096
0.146
4.680
C5H12O
88
C2H6O
46
C4H10O
74
H2O
Total
18
13.035
13.185
234.63
239.411
12.775
12.778
229.95
230.046
0.260
0.407
4.685
9.365
OVERALL REACTION SYSTEM, FLOW DIAGRAM
S3
S17
S18
S9
Liquid
d
liquid
liquid
extra
extrac
ction
tion
Liqui
Distill
ation
colu
mn
S11
Distill
ation
colu
mn
7.8
85
Re
act
or
R
Se
ea
pa
rat
ct
or
or
Ca
tal
ytic
rea
cto
r
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
S2
S10
S12
S15
Overall mass balance is shown below:
Input
S4
Molar flow
Mass flow
kgmole/hr
(kg/hr)
5800
output
Input
S13
Molar flow
Mass flow
Molar flow
(kgmole/hr)
(kgmole/hr)
(kg/hr)
2278.848
Total input
S20
Mass flow
(kg/hr)
216.522
8295.37
Output
S10
Molar flow
Mass
(kgmole/hr
flow
(kg/hr)
129.48
S16
Molar flow
(kgmole/hr)
Total
S26
Mass
flow
(kg/hr)
5710.828
Molar flow
(kgmole/hr)
S21
Mass
flow
(kg/hr)
230.046
Molar flow
(kgmole/hr)
Mass
flow
(kg/hr)
2225.016
8295.37
output
7.6 SCALE-UP FACTOR
Determination of the scale-up factor for the end product (MTBE)
With a basis 100 kgmole/hr of feed at stream S2, the product at stream S12 acquired is
5658.934 kg/hr.
This amount if converted to kg/yr, by conversion unit,
5582.72kg/hr * 7920 hr/yr = 44.215142 * 106 kg/yr of MTBE
Targeted production of MTBE = 300 X106 kg/yr (300,000 metric tonnes/yr)
86
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Therefore, the scale-up factor = Targeted Amount
Actual Amount
= 300,000 x103 kg/yr
44.8188 x106 kg/yr
= 6.785005854
6.785
To determined whether the scale-up factor can proceed or not,
Target amount
Actual Production x Scale-up factor
44.215 x 106 x 6.785
299.9997385 x106
300 x106 kg/yr at stream S14
Therefore, the scale-up factor of 6.785 is acceptable for this process.
CHAPTER 8
ENERGY BALANCE
8.1
ENERGY EQUATION
The equation that we used to calculate the power Q or W at each equipment is:
87
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Q W = HR + (-Hin) + (Hout) + (KE) + (PE)
To calculate H, first we need to find the Cp values for every component in each of the
stream. To find the Cp values, we need to use this equation to find the values of Cp
= a + bT + cT2 + dT3
The values of a, b, c and d are taken from Appendix D, Coulson and Richardson
Chemical Engineering, Volume 6. If the temperature and pressure is more than the
critical temperature and pressure of the component, we need to find the (C p Cpo) for
that specific component. But as for all of our temperatures and pressures none of them
exceed the critical temperature and pressure; we need not to find the (Cp Cpo).
To find the value of H, we use this equation:
H =
T2
C
T1
dT x (n)
Should there is any reaction in the process; we need also to find the values of HR
which takes place in the equipment. The equation, which we used to find HR is:
HR = (F product - F reactant) x n
and if the equipment has KE and PE, we also need to calculate the values by using
this equation:
KE = 0.5 m(vout2 - vin2)
PE = mg x (zout zin)
so, after we have calculated all the values of the energy for each and every of the
stream, we then can calculate the value of Q or W.
And for this sample of calculations, listed are the values of constants in the ideal gas
heat capacity equation based on R. K Sinnot, Coulson & Richardson, Chemical
Engineering, Volume 6, Third Edition, Butterworth Heinemann:
88
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 8.1 Table of Constant in the Ideal Heat Capacity
Component
C5H12O
CH3OH
H2O
C4H8
i-C4H8
i-C4H10
C4H10O
n-C4H10
(CH3)2O
H2
8.2
Delta HF
kJ/kmol.K
kJ/kmol
-1
-4
-8
2.53
5.14 x 10 -2.60 x 10 4.30 x 10 -292990
2.12 x 101 7.09 x 10-2 2.59 x 10-5 -2.85 x 10-8 -201300
32.243
1.93 x 10-3 1.06 x 10-5 -3.60 x 10-9 -242000
-2.994
3.53 x 10-1 -1.98 x 10-4 4.46 x 10-8
-130
16.052 2.8043 x 10-1 -1.091 x 10-4 9.098 x 10-9 -16900
-1.39
3.85 x 10-1 -1.85 x 10-4 2.90 x 10-8 -134610
1
-4.86 x 10 7.17 x 10-1 -7.08 x 10-4 2.92 x 10-7 -312630
9.85
3.31 x 10-1 -1.11 x 10-4 -2.82 x 10-9 -126.23
1.70 x 101 1.79 x 10-1 -5.23 x 10-5 -1.92 x 10-9 -184180
2.71 x 101 9.27 x 10-3 -1.38 x 10-5 7.65 x 10-9
0
ENERGY BALANCE: SAMPLE OF CALCULATIONS
(Methods of calculations are based on, Coulson & Richardson, Chemical Engineering,
Volume 6, page 78).
8.2.1
P-100 (Pump 1)
S2
T = -150C
P = 750 Kpa
(liquid)
S1
T = -180C
P = 450
Calculations
are Kpa
based on Yunus A. Cengel, Micheal A. Boles, Thermodynamics:
(Liquid)
An Engineering Approach, WCB/Mc Graw-Hill, 1989, page, 354-355.
Assumptions:
1. Steady operating conditions exist
2. Kinetic and potential energy negligible
3. The process is to be isentropic
Specific volume:
Isobutane = 0.255 m3/mol
n-butane = 0.263 m3/mol
89
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
(Data of these specific volumes are based on Coulson & Richardson, Chemical
Engineering, Volume 6, Third Edition, Butterworth Heinemann, page 947)
Isobutane
0.255m3/mol x 1000mol/kmol x 1kmol/58kg = 9.11 m3/kg
n-butane
0.263m3/mol x 1000mol/kmol x 1kmol/58kg = 4.53 m3/kg
Vavg = (9.11 + 4.53) m3/kg / 2 = 6.82 m3/kg
(Which remains essentially constant during the process)
2
Win = Vdp
1
= V1 (P2 P1 )
= 6.82m 3 /kg(750 450)kpa(1k J/1kpa.m
= 6.82(300)
= 2046.00kJ/ kg
2046kJ/kg x 39353kg/hr
= 80516238kJ /hr = 22365.62kW
8.2.2
E-100 (Heat Exchanger 1)
S2
T = -15 oC
P = 750Kpa
(Liquid)
S3
T = 117 oC
P = 450Kpa
(Gas)
Stream 2
Component
i-C4H10
n-C4H10
Flowrates
Kmol/hr
675.786
2.714
F
kJ/Kmol
-1.35 x105
-1.26 x105
To
K
298
298
T, K
258
258
H
kJ/hr
-690.04
-2.81
90
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
H =
-692.86
Stream 3
Component
i-C4H10
n-C4H10
Flowrates
Kmol/hr
676.786
2.714
F
kJ/Kmol
-1.35 x105
-1.26 x105
To
K
298
298
T, K
390
390
H
kJ/hr
1902.85
7.66
H =
1910.51
Sample of calculations for i-C4H10 at stream 3
H =
T2
C
T1
T2
C
T1
dT =
dT x (n)
T2
T1
a + bT + cT 2 + dT 3
b(T 2 T1 ) 2 c(T 2 T1 )3 d(T 2 T1 ) 4
a(T
T
)
+
+
+
= 2
1
2
3
4
= ( 1.390 )( 390 298 ) +
+
38 .473 10 2 (390 - 298 ) 2
2
( 1.846 10 4 )( 390 - 298 )3
3
28 .952 10 9 (390 - 298 ) 4
4
= 10100 kJ/kmol
H
= 10100 kJ/kmol x 675.786 kmol/hr
= 6825438.6 kJ/hr
= 1902.85 kW (for i-C4H10)
And as for n-C4H10, the H
So
= 7.66 kW
H = 1910.51
Energy balance,
91
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Q=(
H)
out
H)
in
= 1910.51- (-692.86)
= 2603.37kW
Steam flowrate, Q = mCpT
Cp of isobutane, 2155 J/kg oC (Elementary Principles of Chemical Processes,
W.
Rousseau et. al)
m=
Q
Cp T
2603370 J/s
2155J/g o C x (117 - (-15))
= 9.152g/s
=
Therefore the supply of steam flow rate required is 9.152 g/s.
8.2.3
E-101 (Heat Exchanger 2)
S3
T = 117 oC
P = 450 kPa
(Gas)
S4
T = 250 oC
P = 325 kPa
(Gas)
Stream 3
Component
i-C4H10
n-C4H10
Flowrates
Kmol/hr
676.786
2.714
F
kJ/Kmol
-1.35 x105
-1.26 x105
To
K
298
298
T, K
390
390
H
kJ/hr
1902.85
7.66
H =
1910.51
Stream 4
Component
i-C4H10
n-C4H10
Flowrates
Kmol/hr
676.786
2.714
F
kJ/Kmol
-1.35 x105
-1.26 x105
To
K
298
298
T, K
523
523
H
kJ/hr
5356.57
21.46
92
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
H =
5378.03
Sample of calculations for i-C4H10 at stream 4
H =
T2
C
T1
T2
C
T1
dT x (n)
T2
dT =
T1
a + bT + cT 2 + dT 3
b(T 2 T1 ) 2 c(T 2 T1 )3 d(T 2 T1 ) 4
a(T
T
)
+
+
+
= 2
1
2
3
4
= ( 1.390 )( 523 298 ) +
+
38 .473 10 2 (523 - 298 ) 2
2
( 1.846 10 4 )( 523 - 298 )3
3
28 .952 10 9 (523 - 298 ) 4
4
= 28500 kJ/kmol
H
= 28500 kJ/kmol x 675.786 kmol/hr
= 19259901 kJ/hr
= 5349.97 kW (for i-C4H10)
And as for n-C4H10, the H
So
= 21.46 kW
H t = 5371.43kW
ou
Energy balance,
Q=(
H)
out
H)
in
= 5371.43- (1910.51)
= 3460.92kW
Steam flowrate, Q = mCpT
93
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Cp of isobutane, 2155 J/kg oC (Elementary Principles of Chemical Processes,
W.
Rousseau et. al)
m=
Q
Cp T
3460920 J/s
2155J/g o C x (250 - 118) o C
= 12.17g/s
=
Therefore the supply of steam flow rate required is 12.17 g/s.
8.2.4
R-101 (Snamprogetti Fluidized Bed Reactor)
S5
Air Out
S4
T=250oC
P=325kPa
(gas)
S6
Air In
S7
T=180oC
P=110kPa
(Liquid)
Stream 4
Component
i-C4H10
n-C4H10
Flowrates
Kmol/hr
676.786
2.714
F
kJ/Kmol
-1.35 x105
-1.26 x105
To
K
298
298
T, K
523
523
H
kJ/hr
5356.57
21.46
H =
5378.03
Stream 7
94
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Component
i-C4H8
i-C4H10
n-C4H10
H2
Flowrates
Kmol/hr
237
2.71
439
439
F
kJ/Kmol
-1.30 x10-1
-1.35 x105
-1.26 x105
0.00
To
K
298
298
298
298
T, K
453
453
453
453
H
kJ/hr
1056.59
13.82
2236.41
549.84
H =
3856.66
To calculate the value of HR:
1) i-C4H10 C4H8 + H2
so,
R = (F C4H8) + (F H2) -(F i-C4H10)
= (-130) + (0) (-134610)
= 134480 kJ/kmol
therefore,
HR = (R kJ/kmol x 236.53 kmol/hr)
= (134480 kJ/kmol x 236.53 kmol/hr)
= 31808554.4 kJ/hr
= 8835.71 kJ/s
= 8835.71 kW
Although there is stream flow, but the KE is too small and negligible and there is also
now work so, W is zero and as for the PE, the value is neglected, as it is also too
small.
Now we calculate the value of Q,
Q W 0= HR + (-Hin) +(Hout) +KE 0+ PE0
Q = HR + (Hout) - (Hin)
Q = 8835.71 + (1980.66) - (3856.66)
Q = 6959.71kW (heat have been absorbed)
8.2.5
C-100 (Compressor 1)
95
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
S7
T = 1800C
P = 110 Kpa
(Gas)
S8
T = 1930C
P = 120 Kpa
(Gas-liquid)
T7 = 453K
T8 =?
P7 = 1.1 bar
P8 = 1.2 bar
T4 = 453K,
P4 = 1.1 bar,
P8 = 1.2 bar is based on the literature review of process
Snamprogetti fluidized bed.
By assuming polytropic and ideal gas condition:
T7= T6(P7/P6)m (Coulson & Richardson, Chemical Engineering, Volume 6, page 85)
m = 1/ Ep
= CPmean/CV = CPm/CPm R
Where R = 8.314 kJ/kmol.K
For hydrogen, a = 27.143, b = 97.38 x 10-4, c = -1.31 x 10-5, d = 76.451 x 10-10
1000K
CPHydrogen =
453K
a + bT + cT 2 + dT 3 = 16200 kJ/kmol.K
For butene, a = -2.994 , b = 3.53 x 10-1 , c = -1.98 x 10-4 , d = 4.46 x 10-8 ,
CPbutene =
1000K
453K
a + bT + cT 2 + dT 3 = 89400 kJ/kmol.K
For Isobutane, a = -1.39 , b = 3.85 x 10-1 , c = -1.85 x 10-4 , d = 2.90 x 10-8 ,
CPisobutane =
1000K
453K
a + bT + cT 2 + dT 3 = 103000 kJ/kmol.K
For n-butane, a = 9.85 , b = 3.31 x 10-1 , c = -1.11 x 10-4 , d = -2.82 x 10-9,
1000K
CPn-butane =
Cpmean =
0.25 (Cp , Hydrogen + Cp , butene + Cpisobu tan e + Cp , n bu tan e)
(466 453 )
453K
a + bT + cT 2 + dT 3 = 103000 kJ/kmol.K
0.25(16200 + 89400 + 103000 + 103000)
= 167.5kJ/km ol.K
1000 453
So , = CPm/CPm R = 167.5/(167.5-8.314) = 1.07
96
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
To find Ep(efficiency),
Flow rate =
1117.76kmo l
453
1
x22.4x
x
3600s
273 0.5
= 23 .08 m 3 / s
From Figure 3.6, Coulson & Richardson, Chemical Engineering, Volume 6, page 83,
Ep = 75%
m = (1/ Ep) = 1.07-(1/1.07 (0.75) ) = 0.3690
To determine T6,
T8 = T7(P8/P7)m = 453(1.2/1.1)0.3690 = 466.83K (193oC)
Tc and Pc for H2, isobutene, isobutane and n-butane, Tc = 417.07K, Pc = 38.17 bar
Trmean = (T7 + T8)/2Tc = (453+ 466.83K)/2(417.07) = 1.10 K
Prmean = (P5 + P6)/2Pc = (1.1+1.2)/2(38.17) = 0.030 bar
From Figure 3.8, Compressibility factors (Coulson & Richardson, Chemical
Engineering, Volume 6, page 87).
Z = 1.00
Then find n, n = 1/(1-m) = 1/(1-0.3456) = 1.53
Polytropic work = zRT1(n/n-1)x((P1/P2)(n-1/n) 1)
=
1.53
1.00 (8.314 )( 453 )( 0.53
0.53
1.2 1.53
) x
1
1.1
= 332.69 kJ/kmol
Actual work = Polytropic work / Ep
= 332.69 /0.75
= 443.60 kJ/kmol
Compressor power = 443.60 kJ/kmol x 1117.76 kmol/hr x 1hr/3600s
= 137.73 kW
Therefore the compressor power required to increase the pressure from 1.1 bar to 1.2
bar is 137.73kW.
8.2.6
E-102 (Cooler 1)
S8
T = 193oC
P = 120 Kpa
(Liquid)
S9
T = 530C
P = 100 Kpa
(Liquid)
97
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Sample of Calculation for Cooler 1
Stream 8
Component
C4H8
i-C4H10
n-C4H10
H2
Flowrates
Kmol/hr
4.39x1002
2.37x102
2.71x100
4.39x102
F
kJ/Kmol
-1.69x10-4
-1.35x105
-1.26x105
0.00
To
K
298
298
298
298
T, K
466
466
466
466
H
kJ/hr
2155.41
1323.43
15.15
596.20
H =
4090.20
Stream 9
Component
i-C4H8
i-C4H10
n-C4H10
Flowrates
Kmol/hr
6.47x101
3.49x 101
4.00x101
F
kJ/Kmol
-1.69x104
-1.35 x104
-1.26 x105
To
K
298
298
298
T, K
326.3
326.3
326.3
H
kJ/hr
47.45
27.84
0.32
H =
75.61
Energy balance,
Q=(
H)
out
H)
in
= 75.61 4090.20
= -4014.59 kW (heat is being released to the surrounding)
Steam flowrate, Q = mCpT
98
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Cp of pure water, 4.184 J/g oC (Elementary Principles of Chemical Processes,
W.Rousseau et. al)
Q
mCp T
4014590J/s
=
4.184J/g o C x (193 - 53.3)
=6869.36 g/s
m=
Therefore the supply of steam flow rate required is 6869.36 g/s.
8.2.7 V-100 (Separator)
S10
T = 53.3oC
P = 90 kPa
(gas)
S9
T= 53.3oC
P= 100kPa
(liquid-gas)
S11
T=53.3oC
P=100kPa
Sample of Calculation for Hydrogen Splitter Vessel
Stream 9
Component
C4H8
Flowrates
Kmol/hr
2.37x102
i-C4H10
n-C4H10
H2
2.71x102
4.39x102
4.39x102
F
kJ/Kmol
-1.69x104
1.35Ex105
-1.26x105
0.00
To
K
298
T, K
326.3
H
kJ/hr
166.03
298
298
298
326.3
326.3
326.3
2.17
353.50
99.88
99
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
H =
621.57
Stream 10
Component
H2
Flowrates
Kmol/hr
F
kJ/Kmol
To
K
T, K
H
kJ/hr
4.39x102
0.00
298
326.3
99.88
Flowrates
Kmol/hr
439.26
236.53
2.71
F
kJ/Kmol
-16910
-134610
-126230
To
K
298
298
298
T, K
H
kJ/hr
321.92
188.89
2.18
H =
Stream 11
Component
C4H8
i-C4H10
n-C4H10
326.3
326.3
326.3
H =
512.99
Energy balance,
Q=(
H)
out
H)
in
= (512.99)-(621.57+99.88)
= -208.46 (heat is being released to the surroundings)
8.2.8
R-102 (MTBE Reactor)
S11
T = 53.3oC
P = 2000kPa
(liquid)
S14
T = 27oC
P = 100kPa
(liquid)
S15
T = 101oC
P = 2000kPa
(liquid)
100
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The pressure increases from 100kPa to 2000kPa. As there is a heat exchanger (heater)
in the MTBE reactor.
Stream 11
Component
C4H8
i-C4H10
n-C4H10
Flowrates
Kmol/hr
439.26
236.53
2.71
F
kJ/Kmol
-16910
-134610
-126230
To
K
298
298
298
T, K
326.3
326.3
326.3
H
kJ/hr
321.92
188.89
2.18
H =
512.99
Stream 14
Component
CH3OH
H2O
Flowrates
Kmol/hr
4.84x102
1.76
F
kJ/Kmol
-2.01x104
-2.42x105
To
K
298
298
T, K
300
300
H
kJ/hr
11.81
-4.79
H =
7.02
Stream 15
Component
C5H12O
CH3OH
Flowrates
Kmol/hr
4.30x102
1.09
F
kJ/Kmol
-2.93x105
-2.01 x105
To
K
298
298
T, K
374
374
H
kJ/hr
1339.10
1.07
101
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
H2O
i-C4H8
i-C4H10
C4H10O
n-C4H10
(CH3)2O
1.94
0.176
2.37x102
8.64
2.71
26.3
-2.44 x105
-0.123
-1.35 x105
-3.13 x105
-1.26 x105
-1.84 x105
298
298
298
298
298
298
374
374
374
374
374
374
13.94
0.35
539.59
22.49
6.22
39.56
H =
1962.32
Q=(
H)
out
H)
in
= 1962.32 7.02 512.99
= 1442.31 kW
To calculate the value of HR:
1.) C4H8 + CH3OH
2.) 2CH3OH
C5H12O
C2H6O + H2O
3.) C4H8 + H2O
C4H10O
so,
R 1 = (F C5H12O) - (F CH3OH) + (F C4H8)
= (-292990) ((-201300) + (-130))
= -91820 kJ/kmol
R 2 = (F C2H6O) + (F H2O) -(2 F CH3OH )
= (-242000) + (-184180) (2 x -201300)
= -23580 kJ/kmol
R 3 = (F C4H10O) (F H2O) -(F C4H8)
= (-312630) - (-184180) (-130)
= -128270 kJ/kmol
Therefore,
HR = (R 1kJ/kmol x (63.44kmol/hr) + (R 2kJ/kmol x 0.039kmol/hr) +
(R 3kJ/kmol x 0.624kmol/hr)
=(-91820kJ/kmolx63.44kmol/hr)+(-23580kJ/kmolx0.039kmol/hr) +
102
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
(-128270 kJ/kmol x 0.624kmol/hr)
= -5906020.9 kJ/hr
= -1640.56 kW
Although there is stream flow, but the KE is too small and negligible and there is also
now work so, W is zero and as for the PE, the value is neglected, as it is also too small
Now we calculate the value of Q
Q W 0= HR + (-Hin) +(Hout) +KE 0+ PE0
Q = HR + (-Hin) +(Hout)
Q = -1640.56 + 1442.31 = -198.25 kW
8.2.9
P-101 (Pump 2)
S13
T = 270C
P = 115 Kpa
(liquid)
S12
T = 270C
P = 110 Kpa
(Liquid)
Calculations are based on Yunus A. Cengel, Micheal A. Boles, Thermodynamics: An
Engineering Approach, WCB/Mc Graw-Hill, 1989, page, 354-355.
Assumptions:
4. Steady operating conditions exist,
5. Kinetic and potential energy negligible
6. The process is to be isentropic
Specific volume:
103
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
methanol = 0.118 m3/mol
(Data of these specific volumes are based on Coulson & Richardsons)
Methanol
0.118m3/mol x 1000mol/kmol x 1kmol/32kg = 3.6875 m3/kg
Vavg = 1.78 m3/kg
Which remains essentially constant during the process
2
Win = Vdp
1
= V1 (P2 P1 )
= 3.6875m 3 /kg(115 110)kpa(1k J/1kpa.m
= 18 .44 kJ/kg
18.44kJ/kg x 15462 kg/hr
= 285080.63k J/hr = 79.19 kW
8.2.10 M-101 (Mixer)
S13
S14
T= 27oC
P=115kPa
(liquid)
T= 27oC
P= 115kPa
(liquid)
S29
T=27oC
P=130kPa
(liquid)
Stream 13
Component
CH3OH
Flowrates
To
Kmol/hr
kJ/Kmol
4.83 x102
-2.01 x102
298
T, K
H
kJ/hr
H =
300
11.79
104
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Stream 29
Component
CH3OH
Flowrates
Kmol/hr
kJ/Kmol
9.91 x10
H20
1.76
To
T, K
H
kJ/hr
-2.01 x10
298
300
0.02
-2.42 x10
298
300
0.03
H =
0.06
Stream 14
Component
CH3OH
Flowrates
Kmol/hr
kJ/Kmol
4.84 x10
H20
1.76
To
T, K
H
kJ/hr
-2.01 x10
298
300
11.81
-2.42 x10
298
300
0.03
H =
11.84
Energy balance = out - in
Q=(
H)
out
H)
in
= 11.84 0.06 11.79
= 0.04 kW
8.2.11 EX-100 (Expander 1)
S15
S16
T = 1010C
P = 2000 kPa
(liquid)
T = ?0C
P = 450 Kpa
(Gas-liquid)
T15 = 453K
T16 =?
P15 = 2 bar
P16 = 0.45 bar
105
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
By assuming polytropic and ideal gas condition:
T16= T15(P16/P15)m (Coulson & Richardson, Chemical Engineering, Volume 6, page 85)
m = 1/ Ep
= CPmean/CV = CPm/CPm R
Where R = 8.314 kJ/kmol.K
For MTBE, a = 2.533 , b = 51.372 x 10-2 , c = -2.59 x 10-4 , d = 43.04 x 10-9 ,
CPMTBE =
1000K
374K
a + bT + cT 2 + dT 3 = 151000 kJ/kmol.K
For TBA, a = 3.266 , b = 41.80 x 10-2 , c = -2.242 x 10-4 , d = 46.85 x 10-9 ,
CP(TBA) =
1000K
374K
a + bT + cT 2 + dT 3 = 126000 kJ/kmol.K
For DME, a = 17.015 , b = 19.907x 10-2 , c = -5.23 x 10-5 , d = -1.918 x 10-9 ,
CP(DME) =
1000K
374K
a + bT + cT 2 + dT 3 = 70700 kJ/kmol.K
For CH3OH, a = 21.152 , b = 70.924 x 10-3 , c = 25.870 x 10-6 , d = -2.852 x 10-8 ,
CP(methanol) =
1000K
374K
a + bT + cT 2 + dT 3 = 44900kJ/km ol.K
For H2O, a = 27.143 , b = 92.738 x 10-4 , c = -1.381 x 10-5 , d = 76.451 x 10-10 ,
CP(water) =
1000K
374K
a + bT + cT 2 + dT 3 = 23500kJ/km ol.K
For butene, a = -2.994 , b = 3.53 x 10-1 , c = -1.98 x 10-4 , d = 4.46 x 10-8 ,
CPbutene =
1000K
374K
a + bT + cT 2 + dT 3 = 113000 kJ/kmol.K
For Isobutane, a = -1.39 , b = 3.85 x 10-1 , c = -1.85 x 10-4 , d = 2.90 x 10-8 ,
CPisobutane =
1000K
374K
a + bT + cT 2 + dT 3 = 126000 kJ/kmol.K
For n-butane, a = 9.85 , b = 3.31 x 10-1 , c = -1.11 x 10-4 , d = -2.82 x 10-9,
CPn-butane =
Cpmean=
=
1000K
374K
a + bT + cT 2 + dT 3 = 113000 kJ/kmol.K
0.125(Cp, M TBE+ Cp, TBA + Cp, DM E+ Cp, methanol+ Cp, H2 + Cp, butene+ Cpisobu
(1000 453)
0.125(1510 00 + 126000 + 70700 + 44900 + 23500 + 113000 + 126000 + 113000)
= 153.37kJ/k mol.K
1000 374
So , = CPm/CPm R = 153.37/(153.37-8.314) = 1.06
106
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
To find Ep(efficiency),
Flow rate =
53999.92km ol
374
1
x22.4x
x
3600s
273 0.5
= 920 .61m 3 / s
From Figure 3.6, Coulson & Richardson, Chemical Engineering, Volume 6, page 83,
Ep = 85%
m = 1/ Ep = (1.06-1/1.06 (0.85)= 0.0665
To determine T6,
T16 = T15(P16/P15)m = 374(0.45/2.0)0.0665 = 338.68K (65.6oC)
Tc = 417.07K, Pc = 38.17 bar
Trmean = (T15 + T16)/2Tc = (374+ 228.68K)/2(417.07) = 0.723 K
Prmean = (P15 + P16)/2Pc = (2+0.45)/2(38.17) = 0.0321 bar
From Figure 3.8, Compressibility factors (Coulson & Richardson, Chemical
Engineering, Volume 6, page 87).
Z = 0.8
Then find n, n = 1/(1-m) = 1/(1-0.0665) = 1.07
Polytropic work = zRT1(n/n-1)x((P15/P16)(n-1/n) 1)
=
1.07
1.00 (8.314 )( 374 )( 0.07
0.07
2 1.07
) x
1
0.45
= 4872.06 kJ/kmol
Actual work = Polytropic work / Ep
= 4872.06 /0.80
= 6090.07 kJ/kmol
Compressor power = 6090.07 kJ/kmol x 53999.12x 1hr/3600s
= 91349kW
Therefore the compressor power required to decrease the pressure from 2 bar
(2000kPa) to 0.45 bar (450kPa) is 91349kW.
8.2.12 E-103 (Cooler 1)
S16
T = 193oC
P = 120 Kpa
(Liquid)
S17
T = 64.50C
P = 100 Kpa
(Liquid)
107
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Sample of Calculation for Cooler 1
Stream 16
Component
C5H12O
CH3OH
H2O
i-C4H8
I-C4H10
C4H10O
n-C4H10
(CH3)2O
Flowrates
Kmol/hr
4.30 x102
5.02
1.21 x102
8.16
2.37 x102
6.65 x101
2.71
1.21 x102
F
kJ/Kmol
-2.93 x105
-2.01 x105
-2.42 x105
-1.69 x105
-1.35 x105
-3.13 x105
-1.26 x105
-1.84 x105
To
K
298
298
298
298
298
298
298
298
T, K
466
466
466
466
466
466
466
466
H
kJ/hr
3270.13
11.81
194.19
40.93
1323.40
4.26
15.17
438.54
H =
5298.44
Stream 17
Component
C5H12O
CH3OH
H2O
i-C4H8
I-C4H10
C4H10O
n-C4H10
(CH3)2O
Flowrates
Kmol/hr
4.30 x102
5.02 x102
1.21 x102
8.16
2.37 x102
0.665
2.71
1.21 x105
F
kJ/Kmol
-2.93 x105
-2.01 x105
-2.42 x105
-1.69 x105
-1.35 x105
-3.13 x105
-1.26 x105
-1.84 x105
To
K
298
298
298
298
298
298
298
298
T, K
337.5
337.5
337.5
337.5
337.5
337.5
337.5
337.5
H
kJ/hr
665.50
2.50
44.96
8.45
267.64
0.85
3.09
91.16
H =
1084.15
Energy balance,
108
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Q=(
H)
out
H)
in
= 1084.15 5298.44
= -4214.29 kW (heat is being released to the surrounding)
Steam flowrate, Q = mCpT
Cp of pure water, 4.184 J/g oC (Elementary Principles of Chemical Processes,
W.Rousseau et. al)
Q
mCp T
4214290J/s
=
4.184J/g o C x (193 - 64.5)
=7838.44 g/s
m=
Therefore the supply of steam flow rate required is 7838.44 g/s.
8.2.13 T-101 (Distillation Column 1)
S19
P = 305 Kpa
T = 53.3 oC
(gas)
S17
P = 450 Kpa
T =64.5 oC
( liquid )
S18
P = 400 Kpa
T = 103.3oC
( liquid )
Sample of Calculations:
R = L/D
Overall:
(NL), L = D x 1.5
109
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
= 451.779 x 2.5
= 1129.45 kmol/hr
(NV), V = L + D
= 1129.45+ 451.779
= 1581.23 kmol/hr
Stream 17
Component
C5H12O
CH3OH
H2O
i-C4H8
I-C4H10
C4H10O
n-C4H10
(CH3)2O
Flowrates
Kmol/hr
4.30 x102
5.02
1.21 x102
8.16
2.37 x102
0.665
2.71
1.21 x102
F
kJ/Kmol
-2.93 x105
-2.01 x105
-2.42 x105
-1.69 x105
-1.35 x105
-3.13 x105
-1.26 x105
-1.84 x105
To
K
298
298
298
298
298
298
298
298
T, K
337.5
337.5
337.5
337.5
337.5
337.5
337.5
337.5
H
kJ/hr
665.50
2.50
44.96
8.45
267.64
0.85
3.09
91.16
H =
1084.15
Stream 19
Component
Flowrates
To
T, K
CH3OH
H2O
i-C4H8
(CH3)2O
i-C4H10
n-C4H10
Kmol/hr
1.01E+00
6.83E+00
1.76E-01
2.63E+01
2.37E+02
2.71E+00
kJ/Kmol
-2.01 x105
-2.42 x105
-1.69 x104
-1.84E+05
-1.35 x105
-1.26 x105
K
298
298
298
298
298
298
376.3
376.3
376.3
376.3
376.3
376.3
kJ/hr
1.03
5.04
0.38
40.85
557.49
6.42
H =
611.21
Stream 18
Component
Flowrates
Kmol/hr
F
kJ/Kmol
To
K
T, K
H
kJ/hr
110
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CH5H12O
CH3OH
C4H10O
H2O
4.30E+02
7.50E-02
8.64E+00
1.26E+01
-2.93 x105
-2.01 x105
-3.13 x105
-2.42 x105
298
298
298
298
326.3
326.3
326.3
326.3
469.97
0.03
7.82
3.35
H =
481.17
Energy balance,
Q=(
H)
out
H)
in
= (481.17 + 611.21) 1084.15
= 8.23 kW
8.2.14 Cooler 2 (E-104)
S19
T = 53.3oC
P = 305 Kpa
(Liquid)
S20
T = 400C
P =100 Kpa
(Liquid)
Sample of Calculation for Cooler 1
Stream 19
Component
Flowrates
To
T, K
CH3OH
H2O
i-C4H8
(CH3)2O
i-C4H10
n-C4H10
Kmol/hr
1.01E+00
6.83E+00
1.76E-01
2.63E+01
2.37E+02
2.71E+00
kJ/Kmol
-2.01 x105
-2.42 x105
-1.69 x104
-1.84x105
-1.35 x105
-1.26 x105
K
298
298
298
298
298
298
376.3
376.3
376.3
376.3
376.3
376.3
kJ/hr
1.03
5.04
0.38
40.85
557.49
6.42
H =
611.21
Stream 20
Component
Flowrates
Kmol/hr
F
kJ/Kmol
To
K
T, K
H
kJ/hr
111
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CH3OH
1.01E+00
H2O
6.83E+00
i-C4H8
1.76E-01
(CH3)2O
2.63E+01
I-C4H10
2.37E+02
N-C4H10
2.71E+00
-2.01
x105
-2.42
x105
-1.69
x104
-1.84
x105
-1.35
x105
-1.26
x105
298
313
0.19
298
313
0.96
298
313
0.07
298
313
7.33
298
313
98.29
298
313
1.14
H =
107.97
Energy balance,
Q=(
H)
out
H)
in
= 107.97 611.21
= -503.24 kW
Steam flowrate, Q = mCpT
Cp of pure water, 4.184 J/g oC (Elementary Principles of Chemical Processes,
W.Rousseau et. al)
Q
mCp T
503240J/s
=
4.184J/g o C x (53.3 - 40) o C
=9043.40 g/s
m=
Therefore the supply of steam flow rate required is 9043.40 g/s.
8.2.15 P-102 (Pump 3)
S22
T = 270C
P = 30 kPa
(liquid)
S21
T = 270C
P = 25 kPa
(Liquid)
112
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Calculations are based on Yunus A. Cengel, Micheal A. Boles, Thermodynamics: An
Engineering Approach, WCB/Mc Graw-Hill, 1989, page, 354-355.
Assumptions:
7. Steady operating conditions exist,
8. Kinetic and potential energy negligible
9. The process is to be isentropic
Specific volume:
Water = 0.056m3/mol
(Data of these specific volumes are based on Coulson & Richardsons)
(Which remains essentially constant during the process)
Water
0.056m3/mol x 1000 mol/kmol x 1 kmol/18kg = 3.11 m3/kg
Which remains essentially constant during the process.
2
Win = Vdp
1
= V1 (P2 P1 )
= 3.11m 3 /kg(30 25)kpa(1kJ /1kpa.m 3 )
= 3.11(5)
= 15.55kJ/kg
15.55kJ/kg x 1469kg/hr
= 22851 kJ/hr = 22.85kW
8.2.16 T-102 (Extraction Column)
113
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
S22
T=27oC
P=30kPa
(liquid)
S23
T=40oC
P=250kPa
(liquid)
S20
T=40oC
P=100kPa
(liquid)
S25
T=27oC
P=100kPa
(liquid)
Sample of Calculation for Extraction Column
Stream 20
Component
Flowrates
Kmol/hr
CH3OH
1.01E+00
H2O
6.83E+00
i-C4H8
1.76E-01
(CH3)2O
2.63E+01
I-C4H10
2.37E+02
N-C4H10
2.71E+00
F
kJ/Kmol
-2.01
x105
-2.42
x105
-1.69
x104
-1.84
x105
-1.35
x105
-1.26
x105
To
K
T, K
H
kJ/hr
298
313
0.19
298
313
0.96
298
313
0.07
298
313
7.33
298
313
98.29
298
313
1.14
H =
107.97
Stream 22
Component
H2O
Flowrates
Kmol/hr
F
kJ/Kmol
To
K
T, K
8.16E+01
-2.42
x105
298
300
H
kJ/hr
H =
1.53
114
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Stream 23
Component
Flowrates
Kmol/hr
i-C4H8
1.76E-01
(CH3)2O
2.63E+01
i-C4H10
2.37E+02
n-C4H10
2.71E+00
F
kJ/Kmol
-1.69
x104
-1.84
x105
-1.35
x105
-1.26
x105
To
K
T, K
H
kJ/hr
298
313
0.07
298
313
7.33
298
313
98.30
298
313
1.14
H =
106.83
Stream 25
Component
CH3OH
H2O
Flowrates
Kmol/hr
1.01E+00
8.84E+01
F
kJ/Kmol
-2.01 x105
-2.42 x105
To
K
298
298
T, K
300
300
H
kJ/hr
0.02
1.65
H =
1.68
Energy balance,
Q=(
H)
out
H)
in
= (106.83+1.68) (107.97+1.53)
= -0.91 kW
8.2.17 Pump 4 (P-103)
S24
T = 40OC
P=
300 Kpa
(liquid)
115
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Calculations are based on Yunus A. Cengel, Micheal A. Boles, Thermodynamics: An
Engineering Approach, WCB/Mc Graw-Hill, 1989, page, 354-355.
Assumptions:
1. Steady operating conditions exist,
2. Kinetic and potential energy negligible
3. The process is to be isentropic
Specific volume:
(Data of these specific volumes are based on Coulson & Richardsons)
Specific volumes:
DME = 0.178m3/mol
Butene = 0.240 m3/mol
Isobutane = 0.255m3/mol
n-butane =0.263 m3/mol
DME
0.178m3/mol x 1000mol/kmol x 1kmol/46kg = 3.87 m3/kg
butene
0.240m3/mol x 1000mol/kmol x 1kmol/56kg = 4.29 m3/kg
Isobutane
0.255m3/mol x 1000mol/kmol x 1kmol/58kg = 9.11 m3/kg
n-butane
0.263m3/mol x 1000mol/kmol x 1kmol/58kg = 4.53 m3/kg
Vavg = (3.87 + 4.29 + 9.11 + 4.53) m3/kg / 4 = 5.45 m3/kg
Which remains essentially constant during the process
116
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Win = Vdp
1
= V1 (P2 P1 )
= 5.45m 3 /kg(300 250)kpa(1k J/1kpa.m
= 5.45(50)
= 272.50kJ/k g
272.50kJ/k g x kg/hr
= 4113932.50 kJ/hr = 1142.76kW
8.2.18 Pump 5 (P-104)
S26
T = 27OC
P=
150 Kpa
( liquid
) on Yunus A. Cengel, Micheal A. Boles, Thermodynamics: An
Calculations are based
Engineering Approach, WCB/Mc Graw-Hill, 1989, page, 354-355.
Assumptions:
4. Steady operating conditions exist,
5. Kinetic and potential energy negligible
6. The process is to be isentropic
Specific volume:
Water = 0.056 m3/mol
Methanol = 0.118m3/mol
(Data of these specific volumes are based on Coulson & Richardsons)
Water
0.056 m3/mol x 1000mol/kmol x 1kmol/18kg = 4.26 m3/kg
Methanol
0.118m3/mol x 1000mol/kmol x 1kmol/32kg = 3.6875 m3/kg
117
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Vavg = (4.26 m3/kg + 3.6875 m3/kg)/2
= 3.974 m3/kg
Which remains essentially constant during the process.
2
Win = Vdp
1
= V1 (P2 P1 )
= 3.974m 3 /kg(150 100)kpa(1k J/1kpa.m
= 3.974(50)
= 198.7kJ/kg
198.7kJ/kg x 1624.32kg/ hr
= 322752.384 kJ/hr = 322 .75 kW
8.2.19 T-102 (Distillation Column 2)
S28
T=530C
P=100kPa
(liquid)
S26
T=27oC
P=150kPa
(Liquid)
Sample of Calculations:
S27
T=30oC
P=70kPa
(liquid)
R = L/D
Overall:
(NL), L = D x 1.5
= 88.44 x 1.5
= 132.66 kmol/hr
(NV), V = L + D
118
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
= 132.66 + 88.64
= 221.30 kmol/hr
Stream 26
Component
Flowrates
To
T, K
CH3OH
H2O
Kmol/hr
9.91E-01
1.76E+00
kJ/Kmol
-2.01 x105
-2.42 x105
K
298
298
300
300
kJ/hr
0.02
-4.79
H =
-4.77
Stream 27
Component
Flowrates
To
T, K
CH3OH
H2O
Kmol/hr
2.00E-02
8.67E+01
kJ/Kmol
-2.01 x105
-2.42 x105
K
298
298
300
300
kJ/hr
0.00
-235.48
H =
-235.48
Stream 28
Component
Flowrates
To
T, K
CH3OH
H2O
Kmol/hr
1.10E-02
8.84E+01
kJ/Kmol
-2.01 x105
-2.42 x105
K
298
298
326
326
kJ/hr
0.00
-240.26
H =
-240.26
Energy balance,
Q=(
H)
out
H)
in
= (-235.48+(-240.26)) (-4.77)
= -470.97 kW
8.2.20 Cooler 3 (E-105)
S28
T = 53oC
P = 100Kpa
(Liquid)
S29
T = 270C
P = 130 Kpa
(Liquid)
119
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Sample of Calculation for Cooler 1
Stream 28
Component
Flowrates
To
T, K
CH3OH
H2O
Kmol/hr
1.10E-02
8.84E+01
kJ/Kmol
-2.01 x105
-2.42 x105
K
298
298
300
300
kJ/hr
0.00
-240.26
H =
-240.26
Stream 29
Component
Flowrates
To
Kmol/hr
kJ/Kmol
T, K
CH3OH
9.91E-01
-2.01 x105
298
300
0.02
H20
1.76E+00
-2.42 x105
298
300
0.03
kJ/hr
H =
0.06
Energy balance,
Q=(
H)
out
H)
in
= 0.06 (-240.26)
= 240.32 kW
Steam flowrate, Q = mCpT
Cp of pure water, 4.184 J/g oC (Elementary Principles of Chemical Processes,
W.Rousseau et. al)
Q
mCp T
240320 J/s
=
4.184J/g o C x (53 - 27) o C
= 2209.15 g/s
m=
120
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Therefore the supply of steam flow rate required is 2209.15 g/s.
CHAPTER 9
121
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
HYSYS
9.0
THE DESIGN MADE BASED ON HYSYS SIMULATION
There are two method that was used in calculating the mass balance and energy
balance for the process which is:
i)
Manual calculation
ii)
Hysys simulation
Hysys program was used to see whether the design could be run or not. Using
Hysys the calculation of the process was calculated automatically when the parameter
that needed was insert. Then if the parameter that was insert is logic so Hysys program
can calculated the result and the equipment can converge. If the data that was inserted
was illogical the equipment cannot converge and the calculation cannot be done.
At the back of this page show the simulation using Hysys that was converge
and include with the manual log book.
REFERENCES
Alber V.G Hahn (1970). The Petrochemical Industry Market & Economics, USA
Mc Graw Hill Book Company. 363- 372.
122
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Coulson & Richardsons, Chemical Engineering Volume 6 Third Edition, Butterworth
Heinemann.
Norman N. Barish and Seymour Kaplan, Economic Analysis for Engineering and
Managerial Decision Making, Second Edition, Mc Graw Hill
Robert H. Perry and Don W. Green, Perrys Chemical Engineerings Handbook,
Seventh Edition, Mc Graw Hill.
Encik Mohd Napis Bin Sudin. 2003, Production of MTBE, Malaysia, Kuantan. Interview,
8 July
Puan Masri. 2003. Information of MTBE production, Malaysia, Kuantan. Interview, 28
Jun.
Lanny P. Schmidt (1998), The Engineering of Chemical Reaction, Oxford University
Press.
James, G. Speight, Baki Ozum (1985). Petroleum Refining Process, Apex
Engineering Inc. Marcell Dekkir New York.
MTBE and Oxygenates (1990), An International Marketing Guide Dewitt & Company
Incorporated 16800 Greenport park, Suite 120N Houston, Texas 77060-2386.
Page 51-62.
The 1992-1995 Worldwide Catalyst Product, Process Licensing & Service DirectoryTechnical articl-1992/3. Page 9.
Annual Report 1994, Section 4. Area Summary for asia and the Pacific. Page 135-169.
Ray/Johnston (1989). Chemical Engineering Design Project, a case study approach,
volume 6. Gordon and Breach Science Publishers.
Wentz (1998). Safety, Health, And Environmental Protection, Mc Graw Hill
Companies, Inc.
Wiley (2000). Elementary Principles of Chemical Processes, John Willey & sons,
Inc.
Chemical Week June 11, 2003 Volume 165 page 31
George S. Brandy ,Henry R.Clauser & John A. Vaccari. Material Handbook 4th
editionJoshua D.Tayloy, Jerrey I. Steinfeld and Jefferson W.Tester Ind. Eng. Chem. Res
2001,40, 67-74 page 71.
Monica Bianchi & Rachl Uctas,ECN ACN/CMR/ ECN NPRA Supplement, March 2002.
Petronas Resource Center, Tingkat 4, Menara 2, Menara Berkembar Petronas, Kuala
Lumpur City Center.
123
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Malaysia Industrial Development Authority
(MIDA), Plaza Central, K L Sentral. Kuala
Lumpur.
Pusat Informasi SIRIM Berhad. 1, Persiaran Dato Menteri, PO Box 7035, Seksyen 2,
40911 Shah Alam.
Jabatan Perangkaan Malaysia, Pusat Pentadbiran Kerajaan, Putrajaya.
Tiram Kimia Sdn. Bhd, Tingkat 1, Bangunan Shell, Off Jalan Semantan, Damansara
Heights, 50490 Kuala Lumpur.
http://www.Manufacturing.net/pur/index.asp
http://www.ceh.sric.sri.com/Publichtml
http://ww2.cemr.wvu/edu/~wwwche/publications/project/index.html
http://www.illallc.com/engarticle.html
http://www.huntsman.com/pertochemicals/ShowPage.cfm
http://www.cmt.anl.gov/science_technology/basicci/onestep_phenol.shtml
http://www.illallc.com/engpatent3am.html
http://www.wikipedia.org/w/wiki.phtml
http://www.ccohs.ca/oshanswers/chemicals/chem_profiles/acetone/basic_ace
http://www.atsdr.ede.gov/toxfag.html
http://www.shellchemicals.com/chemicals/products/1,1184,806,00.html
http://www.mida.gov.my
http://eneken.ieej.or.jp/en/data/pdf/142.pdf
http://www.matheson-trigos.com/mathportal/-pdfs/product/isobutane.pdf
http://www.specialgas.com/isobutane.html
http://www.gas.com.pdf/gas.pdf
http://www.boc.com/microsite/america/products/gases/mixed/isobutane.html
http://www.boc.com/microsite/america/products/gases/aps/geiger.html
http://www.airliquide.com/safety/msds/en/129-AlEn.pdfhttp://www.cpchem/msds/specchem/isobutaneinstrumengradi.pdf
124
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
DESIGN
PROJECT II
125
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
SECTION 1
Catalytic Cracking Design
1.1 INTRODUCTION
A bed of solid particles can be fluidized by a stream of gas through it. The fluidization
of solids in a stream of gas occurs only if the gas velocity achieved a certain value
which is called minimum fluidization velocity Umf. Once the gas velocity achieved this
value, the bed expands and pressure drop across the fluidized bed remains constant
once fluidization occurred.
In this commercial fluidized-bed catalytic cracking reactor, catalysts flow up through the
reaction regeneration section in a riser type of flow regime. The over head catalyst
captured by cyclones is returned to the hopper where it is fluidized with air to recapture
any entrained hydrocarbon vapor. The catalyst was then discharged from the hopper,
down through a standpipe. The solids flow through the standpipe was controlled by
slide valve located at the base. From there, the solids went into the riser where they
are carried by stream of air to the regenerator vessel.
The regenerator operation in these plants resembled that of the reactor except for the
systems use of air instead of oil vapor. A portion of the catalyst from the regenerated
catalyst hopper was returned to the regenerator through catalyst fresh feed
exchangers. This action controlled the regenerator temperature and served to preheat
the feed. Another bypass line from the hopper to the regenerator was used to control
the dense bed level or holdup in the regenerator. Catalyst from the regenerated
catalyst hopper flowed through a standpipe back into riser where the feed was injected.
The commercial cracking catalysts used most widely is silica-alumina. High content
catalysts are characterized by higher equilibrium activity level and surface area. These
126
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
catalysts could be offered at a lower price. An advantage of this catalyst grade is that a
lesser amount of adsorbed, unconverted, heavy products on the catalyst were carried
over to the stripper zone and regenerator. As a result, a higher yield of more valuable
products and also smoother operation of the regenerator was achieved.
Basically the design of the fluidized bed system can be divided into several sections:
1. Reaction vessel which included:
Fluidized bed portion
Gas disengaging space or freeboard
Gas distributor
2. Solids feeder or flow control
3. Solids discharge
4. Dust separator for the exit gas
5. Instrumentation and control
6. Gas supply
Figure 1.1 : Illustration diagram of the reactor
127
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
1.2
Estimation of diameter of reactor
The fluidized bed diameter depends on the operating gas velocity. A larger diameter is
required for a low gas velocity while for a high gas velocity, a small diameter is
required.
However the gas velocity must exceed the terminal velocity (Ut) of the particle
transport of solid particles may occur. The operating velocity should be between
minimum fluidization velocity and terminal falling velocity to maintain fluidization of
solids.
Operating gas velocity, Uo =
Where
d p2 g ( p g )
18 g
dp = diameter of particle
= density of particle
= density of gases
= viscosity of gases
g = gravitational acceleration
so, the value of Uo =
(80 10 6 ) 2 9.81 (1282 1.484 )
18 (1.15 10 5 )
= - 0.388 m/s (rising)
= 0.388 m/s
Flowrate of gas stream , Q = 39353 kg/hr
=
39353
m3/s
1.484 3600
= 7.366 m3/s
The bed diameter will be depending on the area of reactor used:
Cross sectional area, A =
=
Q
V
7.366
0.388
= 18.985 m2
128
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Diameter of bed, D =
4A
4 18 .985
= 4.9164 m
5m
1.3
Calculation of the Transport disengagement height, TDH
According to M. Rhodes (1998), the TDH region is considered as the region where
located above the bed surface to the top of disengagement zone. While the
disengagement zone is the region above the splash zone or region just above the bed
surface in which the upward flux and suspension concentration of fine particles
decrease with increasing in height.
There are so many correlations that can be used to find the TDH value. For this
design Amitin et al. (1968) was used.
TDH ( F ) = 0.85U 0
TDH ( F ) = 7.740968
1 .2
( 7.33 1.2 log 10 U 0 )
(1.1)
8m
1.4 Minimum fluidization Velocity
The minimum fluidization velocity (Umf) is determined from Ergun equation:
For Reynolds number, 0.01 < Re < 1000:
According to the Martin Rhodes (1999), Ar =
Re =
d p2 g ( p g ) g
18 g
and
U mf D p f
129
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Ar = 150
And
(1 ) Re + 1.75
1
Re 2
3
(1.2)
By rearranging the equation (1.2) above we can get the new equation for minimum
fluidization, Umf. As a result, the equation is becomes:
Re =
U mf D p f
U mf =
g d p
(1135 .7 + 0.0408 Ar ) 0.5 33 .7
1.15 10 5
(1135 .7 + 0.0408 72 .44 ) 0.5 33 .7
6
1.484 80 10
U mf = 0.00425 m / s
1.5
Calculation for the value of terminal velocity U t
The value of Re
need to be calculated first, then the value of U t can be calculated.
The range of particle size is 65 m to 95 m. The mean particle size is 250 m.
6
When d p = 80 10 m
4 g ( p g ) gd p
C D Ret =
2
3
g
C D Re t
4 1.484 (1282 1.484 ) 9.81 80 10 6
=
2
3
1.15 10 5
C D Re t = 96.227883
2
From the chart of C D Re t and
CD
vs Reynolds number, for value of
Re t
130
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 1.1 : CDRe2 and CD/Re versus Reynold number
2
C D Re t = 96.227883 the value of Re t = 3
Now from this we can calculate the value of U t , where
Re t =
3=
g U t d p
g
1.484 U t 8 10 6
1.15 10 5
U t = 0.290599 ms 1
131
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
6
when d p = 85 10 m
C D Re t
C D Re t
4 g ( p g ) gd p
=
3
2
4 1.484 (1282 1.484 ) 9.81 85 10 6
=
2
3
1.15 10 5
C D Re t = 115.421775 5
From the chart of C D Re t and
CD
vs Reynolds number from figure 1.1 for value
Re t
2
of C D Re t = 115.421775 5 the value of Re t = 3.5
Now from this we can calculate the value of U t , where
Re t =
3=
g U t d p
g
5.54 U t 85 10 6
5.0 10 5
U t = 0.3190899 ms 1
Table 1.1 : Calculation for terminal velocity in different size of dp.
132
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Sieve
Weight,
Weight
size dp,
%, xi
fraction,
3.5
7.5
24
44
9.5
7
4.5
xi
0.035
0.075
0.24
0.44
0.095
0.07
0.045
m
0.000095
0.00009
0.000085
0.00008
0.000075
0.00007
0.000065
1.6
CDReT2
161.1394175
137.0119672
115.4217755
96.22788368
79.28933286
64.46516426
51.61441905
Reynold
Terminal
number
4.5
4
3.5
3
2.5
2
1.8
velocity, Vt
0.367073344
0.344414495
0.3190899
0.29059973
0.258310872
0.221409318
0.214596724
Find the value of K i*
Using the correlation for estimating entrainment rates are reported in the literature. The
entrainment rate can be expressed by the following equation, as follows:
Ei = Ei + (Eio -Ei)exp (-afh)
( 1.3)
Where Ei = entrainment rate at a point h above bed surface
af = a constant in freeboard
Ei = rate of elutriation of the fines with diameter dpi above the TDH
Ei = K i Xi
(1.4)
Where Ki is the elutriation rate constant for which numerous correlations have
been reported. Table 2.2 from Appendix lists various published correlations
for the elutriation rate constant.
The constant, af, in equation (1.2) is independent of the beds composition and can be
evaluated from experimental data for Fi as a function of h. Following Chen et al. (1979),
is the entrainment rate of particles at the bed surface, where:
Eio = KoXi
(1.5)
Where Ko = Elutriation rate constant at bed surface
Xi = weight fraction of the particle cut size dpiA
1.7
Find the value of Eo
133
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.07 10 9 g
Eo
=
Ad eqmt
2 .5
Eo =
3.5
g 0 .5
(U U )
2..5
mf
3.07 10 9 (1.484 ) 3.5 (9.81) 0.5 ( 0.388 0.00425416 7 )
(1.15 10 5 ) 2..5
2.5
(18 .98 )( 5)
Eo = 10.676 kg/m2s
The following correlation is used to calculate the value of E i* by using three different
investigators which is :
Merrick and Highley (1974)
0.5
E i
v U mf
= A +130 exp 10 .4 t
g U
U
U U mf
0.25
Geldart et al. (1979) revised
E i
v
= 23 .7 exp 5.4 t
gU
U
and Colakyan et al (1979)
E i
= 331 t
U
From this correlation we find that the average of the correlation for these three
investigators, shown in the table below:
134
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 1.2 : Correlation of three investigators
Merrick and Highley
Geldart et al.
(1974)
Ki*
2.810456563
3.115064954
3.509080841
4.035580234
4.76909596
5.849694145
6.085514067
(1981)
Ki*
0.008247282
0.011304929
0.016081902
0.023907858
0.037471784
0.062625124
0.068853496
Colakyan (1979)
Ki*
10.27741771
8.238989106
6.227114828
4.299839595
2.545780992
1.100824216
0.8993437
1.8
Ki*, average
4.36537385
3.788452996
3.25075919
2.786442562
2.450782912
2.337714495
2.351237088
Calculation of solid loading
First find the value of Kih ,
K ih = K i* + ( E o K i* ) exp( a i h)
= 4.36537385
= 4.36537385
+ (10.676996 53 - 4.36537385
)(2.69398E
- 12)
kg/m 2 s
R = Ri = RmRi
m Bi =
Fm Fi
( F R ) + K i A
135
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
m Bi =
10 .93 0.035
(10 .93 R ) + 4.36537385
18 .98
10 .93 0.075
(10 .93 R ) + 3.78845299 6 18 .98
10 .93 0.024
(10 .93 R ) + 3.25075919
10 .93 0.0095
10 .93 0.07
+
(10 .93 R ) + 2.45078291 2 18 .98 (10 .93 R ) + 2.33771449 5 18 .98
10 .93 0.0045
(10 .93 R ) + 2.35123708 8 18 .98
18 .98
10 .93 0.044
(10 .93 R ) + 2.78644256 2 18 .98
So the value of Rih calculated by excel is = 0.564147556 kg/s
Eih =
RT
A
0.5641475
=
18.98
= 0.0297 kg / m 2 s
Rti = Kih* A
= 4.36537385
1 8.98
2.78644256
2 1 8.98
2.35123708
8 1 8.98
+ 3.78845299
+ 2.45078291
6 1 8.98
2 1 8.98
+ 3.25075919
+ 2.33771449
1 8.98
5 1 8.98
= 404.8578835 kg/s
Ri = Kih A. Xi
= 4.36537385
2.78644256
2.35123708
= 55.90386
18.98 0.035
+ 3.78845299
6 18.98 0.075
+ 3.25075919
18.98
2 18 .98 0.44
+2.45078291
2 18.98 0.095
+ 2.33771449
5 18.98 0
8 18.98 0.045
kg/s
136
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 1.3 : Data calculation to find solid loading
Total
Mbi
0.004103705
0.009964059
0.03640034
0.076031739
0.018254549
0.013978087
0.008943974
0.167676452
MRi
0.602699773
1.269994045
3.981014237
7.127685256
1.505148839
1.099367014
0.707506537
16.2934157
Ki*
4.36537385
3.788452996
3.25075919
2.786442562
2.450782912
2.337714495
2.351237088
Rti
82.85479568
71.90483787
61.69940943
52.88667983
46.51585967
44.36982112
44.62647993
Ri
2.899918
5.392863
14.80786
23.27014
4.419007
3.105887
2.008192
55.90386
a) Solid loading unreturned = 0.029723 / 0.388
= 0.076606351kg/s
b) solid loading return =
=
55.90386 / 18.98
2.94540903 kg/s
1.9 Calculation of holding time and residence time
The outlet concentration of a plug flow reactor is related to the inlet concentration of the
reactant by the same equation as in a batch reactor with the same residence time -.
For an equilibrium reaction between A and B, is first order.
Based on studied of Khabtou, S., Chevreau, T., and Lavalley, J.C., Micropor. Mat. 3,
133 (1994), express the rate per catalyst mass instead of reactor volume.
k mA =
=
1
0.75
0.35
ln 1 0.35
151 .92 0.75 +1
0.75
4.785 10 3 m 3 kg
hr
where x is the conversion in this process,
x = ([A] 0 - [A]) / [A]0 = 0.35,
137
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
[A] is experssed in concentration in mol/m3, K the rate mol m-3 s-1, kA in s-1.
The value K = [i-C4=]eq/[i-C4]eq = 0.75 and ST = 151.94 g.hr/m3 based on study by
Yamamoto, S. Asaoka, et al (1997).
Then from below equation given, w can find :
k = (1 A ) ln
1
A X A
1 X A
1
0.41(065 )
1 0.65
= (1 0.41 ) ln
= 2256.48/ 4.785x10-3
= 417574.39 hr
= 115.99 s
when the total holding time are calculate then the weight of the bed of fluidized bed can
be calculated. The formula used is as below:
t=
W Bed
FB 0
WBed
830
=
3600
39353
W Bed = 95679.7761 7 kg
1.10
Calculation for the pressure drop PB
The equation that can be used to calculate the pressure drop across the bed is:
138
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
g
W Bed W Bed
p
( p ) =
A
( p ) =
( 95679.78
1.484
9.81 ) ( 95679.78 9.81 )
1282
18 .985
( p ) = 49382 .78 Pa
49 .383 kPa
According to Kunii and Levenspield, the pressure drop across the distributor p d is
10% from the value of pressure drop across the bed when fluidized PB . So the value
of p d is:
( p d ) = 10 %( p B )
( p d ) = 10 % (49 .382 kPa )
( Pd ) = 4.938 kPa
0.5035 kgm 2
According to Kunii and Levenspield (1991), to determine the number of holes in the
distributor the Re
need to be calculated first:
Re t =
g U 0 Dt
g
Re t =
1.484 0.388 5.0
1.15 10 5
Re t = 250344
1.11
Determine the direction and flow rate of gas passing between the vessels.
139
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Assuming that in fluidized flow the apparent weight of the solids will be supported by
the gas flow, the equation below gives the pressure gradient for fluidized bed flow:
( P ) = (1 ) ( ) g
p
g
H
( P )
= (1 0.42 ) (1282 1.484 ) 9.81
= 7411.499
Pa / m
Actual pressure gradient =
( 3.25 2.89 ) 10 5
10
= 3600 Pa / m
Since the actual pressure gradient is well below that for fluidized flow, the standpipe is
operating in packed bed flow.
The pressure gradient in packed bed flow is generated by the upward flow of gas
through the solids in the standpipe. The Ergun equation above provides the
relationship between gas flow and pressure gradient in packed bed.
Knowing the required pressure gradient, the packed bed voidage and the particle and
gas properties, equation below can solve for IUrelI, the magnitude of the relative gas
velocity:
( P )
H
2
f (1 )
(1 )
2
= 150 2
!
U
!
+
1
.
75
!U rel !
rel
2
x sv
x sv
For standpipes it is to take downward velocities as positive. In order to create the
pressure gradient in the required direction, the gas must flow upwards relative to the
solids. Hence, IUrelI is negative:
IUrelI = -0.291 m/s
140
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
From the continuity for the solid,
Solid flux, =
Gp
A
= U p (1 ) p
The solid flux calculated is 55.9 kg/m2s as and so
Up =
55 .9
(1 0.41 ) 1282
= 0.072715 m /s
Solids flow is downward, so Up = + 0.072715 m/s
The relative velocity, Urel = Uf - Up
Hence, actual gas velocity, Uf = - 0.291 + 0.072715
= - 0.21829 m/s (upwards)
Therefore the gas flows upwards at a velocity of 0.21829 m/s relative to the standpipe
walls. The superficial gas velocity is therefore :
U = Uf = -0.0895 m/s (upward )
From the continuity for the gas, mass flow rate of gas,
Mf = U f p A
= -0.10431 kg/s
So, for the standpipe operate as required, 0.10431 kg/s of gas must flow from the lower
vessel to the upper vessel.
1.12
Design of cyclone
Size a cyclone separator for removing particles above 80 m in diameter entrained in a
flue gas stream. The following information is supplied:
141
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
0.21
0.66
dp =
0.00008 m
gas volumetric flow =
1.8 m3/s
particle density =
1282 kg/m3
gas density =
1.484 kg/m3
viscosity gas = 0.0000115 Ns/m
kinematic viscosity v = 0.0000278 m2/s
inlet gas velocity =
0.388 m/s
specific wall thickness
=
5 mm
The particles are approximately round with a shape factor of 0.77
All dimensions of a cyclone of any design are selected depending on the width of inlet
duct b or on the diameter of cyclone Dc. The problem is to properly select one of these
dimensions from which the other dimensions are proportionally evaluated.
The cyclone diameter, settling velocity, gas velocity, and parameters of the suspension
to be separated are all interrelated parameters. Therefore, we select a preliminary
diameter for approximate calculations and then refine our estimate to a more exact
design.
According Nicholas P. Cheremisinoff at al. (1984), the relative dimensions of the
cyclone are specified as:
b = DC
and
hin = DC
For the chosen cyclone = 0.21 and = 0.66
The continuity equation for the inlet nozzle is:
bh =
Vsec
Win
(1.5)
142
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
where win is the inlet gas velocity; which for a primary cyclone operation is typically 18
to 22 m/s. Expressing b and hin in terms of diameter DC, equation above is rearranged
to solve for the cyclone diameter:
Vsec
DC =
w
in
0.5
0.5
1.8
0.21 0.66 18
= 0.85 m
For design purposes, assume a value of 0.9 m for DC.
The diameter of the discharge pipe is:
Dd = 0.58Dc = 0.58 x 0.9 = 0.52 m
The gas velocity in discharge pipe is thus:
Wd =
4 Vsec
4 1.8
=
2
Dd
3.142 0.52 2
= 8.5m / s
Specifying a wall thickness = 5 mm for the gas discharge pipe, its outside diameter
will be:
Dd ,out
Dd + 2
= 0.52 + 2(0.005 )
= 0.53 m
The width of the circular gap between the pipe and cyclone shell is:
Dc Dd ,out
2
2
= 0.45 0.265
= 0.185 m
The height of the circular gap from a spiral surface to the lower edge of discharge pipe
is:
H = 0.775 Dc = 0.775 X 0.9
= 0.7 m
The calculated dimensions of the cyclone can be checked by comparing the particle
settling time:
143
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
0 =
Rc Rd ,out
wo
wo
to the residence time of gas in the cyclone:
2 Rav n
wg
where Rc and Rd,out are the radii of the cyclone and discharge pipe, respectively; n =
number of gas rotations around the discharge pipe (we may assume n = 1.5)
The peripheral velocity of gas is:
wg =
Vsec
=
H
1 .8
0.7 0.185
= 13 .9 m / s
For this cyclone, this value must be in the range of 12 to 14 m/s
The average radius of the gas rotation is :
Rav =
Dd ,out
0.53 0.185
+
2
2
= 0.357 m
The centrifugal acceleration (at the average radius) is:
a=
w g2
Rav
13.9
= 542 m / s 2
0.357
The separation criterion is:
Ks
a
g
542
9.81
= 55 .2
In this case, the centrifugal field in the cyclone is 55.2 times more intensive than the
gravitational.
The Archimedes number is:
144
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
g d 3 p g
Ar =
g
2
9.81 (80 10 5 ) 3 (1282 1.484 ) 1.484
=
(1.15 10 5 ) 2
= 77.44
The settling number is:
S1 = Ar x 1 x
Ks = 72.44 x
55.08843
= 3999
Because
3.6 < S1 < 82,500 the flow regime through the cyclone is transitional.
Therefore, the theoretical velocity of the particles is:
f 2
w = 0.22 d
0.333
f 2
= 0.22 d
0.333
5.42 10 2 1282
= 0.22 10 10 5
1.15 10
4.638 m / s
The particles have a shape factor of = 0.77 and the gas inlet gas stream contains a
low volume of solid particles. Based on the operating conditions specified, the settling
velocity is
Ws = R w = 0.77 x 4.634 = 3.568 m/s
Because the concentration of the suspension is low, we may assume R = 1
145
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The settling time is therefore:
0 =
= 0.0518s
The residence time for the gas is:
2Ravg n
wg
2 3.142 0.357 1.5
= 0.2424 s
13 .9
Since 0 <
the diameter of the cyclone selected is acceptable and we may now
specify the other dimensions as based on the recommended proportions.
As a final calculation for the design, we evaluate the hydraulic resistance of the
cyclone:
P=
1
C D win2 Where CD is the typical number of cyclone.
2
= 0.5 x 1 x 1.484 x 182
= 240.4 N/m2
1.13
Calculation for mechanical design
For mechanical design, the temperature and pressure are imperative properties in
calculate the thickness and the stress of the material. For that reason, the safety factor
also required as safeguard and determined by certain consideration such as corrosion
factor, location and process characteristic.
From Hysys data, the operating temperature inlet into the reactor is 250oC and
regenerator is 180oC. The design temperature is related to the operating temperature.
The design pressure and temperature for this reactor are showed as follow:
Reactor
1. Design Pressure
Operating pressure
2.89 bar
0.289 N/mm2
146
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
For safety reason take pressure 10% above operating pressure
Design Pressure, Pi
Design Temp. , T
0.289 N/mm2x 1.1
0.3179 N/mm2
200C
2. Material Construction
The material used is stainless steel (18Cr/8Ni, 304). For this material, the design stress
at 200 C, R.K.Sinnot (1999).
Design stress, f
115 N/mm2
Diameter vessel, Di
5.0 m
Tensile strength,
510 N/mm2
3. Vessel Thickness
e
Pi Di
2 jf Pi
(0.3179 )( 5000 )
2(1)(115 ) (0.3179 )
7 mm + 4 mm
11 mm
From R.K.Sinnot (1999), this value should not be less than 12 mm (including 2 mm of
corrosion allowance). For vessel diameter around 5 m, this take e = 15 mm. A much
thicker wall will be needed at the column base to withstand the wind and dead weight
loads.
4.
Heads and Closure
This section covers the choice of closure to be used in the design. Basically there are
two types of ends, which are domed ends. A standard torispherical heads and
ellipsoidal heads as well as the flat heads are calculated in order to select the most
economical head regarding its thickness. All the calculation is referring to the R.K.
Sinnot, Coulson and Richardson Vol.6 page 815-817.
Take, crown radius, Rc
= Di
= 5.0 m
147
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Knuckle radius, Rk
= 6% Rc
= 0.03 m
A head of this size would be form by pressing: no joints, so J = 1.0
Cs
1
3+
4
Rc
Rk
1
3 +
4
5 .0
0 .3
= 1.77
Therefore, minimum thickness:
=
=
Pi Di
2 Jf Pi ( Cs 0.2)
( 0.3179 )( 5000 )
2(1)(115 ) 0.3179 (1.77 0.2 )
= 11 mm
5.
Column Weight
Dead weight of vessel, Wv
For a steel vessel,
Wv
240 Cv Dm (Hv + 0.8Dm) t
mean diameter, m
(Di + t)
Cv
a factor, take 1.15
Hv
height or length between tangent lines, m
wall thickness, m
Where,
Dm
148
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
To get a rough estimate of the weight of this vessel is by using the average thickness,
11mm
Therefore,
Dm
5 + 2 x 0.011
5.022 m
240 (1.15) (5.022) [7.6 + 0.8(5.022)] 0.011
177.13 N
0.17713 kN
So,
Wv
Weight of insulation, WI
Assume material is Mineral wool.
of Mineral wool
thickness
75 mm
130 kg/m3
Volume of insulation
=
(5.022) (7.622) (0.075)
9.018967m3
x Dm x Hv x thickness of insulation
Weight of insulation, WI
=
Volume of insulation x x g
9.018967 x 130 x 9.81
11501.89N
11.50189kN
Double this value to allow fittings, so weight of insulation will be = 23.004 kN
Weight of bed
149
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Weight of bed in reactor
WB
= 95679.78 x 9.81
= 938618.6 N
= 938.6186 kN
Weight of cyclone
Volume of cyclone in reactor = 2.438 m3
Weight of cylone = 2.438 x 9.81 x 1282
= 30661.31 N
= 30.661 kN
There fore,
Total weight
= Wv +WI +WB + Wc
= (177.1311 + 23003.78 + 938618.6 + 30661.31)N
= 992.461 kN
For Regenerator
1.
Design Pressure
Operating pressure
2.6 bar
0.26 N/mm2
For safety reason take pressure 10% above operating pressure
Design Pressure, Pi
Design Temp. , T
2.
0.26 N/mm2x 1.1
0.286 N/mm2
180C
Material Construction
The material used is stainless steel (18Cr/8Ni, 304). For this material, the design stress
at 200 C, R.K.Sinnot (1999).
Design stress, f
121 N/mm2
Diameter vessel, Di
6.5 m
150
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Tensile strength,
3.
e
510 N/mm2
Vessel Thickness
=
Pi Di
2 f Pi
(0.286 )( 6500 )
2(1)(121 ) (0.286 )
8 mm + 4 mm
12 mm
Heads and Closure
This section covers the choice of closure to be used in the design. Basically there are
two types of ends, which are domed ends. A standard torispherical heads and
ellipsoidal heads as well as the flat heads are calculated in order to select the most
economical head regarding its thickness. All the calculation is by referring to the R.K.
Sinnot, Coulson and Richardson Vol.6 page 815-817
Take, crown radius, Rc
= Di
= 6.5 m
Knuckle radius, Rk
= 6% Rc
= 0.39 m
A head of this size would be form by pressing: no joints, so J = 1.0
Cs
1
3+
4
Rc
Rk
1
3 +
4
6.5
0.39
= 1.77
Therefore, minimum thickness:
Pi Di
2 Jf Pi ( Cs 0.2)
151
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
( 0.286 )( 5000 )
2(1)(121 ) 0.286 (1.77 0.2 )
= 14 mm
5.
Column Weight
Dead weight of vessel, Wv
For a steel vessel,
Wv
240 Cv Dm (Hv + 0.8Dm) t
mean diameter, m
(Di + t)
Cv
a factor, take 1.15
Hv
height or length between tangent lines, m
wall thickness, m
Where,
Dm
To get a rough estimate of the weight of this vessel is by using the average thickness,
12 mm
Therefore,
Dm
6.5 + 2 x 0.012
6.524 m
240 (1.15) (6.524) [8 + 0.8(6.524)] 0.012
285.6337 N
0.2856 kN
So,
Wv
Weight of insulation, WI
Assume material is Mineral wool.
of Mineral wool
130 kg/m3
152
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
thickness
75 mm
Volume of insulation
=
(6.524) (8) (0.075)
12.29745 m3
x Dm x Hv x thickness of insulation
Weight of insulation, WI
=
Volume of insulation x x g
12.29745 x 130 x 9.81
15682.94N
15.68294kN
Double this value to allow fittings, so weight of insulation will be = 31.36588kN
Weight of bed
Weight of bed in reactor
WB
= 75060.19 x 9.81
= 736340.5 N
= 736.3405 kN
Weight of cyclone
Volume of cyclone in reactor = 2.438 m3
Weight of cylone = 2.438 x 9.81 x 1282
= 30661.31 N
= 30.661 kN
There fore,
Total weight
= Wv +WI +WB + Wc
= 0.2856 + 31.36588 + 736.3405 + 30.661
= 798.652 kN
153
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Total weight for reactor and regenerator = 992.461 + 798.652 kN
= 1791.113 kN
6.
Wind Loads
Take,
Win speed, Uw =
160 km/hr
For a smooth cylindrical column stack, the following semi-empirical equation can be
used to estimate wind pressure.
Pw
0.05Uw2
0.05(160)2
1280 N/m2
Loading per Unit Length of column, Fw
Fw
Pw Deff]
Where,
Deff
= Effective column diameter
= Diameter + 2(tshell + tinsulation )
= 6.5 + 2(12 + 75 ) x 10-3
= 6.68 m
Therefore,
Fw
1280 x 6.68
8550.4 N/m
Bending Moment
Mx
Fw ( X ) 2
2
154
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Where,
X
= Distance measure from the free end
=8m
Therefore,
Mx
7.
8550 .4 (18 ) 2
2
1385164.8 Nm
1385.1648 kNm
Analysis of Stress
From bottom tangent line,
Longitudinal pressure stress,
Pi Deff
2t
(0.286 )( 6680 )
2(12 )
79.6033N/mm2
Circumferential pressure stress,
Pi Deff
4t
(0.286 )( 6680 )
4(12 )
39.802 N/mm2
Dead weight stress,
( Di + t )t
155
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
1791 .113 10 3
(6500 +12 )12
7.2958 N/mm2
Bending Stress,
M Di
+ t
Iv 2
where,
M
= total bending moment
Iv
Iv
= second moment of area
64
(D
Di
which,
Di
= 6500 mm
Do
= (6500+ 2(12))
= 6524 mm
so,
Iv
(6524
64
1.3013 x 1012 mm4
6500 4 )
Therefore,
1385164 .8 1000 6500
+ 12
12
1.3013 10
2
3.47 N/mm2
The resulted longitudinal stress, z is:
z(upwind)
39.802 - 7.2958 + 3.47
35.9762 N/mm2
156
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
z(downwind)
39.802 - 7.2958 - 3.47
29.0363 N/mm2
=
8. Elastic Stability
Critical bulking stress
t
= 2 x 104
D
m
12
= 2 x 104
6512
= 36.855 N/mm2
Maximum compressive stress will occurs when the vessel not under pressure
=
= 7.2958 + 3.472
= 10.768 N/mm2
This is below critical bulking stress, so acceptable.
9. Vessel Support Design (Skirt Design)
Type of support
: Straight cylindrical skirt
: 80
Material construction : Carbon steel
Design stress, fs
: 135 N/mm2 at ambient temperature, 20C
Skirt height
: 4.0 m
Young modulus
: 200, 000 N/mm2
Therefore,
The total weight of vessel from calculation before = 1791.113 kN
Wind load,
Fw
= 8550.4 N/m
= 8.5504 kN/m
157
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Bending moment at skirt base,
Ms
( H v + H skirt ) 2
= Fw
(18 + 2 ) 2
= 8.5504
= 1710.08 kNm
As a first trial, take skirt thickness as same as the thickness of the bottom section of
the vessel, ts = 12 mm
Bending stresses in skirt,
bs
4 Ms
[ ( Ds + ts )ts Ds ]
Where,
Ms
= maximum bending moment (at the base of the skirt)
ts
= skirt thickness
Ds
= inside diameter of the skirt base
= 3.0 m
Therefore,
bs
4(1710 .08 )(1000 )(1000 )
[ ( 6500 +12 ) (12 )( 6500 )]
= 4.287 N/mm2
Dead weight stress in the skirt,
ws
2W
[ ( D s + t s ) t s ]
Where,
158
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
= Total weight of the vessel and content
= 1791.113 kN
Therefore,
(test)
ws
2 1791 .113 1000
[( 6.5 + 0.012 ) (0.012 )]
= 14591753.05 N/m2
= 14.592 N/mm2
bs,
(operating)
1791 .113 1000
[( 6.5 + 0.012 ) (0.012 )]
= 7295876.525 N/m2
= 7.2958 N/mm2
Thus, the resulting stress in the skirt,
Maximum s (compressive) =
ws
(test) +
bs
= 14.592 + 4.287
= 18.879 N/mm2
Maximum s (tensile)
bs
ws
(operating)
= 4.287 - 7.2958
= 0.0323 N/mm2
10.
General consideration for design
Take the joint factor J as 0.85,
159
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
s (tensile)
< fs J sin s
s (compressive)
< 0.125 E
ts
sin s
Ds
Where ,
fs
= maximum allowable design stress for the skirt material
= 135 N/mm2
= weld joint factor
= base angle of a conical skirt
= modulus young = 200, 000 N/mm2
Therefore,
s (tensile)
< 135 x 0.85 sin 80
0.1892 N/mm2
< 113.007 N/mm2
s (compressive)
< (0.125)(200,000)
0.4014 N/mm2
< .1214.473 N/mm2
148
sin 80
3000
Both criteria are satisfied, add 2 mm for corrosion, give design thickness of 150 mm
11.
Base Rings and Anchor Bolts
Assume pitch circle diameter
= 5.0 m
Circumference of bolt circle
= 5000
Bolt stress design, fb
= 125 N/ mm2
Recommended spacing between bolts
= 600 mm
160
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Minimum number bolt required, Nb
5000
600
= 26.18
Closest multiple of 4
= 28
Bending moment at base skirt, Ms
= 212.101 kNm
Total weight of vessel, W
= 236.7534 kN
Area of bolt,
Ab
4M s
W
N b f b Db
1
4( 212 .101 )(1000 )
(184 .046 )(1000 )
28 (125 )
3. 0
E.1.19
= 28.22 mm2
bolt root diameter,
28 .22 4
= 6 mm
Total compressive load on the base ring per unit length,
Fb
4M s
Ds
Ds
4 (212 .101 ) 1000
236 .7534 1000
+
=
2
(3)
(3.0)
= 55.12 kN/m
Assuming that a pressure of 4 N/mm2 is one of the concrete foundation pad, fc
Minimum width of the base ring,
Lb
Fb
1
3
fc
10
161
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
55 .12 10 3
4 10 3
= 13.78 mm
12.
Feed Nozzle Sizing
Optimum duct diameter,
dopt,t
= 293G0.53
-0.37
Where,
G
= flow rate
= 1.32121 x 105kg/hr
= 36.7 kg/s
= density
= 30.698 kg/ m3
Therefore,
Dopt
= 293 (36.7)0.53 (30.698)-0.37
= 500 mm
Nozzle thickness,
Ps d opt
20 + Ps
Where,
Ps
= Operating pressure
= 2.74945 N/mm2
= Design stress at working temperature
= 30 N/mm2
Therefore
162
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
( 2.74945 )(118 )
20 (30 ) + 2.74945
= 0.2 mm
So, thickness of nozzle
= corrosion allowance + 0.2 mm
= 4 + 0.2 mm
= 4.2 mm
5 mm
13.
Top Product Nozzle Sizing
Optimum duct diameter,
dopt,t
= 260G0.52
-0.37
Where,
G
= flowrate
= 223938.68 kg/hr
= 62.205 kg/s
= density
= 56.69 kg/m3
Therefore,
dopt
= 226(62.205)0.50 (56.69)-0.35
= 500 mm
Nozzle thickness,
Ps d opt
20 + Ps
163
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Where
Ps
= Operating pressure
= 2.74945 N/mm2
= Design stress at working temperature
= 30 N/mm2
Therefore
( 2.74945 )(1000 )
20 (30 ) + 2.74945
= 2 mm
So, thickness of nozzle
= corrosion allowance +2 mm
=4 +2
= 6 mm
6 mm
Table 1.4 : Summary of the Mechanical Design
Design Pressure
Reactor Operating Pressure
2.89 bar
Reactor Operating Temperature
160
Reactor Design Pressure
3.179 bar
164
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Reactor Design Temperature
200
Regenerator Operating Pressure
2.6 bar
Regenerator Operating Temperature
150
Regenerator Design Pressure
2.86 bar
Regenerator Design Temperature
180
Safety Factor
0.10
Design of Domed Ends
Types
Torispherical head
Crown Radius
Knuckle Radius
Joint Factor
Stress Concentration Factor
Minimum Thickness
Corrosion Allowance
Column Weight
Dead Weight of Vessel
Weight of Bed
Weight of cyclone
Weight of Insulation
Total Weight reactor and regenerator
Wind Pressure
Loading
Bending Moment
6.5
0.039
1
1.77
14
4
389
736.34
30.66
31.366
1791.11
1280
8550.4
1385.1648
m
m
mm
mm
kN
kN
kN
kN
kN
N/m2
N/m
kNm
REFERENCES
Cheremisinoff, Nicholas P., 1984, Hydrodynamics of Gas-Solids Fluidization, 279 - 231
Amitin et al. 1985, The Hydrodynamic of Fluidization, Powder Technology,(42):
67-78
Geldart, D ,. et al., 1979, Transition Institute Chemical Engineers, 57-269.
Dolignier J. C, Marty E., Martin G. & Delfosse L., 1998, Modelling of gaseous pollutants
emission in circulating fluidized bed of municipal refuse. Elsevier Science
Ltd(77): 1399-1409
Gregory et al, 1985, The design of distributor for gas-fluidized bed, Powder
Technology. (42): 100-145
165
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Horio et al. 1980, The Hydrodynamic of Fluidization, Powder Technology,(42):
67-78
Sinclair, J.L. and R. Jackson, Gas-Particle Flow in a Vertical Pipe with Particle-Particle
Interactions, AIChE J., 35, 1473-1486 (1989)
Sinclair, J.L., Hydrodynamic Modeling, in Circulating Fluidized Beds, ed. Grace,
J.R., Avidan, A.A. and T. M. Knowlton, Chapman and Hall, Great Britain (1997)
Wang, Z., D. Bai and Y. Jin, "Hydrodynamics of Concurrent Downflow Circulating
Fluidized Bed (CDCFB)", Powder Technology, 70, 271-275 (1992)
Wei, F., Wang, Z., Jin, Y., Yu, Z. and W. Chen, Dispersion of Lateral and Axial Solids
in a Cocurrent Downflow Circulating Fluidized Bed, Powder Technology, 81, 2530 (1994)
Himmelblau M. D. 1996, Basic Principles and calculation in Chemical Engineering.
Sixth edition. United States of America: Prentice Hall International, Inc.
Levinspiel O. 1999. Chemical reaction engineering. Third edition. United States of
America: John Wiley & Sons.
Rhodes, M. 1998. Introduction to particle technology. Chichester England. John Wiley
& Sons 97-130.
Sinnot, R.K. 1999.Chemical engineering volume 6. Third edition. Great Britain:
Butterworth-Heinemann.
Zhang H, Hydrodynamics of a Gas-Solids Downflow Fluidized Bed Reactor, Ph.D.
thesis, The University of Western Ontario (1999)
Zhang, H., Zhu, J-X., Hydrodynamics in Downflow Fluidized Beds (2): Particle Velocity
and Solids Flux Profiles, Chemical Engineering Science, 55, 4367-4377 (2000)
Matsen, J. M. "Some Characteristics of Large Solids Circulation Systems". In
Fluidization Technology, Keairns, D. L., Ed.; Hemisphere: New York, Vol. 2,
Chapter1 (1976)
166
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Ouyang, S. Potter, S.E., Consistency of Circulating Fluidized Bed Experimental Data,
Ind. Eng. Chem. Res., 32, 1041-1045 (1993)
Wei, F., R. Xing, Z. Rujin, L. Guohua, J. Yong, A Dispersion Model for Fluid Catalytic
Cracking Riser and Downer Reactors, Ind. Eng. Chem. Res., 36, 5049-5053
(1997)
Yang Y.L., Y. Jin, Z.Q. Yu, Wang, Z.W., Investigation on Slip Velocity Distribution in
the Riser of Dilute Circulating Fluidized Bed, Powder Tech., 73, 67-73 (1992)
http://thor.tech.chemie.tu_muenchen.de/~tc2/eng/teaching/industr_chem_process/crac
king%20lecture%201.pdf
http://www.refiningonline.com/EngelhardKB/npra/NPR8851.htm
http://iglesia.cchem.berkeley.edu/ChemicalCommunications_1764_2003.pdf
http://www.caer.uky.edu/energeia/PDF/vol10-3.pdf
http://www.netl.doe.gov/publications/proceedings/96/96ps/ps_pdf/96ps3_2.pdf
http://tetra.mech.ubc.ca/CFD03/papers/paper29AF3.pdf
http://www.netl.doe.gov/products/r&d/annual_reports/2001/stpt/cfb%20operating
%20regimes%20cork.pdf
http://www.flotu.org/~weifei/twophase-ces.pdf
http://www.gtchouston.com/articles/GTC%20online%20reprint%2011-99.pdf
167
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
SECTION 2
MULTITUBULAR FIXED BED REACTOR
2.1
CHEMICAL DESIGN
2.1.1 INTRODUCTION
168
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Fixed bed reactors are the most important type of the reactor for the synthesis of large
scale basic chemicals and intermediates. In these reactors, the reaction takes place in
the form a heterogeneous catalyst. In addition to the synthesis of valuable chemicals,
fixed bed reactors have been increasingly used in recent years to treat harmful and
toxic substances. The most common arrangement is the multi tubular fixed bed reactor,
in which the catalyst is arranged in the tubes, and the heat carrier circulates externally
around the tubes. Fixed bed reactor for industrial synthesis are generally operated in a
stationary mode under constant operating conditions over prolonged production runs,
and design therefore concentrates on achieving an optimum stationary operation.
However, the non stationary dynamic operation mode is also great importance for
industrial operation control.
CATALYST FORM FOR FIXED BED REACTOR
The heart of fixed bed reactor and the site of the chemical reaction is the catalyst. The
processes taking place on the catalyst may formally be subdivided into the following
separate steps:
1. Mass transfer of reactants from the main body of the fluid to the gross exterior
surface of the catalyst particle.
2. Molecular diffusion /Knudsen flow of reactants from the exterior surface of the
catalyst particle into the interior pore structure.
3. Chemisorption of at least of the reactants on the catalyst surface.
4. Reaction of the surface
5. Desorption of absorbed species from the surface of the catalyst.
6. Transfer of products from the interior catalyst pores to the gross exterior
surface of the catalyst by ordinary molecular diffusion/Knudsen flow.
7. Mass transfer of products from the exterior surface3 of the particle into the bulk
of the fluid.
169
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
For industrial use, a particle size is a compromise between the speed of the exchange
reaction (which is greater with small beds) and high flow rates (which require coarse
particles to minimize the head loss). Standard resins contain particle with diameter
from 0.3 to 1.2 mm, but coarser or finer grades are available. For the MTBE production
process, the fine sulphonic ion exchange resin particles with its size less than 1.0 mm
have to be enveloped in various conceivable shapes
The catalyst properties are as below:
Shape of Catalyst
Spheres
Diameter of catalyst (dc)
0.6 mm (ref: Jon J.Ketta)
Effective Diameter surface dp
0.5mm
Bulk Density of Catalyst (b)
810 kg/m3
Specific Solid Sphere surface
34.25 m2/g
(ref:PerrysHandbook,pg 16.10)
2.1.2
Voidage (b)
0.32 (ref:Tech Info.Buletin)
Surface Area (Sa)
45 m2/g
Specific Surface
0.034 m2/g
Specific Gravity
in range 1 to 1.4
Internal Void Fraction (p)
0.54
Molecular Weight
98 g/mole
PARTICLES SOLID DENSITY
Particle solid density (p) can be obtained from the equation below (ref: Particle
Technologys book):
b
1-b
810
1 - 0.32
1191.2
kg/m3
170
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
2.1.3
VOID VOLUME OF CATALYST
Void volume of catalyst, Vg can be determined as below:
Vg
2.1.5
p
p
0.32
1191.2
0.268 cm3/g
(2.1)
PORE RADIUS OF CATALYST
Pore radius of particle, r is the determined,
r
2.1.6
2. Vg
Sa
2 * 0.268
45 x 104
1.194 x10-5 m
(2.2)
KNUDSEN DIFFUSIVITY
Knudsen Diffusivity is given by the equation below:
Dk
where M
T
Dk
9.7 x103 r (T)1/2
(M)
molecular weight of the catalyst
98 g/mole
operating temperature
393 K
9.7 * 103 * 1.194 *10-5 *( 393)1/2
(2.3)
171
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
98
=
0.013 cm2/ s
The effectiveness factor is given in term of Thiele Modulus as:
2.1.7
3 ( - 1)
2 tan h
(ref: Jon J.Mc Ketta)
(2.4)
0.928 where = 1.1 (ref: Perry,s Handbook)
REACTION RATE
The synthesis of MTBE from methanol and isobutylene catalyzed by Amberlyst-15
or similar sulphonic ion exchange resin catalyst is a reversible etherification as shown
in equation 1:
iC4H8 (isobutene) + CH3OH (methanol)
C5H12O (MTBE)
k1
CH3C(CH3)=CH2(B) + CH3OH
CH3C(CH3)2OCH3 (M)
K2
Reaction kinetics:
According to Yang et al, the forward reaction of reaction above is first order with
respect to the isobutylene concentration and zero order with respect to the methanol
concentration, respectively, and the reverse reaction is first order with respect to the
MTBE concentration as shown below:
Table 1:
Arhenius parameters of rate constant K1 and K2 for MTBE
synthesis catalyzed by Sulphonic ion exchange acidic resin catalyst. (Ref: Chem. Eng.
Journal)
172
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
A1
A2
E1(J/mole)
E2(J/mole)
6.50E+05
1.36E+08
4.74E+04
7.04E+04
-rB = k1CB k2CM
where: k1 = A1 exp (-E1/RT) & k2 = A2 exp (-E2/RT)
k1 = A1 exp (-E1/RT)
(2.5)
= 6.5 * 105 exp (-4.74*104/ 8.314 * 326 K)
= 0.0165 / min
k2 = A2 exp (-E2/RT)
(2.6)
= 1.36 * 108 exp (-7.04 * 104 / 8.314 * 326 K)
= 0.00071/ min
The main side reactions are the dimerization of isobutylene to diisobutylene, and the
hydration of isobutylene to tert-butyl-alcohol (TBA) as shown below:
2CH3C(CH3)=CH2
CH3C(CH3)=CH2 + H2O
CH2=C(CH3)CH2C(CH3)2CH3 (DIB)
CH3C(CH3)2OH (TBA)
(3)
(4)
The kinetic study shows that reaction (3) can only take place when the addition of
methanol is unsufficient. Since the methanol addition is carefully arranged to allow the
molar ratio of methanol to isobutylene to be higher than 0.8, the reaction (3) can be
173
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
neglected. For reaction (4), the minor water in hydrocarbon and methanol feedstock is
consumed in the pre-reactor before it is fed into reactor. Therefore, reaction (4) can
also neglect. The products of the two side reaction are considered in the vapor-liquid
equilibrium calculation, whereas the reaction kinetics is not included in the calculation.
-rB = k1CB k2CM
= k1(CBo CBXB) k2( MCBo CBXB)
CB = CBo since Pi = 2000Kpa and Ti = 326 K
CBo = Pi /RT
= 73.79 mol /m3
M = CMo / CBo = 0.138
Substitute all the value, -rB = 173.8 mole/ m3hr
2.1.8 WEIGHT OF CATALYST
The weight of catalyst can be determined from the equation,
W = dX
F
r
2.7)
Where r = overall reaction rate
W = weight of catalyst needed for the conversion
F = mole flow rate of the feed to the reactor
Substitute all the value and the by integration, the weight of catalyst is found to be
2998 kg.
2.1.9 DETAIL DESIGN OF THE REACTOR
2.1.9.1 Heat Exchanger for Reactor
174
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
For isothermal operation, heat may be supplied or removed continuously along with the
reaction path. In order to accomplish effective heat transfer with the packed bed, the
width of the bed must be small. In other words, isothermal reactors usually consist of a
number tube arranged as in large heat exchangers with the catalyst inside the tubes
and the cooling or heating medium outside the tubes.
2.1.9.2 Direction of the Reactant Flow
For the fixed bed reactor to be designed so that reactants remain isothermal, the rate
of heat required for the exothermic reaction must be exactly balance the heat transfer
from the heating medium. For any isothermal reaction of positive order, the reaction
rate falls as the reaction approaches equilibrium. Therefore a more rapid heating is
need at the reactant entrance than the reactant exit. Therefore the reactors have to be
designed as co-current to match the requirement and heat transfer.
2.1.9.3 Volume of Catalyst Bed
Volume of catalyst bed (Vb)
= W / b
= 2998
810
= 3.70 m3
(2.8)
2.1.9.4 Pressure Drop in the Bed
Pressure drop is an important variable in the rate equations. The maximum allowable
pressure drop criteria below have to be concerned.
1. The resulting force must not be exceeding the crushing strength of the
particle. For the down flow bed this force created by the pressure drop is
transmitted by contacting solid to the bottom of the bed.
2. Mass velocity through the bed must be high enough to minimize interphase
gradients and assure good distribution. Incremental increases in pressure
175
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
drop however should not exceed savings release from improved reactor
performance. In many bed systems the maximum economical pressure drop
is in the range of 3 to 5% of the total pressure.
2.1.9.5 Estimating Pressure Drop
(P)
L
Where
u2 f
dp
(2.9)
= friction factor, the correlation of Ergun (1952) will be used
= [1.75 + 150 / (1-b) / Re] (1-b)/ b3
Re
= dp u f = dp G
f
f
(2.10)
Where G is mass velocity = m/ A since m= 12.12 kg/s and G = 1.968 kg/m2s
f
= ( 0.28 E-6 + 1.001 E-3 +7.86 E-6)/3
= 3.36 E-4 kg/ms
Substitute the value, so Re = 2.928 (transition region) and = 73.75.
Since f = 796.57 kg/m3 and u= 0.0178x4/ x2.82 =0.003 m/s,
(P) will be 1060 N/m2
2.1.9.6 Height of Bed
From the criteria shown above, the optimum value of pressure drop is between 3 to
15% of the total pressure. Let the height of bed equals to 4 m, the pressure drop in the
bed is 4240 N/m2 which is equal to 4.2% of the operating pressure. Therefore, the
height of bed is taken as 4m
2.1.9.7 Total cross Section Area of the Tube
Total cross section area of the tube can be obtained by dividing the volume of the bed
with the height of the bed.
At
3.70
176
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
0.925 m2
(2.11)
2.1.9.8 Tube Diameter
If the tube diameter to particle diameter ratio is less than 10, the effect of wall can
become predominate, the void fraction and thus fluid velocity near the wall become
more dominant. However, if high value of tube to particle diameter ratio is obtained, the
heating effects through the tube wall will not be efficient. Therefore, tube with the inside
diameter Di =0.1143m and thickness 0.005m is used in this reactor, where the ratio is
approximately 11.5.
The inside diameter of the tube (Di) =
=
0.1143 2 x 0.005
0.1043 m
The cross section of the tube is then obtained from the equation:
At
Di 2 /4
0.009 m2
(2.12)
2.1.9.9 Total Number of Tubes
Total number of tube can then obtained by dividing the total cross section area of one
tube.
Nt
0.925
0.009
103 tubes
(2.13)
2.1.9.10 Tube Arrangement
The tubes in an exchanger are usually arranged in an equilateral triangular, square or
rotated square pattern. Since the triangular pattern gives higher heat transfer rates, it is
recommended for this reactor.
177
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
2.9.1.11 Pitch of the Tube
From reference (Process Heat Transfers book), the pitch of the tube is recommended
to be 1.25 times of the outside tube diameter Do.
Therefore,
Pitch of the tube (Pt)
1.25Do
1.25 x 0.1143
0.143 m
(2.14)
2.1.9.12 Bundle Diameter
The bundle diameter depends not only on the number of tubes but also can also on the
number of tube passes. For a single pass heat exchanger type reactor, the bundle
diameter can be obtained from the empirical equation base on standard tube layout as
shown:
Bundle diameter, Db =
Do ( Nt)n-1
K1
(2.15)
Where n1 and K1 = constant for use in the equation above given in reference (Process
Heat Transfers book). For single pass, n1 and K1 is given as 2.142 and 0.319
respectively. Hence,
Db =
=
0.1143 x (103 / 0.319) (1/2.142)
1.70 m
2.1.9.13 Number of Tubes In the Centre Row
The number of tubes in the centre row is then given by equation:
Nc =
=
Db / Pt
1.70/ 0.143
178
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
11.8 or 12 tubes
(2.16)
2.1.9.14 Shell Diameter
By using split ring floating head type from Fig 12.6 in Reference (Chem. Eng. Vol.6s
book),
Ds Db
95.14 mm
Where Ds
Shell Diameter
Ds
1.7 + 0.09
1.79 m
2.1.10 Baffles
Baffles are used in the shell to direct the fluid stream across the tubes, increase the
fluid viscosity and create turbulence so as to improve the rate of heat transfer. The
baffles used in this reactor are a common type i.e the single segmented baffles with
baffles cut 35 %.
2.1.11 Baffles Spacing
The optimum baffles spacing will usually be between 0.3 to 0.5 times of the shell
diameter. Here, it is taken as 0.3 times of the shell diameter.
Bs
0.3 Ds
0.3 x 1.79
0.55 m say 0.6 m
(2.17)
2.1.12 Number of Crosses
Number of crosses in the shell side,
179
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Nc
=
=
L -1
Bs
4
(2.18)
(Pt Do) * Ds * Bs
Pt
(2.19)
Area for Cross Flow
As
=
=
(0.143 - 0.114) * 1.79 * 0.6
0.143
2
0.218 m
2.1.14 Shell side heat transfer and pressure drop calculation
The flow pattern in the shell of a segmental baffled heat exchanger type of reactor is
complex, and this makes the prediction of the shell side heat transfer coefficient and
pressure drop much more difficult than for the tube side. However, Kern has developed
a method base on experimental work on commercial exchangers with standard
tolerances and gives a reasonably satisfactory prediction of the heat transfer coefficient
and pressure drop for standard design.
2.1.15 Shell side Mass Velocity
Mass velocity, Gs
Gs =
Ws
As
G =
2.211
0.218
G s =10.14 kg / m 2 s
(2.20)
Shell side velocity
180
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Gs
us =
us =
10.14
1209
u s = 0.01 m / s
(2.21)
Shell side equivalent diameter for triangular pitch arrangement
= 4(Pt/2).0.87.Pt .Do2/8
. Do/2
Das
= 0.084 m
(2.22)
Reynolds number
Re =
Re =
G s d as
(10.14)(0. 084)
0.00034
(2.23)
Re = 2505
Prandtl number
Pr =
Pr =
CP
kf
(3124.5)(0 .00034)
0.086
(2.24)
Pr =12.3
Choose buffle cut of 35%, from figure 12.30 (Coulson & Richardsons Chemical
Engineering), we can obtained
Jf
=1.3 x 10-1
181
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Assumed that the viscosity correction is negligible
hs =
k f jf Re Pr 1 / 3
de
hs =
(0.086)(0. 13)(2505)( 12.3 1/3 )
0.084
h s = 763.2 W / m 2
(2.25)
Shell side pressure drop
Reynolds number
Re = 2505
From figure 12.30 (Coulson & Richardsons Chemical Engineering),
Jf = 1.3 x 10-1
Shell side pressure drop can be calculated using equation below
P =8j f ( D d / d e )( L/I B )( s / 2 )( / w )
P =8.93kPa
-0.14
(2.26)
2.1.16 Tube side coefficient, hi
Mean temperature of the tube
182
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
t mean =
t1 + t 2
2
t mean =
53 + 27
2
(2.27)
t mean = 80 o C
cross sectional area = d12/ 4
= (3.142)(0.10432) / 4
= 0.009 m2
Tube / pass = 103 / 1
= 103 tube / pas
(2.28)
Total flow rate area = (cross sectional area)(tube / pass)
= (0.009)(103)
= 0.927 m2
velocity, Gt
= (2.211)/(0.927)
= 2.05 kg / sm2
= 0.002 kg / ms
Ratio of L / di = 4 / 0.104
(2.29)
= 38.46
Reynolds number, Re
Re =
d i
Re =
(1209) (0.002 ) (0.1043)
0.00034
Re =1000
183
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Heat transfer factor figure 12.31 (Coulson & Richardsons Chemical Engineering),
Jh = 2 x 10-2
Prandtl number, as 12.3
Pr =12.3
Tube side coefficient, hi can be calculated using equation below.
hi =
jh k f Re Pr 0.33
di
hi =
(2x 10 -1 )(0.086)(1 000)(12.3)
0.104
(2.30)
0.33
h i = 393 W / m o C
Tube side pressure drop
Reynolds number, Re = 1000
From figure 12.24 (Coulson & Richardsons Chemical Engineering),
jf = 2 x 10-1
Neglect the viscosity correction term
Pt = N p 8 jf ( L/d i )( / W ) - m + 2.5
Pt = 2 8(5 x 10 -3 )(4/0.104) + 2.5
] 2
i
] (1209 )( 02.00034 )
(2.31)
Pt =10.89 kPa
2.1.17 Correction for Tube Heat Transfer Coefficient
184
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Since the tube side heat transfer coefficient calculated is based on the inside diameter
of the tube, correction has to be done to obtain the heat transfer coefficient for outside
diameter of tube.
HO
=
=
hI . Di
Do
192 W/ moC
(2.32)
2.1.18 Overall Heat Transfer Coefficient
Uc
HO . hS
HO + hS
100.1 W/ moC
(2.33)
2.1.19 Reactor cooling system
mfCPf (t1 - t2)
mcCpc (t1 - t2)
(2.34)
Log Mean Temperature difference (LMTD) :
TLMTD
Ti To
ln(Ti-To)/(Ti-To)
(2.35)
45 oC
From this value can get mc = 39.63 kg/s where CPc=4220 and ti-t2 =135 oC
2.2
MECHANICAL DESIGN
2.2.1 INTRODUCTION
The mechanical design of chemical plant are of particular interest to chemical
engineers, but not usually be called on to undertake the detailed mechanical design of
185
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
the plant, especially the reactor vessels. However, the chemical engineer will be
responsible for developing and specifying the elementary design information for the
reactor, and need to have a general appreciation of pressure vessel design to work
effectively with the specialist designer.
Therefore, the design of wall thickness, head, column support, flange joint,
reinforcement and maximum allowable pressure are considered here.
Material of construction:
In this design, reference will mainly be based on the current British Standard BS 5500
and Bs 1515 where the current addition of Bs 5500 covers vessels fabricated in carbon
steel. The most common types used in the petroleum industry are Types 304, 316,321,
and 347.Because of their inherent high temperature strength propertied and high
corrosion resistance, they are particularly suitable for use in this process, in areas of
moderate and high temperature, and where substantial resistance such as in heater
tubes, reactors, reactor effluent exchangers and piping. In this design, material of
construction: can be constructed by using carbon steel. Type 304
DESIGN STRESS
It is necessary to decide a value for the maximum allowable stress that can be
accepted in the material of construction, for example, it can withstand without failure
under standard test conditions. The nominal design strengths (allowable design stress)
for the range of materials covered are listed in BS 5500. By using carbon steel (semi
killed or silicon Killed), the design stress is given, D as 125 N /mm2 at design
temperature.
WELDED JOINT EFFICIENCY
The strength of a welded joint will be depending on the type of joint and the quality of
the welding. The soundness of welds is checked by visual inspection and non
destructive testing (radiography).
For the reactor, it is assumed that the joint is equally as strong as the virgin plate; this
is achieved by radio graphing the complete weld length and cutting out and remarking
186
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
any defects. Therefore, the non destructive testing is assumed to be 100% and the
joint efficiency, J is taken as 1.0.
2.2.4 CORROSION ALLOWANCE
1. Corrosion and erosion or scaling will cause material lost, so an additional thickness
of material called corrosion allowance must be added to be calculated wall
thickness.
MINIMUM THICKNESS OF CYLINDRICAL SECTION OF SHELL
The minimum thickness of cylindrical section of shell to resist the internal pressure can
be determined by using equation below:
e
PD DIs
2J D - PD
2.0*1.8
2.125-2
0.015 m
(2.36)
By adding corrosion allowance 2 mm,
e
0.015 + 0.002m
0.017 m
MINIMUM THICKNESS OF DOMED HEAD
There are three types of commonly used domed head:
1. Hemispherical heads
2. Ellipsoidal heads
3. Torispherical heads
187
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The selection of head depends on the cost and the thickness required for the head.
The design equations and charts for the various types of domed head are given in the
codes and standards (BS 5500) used in this design.
A standard dished head (torisphere) is used as first trial. The crown radius of this head
equals to the diameter of the shell, DIS. On the other hand, the knuckle radius is taken
as 6% of the crown radius. Since this type of head is formed by pressing, no joint is
needed. Therefore, the joint factor is taken as 1.
For the torispherical head, the minimum thickness of the head can be determined from
equation below:
eh
P D RC CS
2 J TD + PD(CS 0.2)
(2.37)
where Rc is the crown radius, equal to DIS in this case.
CS = stress concentration factor for torispheriical head.
It is given by equation below:
CS
1 / 4(3 + (Rc + Rk) 1/2)
(2.38)
Since Rk is 0.06 of Rc,
CS
1 / 4 (3 + (1/ 0.06)1/2)
1.771
2.0 x1.8 x1.771
2 x 1x125 + 2.0 (1.771 - 0.2)
0.025 m
Therefore,
eh
However, for a standard ellipsoidal head,
eh
PDDIs
2J D - 0.2PD
0.014 m
(2.39)
Therefore, an ellipsoidal head would probably be the most economical to be used. For
convenience, the thickness is taken to be as same of wall thickness 17 mm.
188
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
LOADING STRESSES
2.2.7.1 Dead Weight of Loading
2.2.7.2 Dead Weight Of vessel
The major source of dead weight loads are:
1. vessel shell
2. vessel fitting : manway, nozzles
3. internal fittings: where the main item is tube
4. insulation
5. catalyst
The preliminary calculation of the approximate weight of a cylindrical vessel with
domed ends, and uniform wall thickness can be estimated from the following equation,
Wv
Cv..m.Dm.g.(Hv + 0.8Dm)t x 10-3
Where Wv
total weight of the shell, excluding internal fitting
Cv
a factor to account for the weight of nozzles, internal
support etc.
Cv is given as 1.08 for vessels with only a few internal
fitting.
Hv
height between 2 tangent lines, 4m in this case
gravitational acceleration, 9.81 m/s2
wall thickness, mm
density of vessel material, kg/m3. by using carbon steel,
m = 6870 kg/m3
Dm
mean diameter of vessel
DIs + t * 10-4
Since the wall thickness = 0.017 m
Therefore, Dm = 1.8 + 0.014
= 1.817 m
As a result, by substituting into the equation above:
Wv
39000 N
39 KN
189
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Weight of the Tubes
From BS 3059, the mass per length of tubes is equal to 13.5 kg/ m for carbon steel.
Therefore, the weight of one tube
=
13.5 x 3 x g
4.529 * 104 N
45 KN
(2.40)
Total weight of the tubes,
Wt
103 x 13.5 x3 x9.81
4.1x104
Weight of Insulation
To avoid heat loss from the surface of the shell, mineral wool is used as the insulator.
From (Chem. Eng. Vol. 6s book),
Density of mineral wool
= 130 kg/m3
Let the thickness of mineral wool
= 75 mm
The approximate weight of the insulator,
Wi
2Hvtiig
2 x3 x0.075 x30 x9.81
1.803 x103 N
(2.41)
This value should be double to allow for fittings, etc.
=
3.6 x103 N
190
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Weight of Catalyst
Weight of catalyst, Wc = 2998 kg
= 2.998 x104 N
Total Weight
Since only 4 baffles are used in the reactor, their weight is neglected compared to
others.
W
Wv + Wi + Wt +Wc
39000N+ 4.1x104 N+ 3.6x103 N +2.998x104 N
1.1x 105 N
Wind Loading
Take dynamic wind pressure as 1300 N/m2
Mean Diameter, including insulation = 2+2(17+75) x10-3
= 2.18 m
The wind loading is then given by equation below:
Fw
P w Dm
1300 x2.18 m
2.8 x 103 N/m
(2.42)
Therefore, the bending moment at bottom tangent line can be determined from
equation below:
Mx
Fw L2
191
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
(2.43)
2.8 x 103 x 42
2
2.24 x104 N
2.2.7.8 ANALYSIS OF STRESSES
Uniform thickness is used for analyzing stresses in the column. If it is found satisfactory
at the bottom of the vessel which is the highest loading point, then the entire column
structure is feasible.
At bottom tangent line, the longitudal and circumferential stresses due to pressure is
given by:
Longitudal,
Circumrerential, h
P i Di
4t
2.0 x1.8
4 x 0.017
53 N /mm2
P i Di
4t
2.0 x1.8
2 x 0.017
106 N /mm2
(2.44)
Dead Weight Stress
The direct stress is mainly due to the weight of vessel, its contents and any attachment
which is often called the dead weight stress. The stress is compressive since it is at the
bottom of the vessel to support the direct loading.
192
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Dead Weight stress, W =
=
=
W
(Di + ti) * t
1.1 x 105
(1800 + 17) x 17
(2.45)
1.13 N /mm2 (compressive)
Bending Stress
The second moment of area of the vessel about the plane of bending,
Iv
Where Do
Iv
(Do4 Di4)
64
outer diameter of vessel
Di + 2t
1.8 + 2 x 0.017
1.834 m
(18344 18004)
64
4.0 x1010 mm4
(2.46)
Therefore, the bending stress is then given by equation below:
b
=
=
Mx (Di /2 + t)
Iv
(2.47)
0.51 N/mm2
The resultant longitudal stress
Z
L + W + b
53 1.13 + 0.51
52.38 N/mm2
For upwind,
For downwind,
193
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
53 1.13 - 0.51
51.36 N/mm2
Pi = 2.0
2
2
Radial Stress
(2.48)
1.0 N/mm2
Since radial stress obtained is a small value and there are torsional stress in the
system, therefore the principle stress will be Z and h
52.38 N/mm2
Figure 2.1:
51.36 N/mm2
Analysis of Stresses
194
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Therefore, the greatest difference between the principles stresses,
d
h - Z ( downward)
106 51.36
54.64 N/mm2
The value obtained is well bellow the maximum allowable design, 125 N/mm2.
2.2.11 CHECK ELASTIC STABILITY
The design of this vessel have to be checked to ensure that the maximum value of the
resultant axial stress does not exceed the critical value at which buckling will occur.
By applying a factor of safety of 12, the critical buckling stress gives:
C
2x104 (t/Do)
185.4 N/mm2
(2.49)
The maximum compressive stress will occur when the vessel is not under pressure,
=
W + b
1.13 + 0.51
1.64 N/mm2
Which is well below the critical buckling stress, C .
2.2.12 VESSEL SUPPORT
The method used to support a vessel will depend on the size, shape and weight of the
vessel; the design temperature and pressure; the vessel location and arrangement;
and the internal and external fittings and attachment. Since the reactor is a vertical
vessel, skirt support is used in this design.
195
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
A skirt support consists of a cylindrical or conical shell welded to the base of the
vessel. A flange at the bottom of the skirt transmits the load to the foundations. The
skirt may be welded to the bottom, level of the vessel.
Skirt supports are recommended for vertical vessels as they do not imposed
concentrated loads on the vessel shells; they are particularly suitable for use with tall
columns subject to wind loading.
2.2.13 SKIRT THICKNESS
The skirt thickness must be sufficient to withstand the dead weight loads and bending
moments imposed on it by the vessel; it will not be under the vessel pressure.
The resultant stresses in the skirt will be:
S (tensile)
bS - WS
S (compressive)
bS + WS
and
where bS
=
=
WS
=
=
bending stress in the skirt
4Ms
(Ds + ts) tsDs
(2.50)
the dead weight stress in the skirt
W
(Ds + ts) ts
Where Ms
maximum bending moment, evaluated at the base of the skirt.
total weight of the vessel and contents
Ds
inside diameter of the skirt, at the base
ts
skirt thickness
196
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The skirt thickness should be such that under the worst combination of wind and dead
weight loading the following design criteria are followed:
S (tensile)
< fs.J sin s
S (compressive)
< 0.125 E (ts/Ds) sin s
Where fs is the maximum allowable design stress for the skirt material, normally taken
at ambient temperature, 20oC.
J
weld joint factor
base angle of conical skirt, 90o is used in this design
The maximum thickness should not less than 6mm.
2.2.14 HEIGHT OF THE SKIRT
The height of the skirt, Hs is taken to be 1m.
2.2.15 BENDING STRESS AT BASE OF THE SKIRT
Mbs
=
=
Fw (Hv + Hs)21
2
(2.51)
2.8x 103 (4 + 1)2
2
3.5 x 104 Nm
BENDING STRESS IN THE SKIRT
As the first trial, the thickness of skirt is taken to be 20 mm.
Substitute into the equation (19),
bS
4 x 2.24 x104
(1.9 + 0.020) 1.9x0.020
3.9 x 105 N/m2
197
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
WS
1.1 x 105
(1.9 +0.020)*0.020
9.1 x 105 N/m2
The maximum stress (compressive),
S
bS + WS
3.9 x 105 N/m2 +9.1x 105 N/m2
1.3 x 106 N/m2
The maximum stress (tensile),
S
bS - WS
3.9 x 105 N/m2 9.1 x 105 N/m2
5.2 x 105 N/m2 (negative)
Let the joint factor for skirt support, J = 0.85
Criteria for design,
fs.J sin s
125 x 0.85 xsin( 90o)
10.63 * 107 N/m2
>
S (tensile) , therefore it satisfied.
(2.52)
Modulus Young = 200,000 N/mm2 at ambient temperature, (from Bs 5500:1998),
0.125 E (ts/Ds) sin s =
0.125x2x105 (0.02/1.9) sin 90o
26.3 x 107 N/m2
>
S (compressive) , therefore it satisfied
Both criteria are satisfied, add 2mm for corrosion allowance, and give a design
thickness of 22 mm.
198
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
BASE RING AND ANCHOR BOLT DESIGN
The loads carried by the skirt are transmitted to the foundation slab by the skirt base
ring (bearing plate). The moment produced by wind and other lateral loads will tend to
overturn the vessel. Since the reactor can be considered as the small vessel, the
simplest types, rolled angle ring are used. The preliminary design of base ring is done
by using Scheimans short cut method.
The anchor bolts are assumed to share the overturning load equally, and the bolt area
required is given by:
=
1 (4.Mbs W)
Nbfb
Db
(2.53)
Where Ab
area of one bolt at the root of the thread, mm2
Nb
number of bolts
fb
maximum allowable bolts stress, typical design = 125 N/mm2
Mbs
bending (overturning) moment at the base, Nm
weight of the vessel, N
Db
bolt circle diameter, m
Scheiman gives the following guide rules which can be used for the selection of the
anchor bolts.
1. bolts smaller than 25 mm diameter should not be used
2. minimum number of bolts = 8
3. use multiples of 4 bolts
4. bolts pitch should not be less than 600 mm
The base ring must be sufficiently wide to distribute the load to the foundation. The
total compressive load on the base ring is given by:
fb
(4 Mbs + W ) = 28255 N/mm
Ds2
Ds
(2.54)
Where Fb is the compressive load on the base ring and Ds = skirt diameter, m
The minimum width of the base ring is given by:
199
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Lb
Where Lb
fc
fb
fc
1
103
(2.55)
= base ring width, mm
= the maximum allowable fc on the concrete foundation pad,
And typically range from 3.5 to 7 N/mm2
fb
= 28255 N/mm
Take the bearing pressure as 5 N/mm2, fc = 5 N/mm2
Substitute into the equation above,
Lb
=
=
28255
5 x103
5.65 mm
This is the minimum width required; actual width will depend on the chair design
Actual width required= Lr + ts +50 mm
Where Lr = the distance from the edge of the skirt to the outer edge of the ring, mm
= 64 mm (from BS 4190:1967)
Therefore, actual width required = 64 + 22 +50
= 136 mm
Actual bearing pressure on concrete foundation:
fc
=
=
tb
28255
136 x103
0.21 N/mm
64 ( 3 x 0.21)
140
(2.56)
200
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.3 mm
COMPENSATION FOR OPENING AND BRANCHES
All process vessels will have opening for connections, man ways, sight holes, hand
holes and instrument fittings. The presence of an opening weakens the shell, and gives
rise to stress concentrations. The stress at edge of a hole will be considerably higher
than the average stress in the surrounding plate. To compensate for the effect of an
opening, the wall thickness is increased in the region adjacent to the opening.
Sufficient reinforcement must be provided to compensate for the weakening effect of
opening without altering the general dilation pattern of the vessel opening.
2.2.19 COMPENSATION FOR OTHER NOZZLES
Pipe size for inlet and outlet of the reactor are all less than 10 mm, therefore, the
reinforcement area can be usually is provided by increasing the wall thickness of the
branch pipe. This already done on piping, where extra thickness is provided, thus no
compensation area needed.
2.2.20 BOLTED FLANGE JOINT
Flanged joints are used to connect pipes and instruments to vessels and from
removable vessel heads when ease of access is required. Flanges may also be used
on the vessel body, when it is necessary to divide the vessel into section for transport
or maintenance. Flanged joints are also used to connect pipes to other equipment,
such as pumps, valves.
2.2.20.1 Type of Flanges Selected
a) Welding neck Flanges
201
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Have a long tapered lub between the flange ring and the welded joint. This gradual
transition of the section reduces discontinuity stresses between flange and branch,
and increases the strength of the flange assembly.
Welding neck flanges are suitable for extreme service conditions, where the flange
is likely to be subjected to temperature, shear and vibration loads.. For this reactor,
the welding neck flanges are suitable for use in connecting the inlet and outlet
piping of reactor.
b) Gasket
Gaskets are used to make a leak tight joint between two surfaces. It is impractical to
machine flanges to the degree of surface finish that would be required to make a
satisfactory seal under pressure without a gasket. Gasket are made from semi plastic
materials, which will deform and flow under load to fill the surface inequalities between
the flange faces, yet retain sufficient elasticity to take up the changes in the flange
arrangement that occur under load.
Several factors must be considered when selecting a gasket material:
1. The process condition: pressure, temperature, corrosive nature of
process fluid.
2. Whether repeated assembly and disassembly of the joint is required
3. The type of flange and flange face.
Judging from process conditions, where the operating temperature is quite high, 393K,
metal reinforced gaskets is recommended, since it have a quite good heat resistance
property.
2.2.21 Flange Face
The raised face, narrow feed flange are used for all the flanges. Where the flange has
a plain face, as for the flange faces mentioned above, the gasket is held in place by
friction between the gasket and flange surface.
202
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
SUMMARY OF CHEMICAL DESIGN PARAMETERS
Reactor Design
Catalyst weight required
2998 kg
Volume of Catalyst bed
3.7 m3
Height of Bed
4.0 m
Diameter of Bed
1.817 m
Tube inside pressure drop
1.06E-4 N/m2
% of pressure drop
0.042
Tube inner diameter
0.1043 m
Total number of tubes
103 tubes
Tube arrangement
equivalent triangular pitch
Tube side heat transfer coefficient
293 W/moC
Pitch of tube
0.143 m
Bundle diameter
1.70 m
203
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Shell diameter
1.79 m
Baffles cuts
0.35
Baffles Spacing
0.6 m
Number of crosses
Inlet flow rate
0.647 kg/s
Inlet temperature
326 K
Outlet temperature
373 K
Shell side heat transfer coefficient
191 W/moC
Shell side pressure drop
0.557 kPa
Design overall heat transfer area
100.1 W/moC
SUMMARY OF MECHANICAL DESIGN PARAMETERS
204
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Operating Conditions and Material of construction
Design pressure
2.0 bar
Design temperature
323 K
Material of construction
Carbon Steel plate (Type 304)
Design Stress
125 N/mm2
Welded joint efficiency
Corrosion allowance
2 mm
Designed Column Diameter
Shell thickness
17 mm
Domed end thickness(ellipsoidal heads)
17 mm
Vessel Support (skirt)
Skirt thickness
22 mm
Skirt diameter
1.9 m
Skirt height
1m
Base Ring and Anchor Bolt
Base ring
Rolled angle rings
Minimum ring thickness
4.5 mm
Minimum base ring width
7.06 mm
Anchor bolt
M24
Number of bolts
Bolt root diameter
21.2 m
205
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
SECTION 3
MTBE DISTILLATION COLUMN
3.1
INTRODUCTION
Distillation is a method used to separate the components of a liquid solution, which
depends upon the distribution of these various components between a vapor
and a liquid phase. All components are present in both phases. The vapor
phase is created from the liquid phase by vaporization at the boiling point.
MTBE is our main product that needs to be separated. For individual design,
MTBE distillation column was chosen. The characteristics required in the chosen types
of distillation column are the separation objective satisfied in the column, the cost of
construction and the design of the selected distillation column.
R e lief Va lve
C on den se r
T=53.3oC
T=6 4.5oC
TC
LC
R e bo iler
T=10 3.3oC
Figure 3.1 MTBE Distillation Column
3.2
SELECTION OF CONSTRUCTION MATERIAL
206
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Materials chosen are based on the characteristics of the component in the distillation
column, the location and the environmental consideration of the MTBE plant. Stainless
steel 304 is used in construction of the MTBE distillation column - The stainless steels
are the most frequently used corrosion resistant materials in the chemical industry
Coulson & Richardson, Chemical Engineering, Volume 6, page 295.
Carbon steel is used in skirt support material and the insulation material used is
fiberglass. The selection is based on the chemical and mechanical design as stated in
Coulson & Richardson, Chemical Engineering, Volume 6. Most parameters used in
design were referred to mass, energy balance data and also data generated by
Chemical Engineering Simulation Software; HYSIS Version 3.2. Other materials
chosen were based on the British Standard BS 5500, BS4505 and BS 750.
3.3
CHEMICAL DESIGN
In the MTBE distillation column design, the McCabe-Thiele method in
determining the number of stages needed was used.
The McCabe-Thiele method is
based upon representation of the material-balance equations as operating lines on the
X-Y diagram. The lines are made straight (and the need for the energy balance
obviated) by the assumption of constant molar overflow. The liquid-phase flow is
assumed to be constant from tray to tray in each section of the column between
addition (feed) and withdrawal (product) points. If the liquid rate is constant, the vapour
rate must also be constant.
Table 3.1 The Composition in Feed Stream
FEED
Component
T (K)
Operating
Op Pressure,
Feed Flowrate
Fraction, zi
207
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
T (K)
kPa
kmol/hr
i-C4H10
313
337.5
450
34.860
0.3261
n-C4H10
313
337.5
450
0.400
0.0037
C4H8
DME
313
313
337.5
337.5
450
450
0.026
3.880
0.0002
0.0363
CH3OH
313
337.5
450
0.160
0.0015
H2O
313
337.5
450
2.866
0.0268
MTBE
TBA
380
380
337.5
337.5
450
450
63.44
1.274
=106.906
0.5934
0.0119
= 1.0000
Table 3.2 The Compositions in Top Stream
Top
Component
T (K)
Operating
T (K)
Op Pressure,
kPa
Top Flowrate
Yi
kmol/hr
S16
(top)
i-C4H10
313
326.3
305
33.860
0.839760919
n-C4H10
313
326.3
305
0.400
0.009920389
C4H8
313
326.3
305
0.026
0.000644825
DME
313
326.3
305
3.880
0.096227772
CH3OH
313
326.3
305
0.149
0.003695345
H2O
313
326.3
305
1.006
0.024949778
MTBE
313
326.3
305
1.000
0.024800972
=40.321
=1.0000
Table 3.3 The Composition in Bottom Stream
Bttm
S14
(bttm)
Component
T (K)
Operating
T (K)
Op Pressure,
kPa
Bttm
Flowrate
kmol/hr
Xi
i-C4H10
380
376.3
400
1.000
0.015018398
MTBE
380
376.3
400
62.44
0.937748742
TBA
380
376.3
400
1.274
0.019133438
CH3OH
380
376.3
400
0.011
0.000165202
H2O
380
376.3
400
1.860
=66.59
0.027934219
=1.0000
* The T(K) is the stream temperature, while the Operating T(K) temperature is the
temperature which should be achieved by controlling the pressures.
208
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.3.1
DETERMINATION OF THE NUMBER OF PLATES USING McCABETHIELE METHOD
The components in the feed to the MTBE distillation column are i-C4H10, n-C4H10, C4H8,
DME, CH3OH, H2O, MTBE and TBA, and the feed is assumed as multicomponents
feed. By using the Hengstebecks and McCabe-Thiele method, the number of stages
required and the position of the feed in the MTBE distillation column can be
determined.
The determination of the plate by using McCabe-Thiele method was simply
because as explained in J.M Coulson, J.F Richardson, Chemical Engineering Volume
2, Third Edition, the Pergamon Textbook, page 429, which stated that This method is
one of the most important concepts in chemical engineering and is an invaluable tool
for the solution of distillation column. The assumptions of constant molar overflow is
not limiting since in very few systems do the molal heats of vaporizations differ by more
than 10 percent. The method does have limitations, however, and should not be
employed when the relative volatility is less than 1.3 or greater than 5, when the reflux
ratio is less than 1.1 times the minimum, or when more than twenty-five theoretical
trays are required. In these circumstances, the Ponchon-Savarit method should be
employed.
The vapor pressure can be determined by using the Antoines equation as follows:
Log10 P* = A -
B
T +C
(3.1)
With related at equilibrium, constant K,
Ki = P* x P
(3.2)
And related with concentration,
209
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Ki =
yi
xi
(3.3)
Where yi = concentration in vapor phase
xi = concentration in liquid phase
Calculation for the relative volatility, ,
= KLK / KHK
(3.4)
Where KLK = Light key component
KHK = Heavy key component
In this case, MTBE is as the heavy key and i-C4H10 is as the light key components. By
using goal seek in the excel programme, the value of the bubble point at bottom
column = 103.3oC and dew point at the top column is = 53.3 oC, from the values of Ki
related to the pressure, the values of relative volatilities could be determined, listed are
values of the relative volatilities for components at the top and bottom of the distillation
column.
Table 3.4 Average Relative Volatility,
Bttm,
Top,
i-C4H10 (LK)
7.699631
n-C4H10
5.654994
2.827496955
C4H8
DME
MTBE (HK)
TBA
6.948741
14.10614
1.00000
-
1.0000
0.149333
3.474370398
7.05306993
1.00000
0.074666444
CH3OH
0.67395
0.99561
0.834780143
H2O
0.152422
0.288322
0.220371796
5.084017
Avg,
Component
6.39182391
Calculations for the non-key flows,
Table 3.5 The Non-key Flow of the Top Stream
210
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
i
2.827497
3.47437
7.05307
0.83478
0.220372
TOP
n-C4H10
C4H8
DME
CH3OH
H2O
di
0.4
0.026
3.88
0.149
1.006
li = di / ( i-1)
0.218878614
0.010507724
0.640997055
-0.901828648
-1.290358653
Vi = li + di
0.618878614
0.036507724
4.520997055
-0.75282865
-0.28435865
= -1.321803909
= 4.139
Table 3.6 The Non-Key Flow of the Bottom Stream
BOTTOM
bi
TBA
CH3OH
H2O
0.074666
0.83478
0.220372
1.27
0.01
1.86
Vi= ibi / (
LK
li = vi + bi
i)
0.01506000
0.001652422
0.066417357
= 0.083127984
1.2900
0.0100
1.9300
= 3.23
Flows of combine key,
Le
= L - li
(3.5)
= (2.5 X 40.321) (- 1.3218)
= 102.1243
Ve
= V - Vi
(3.6)
= (2.5+1) x 40.321 4.139
= 136.985
Calculation of the slope for top operating line,
Le
Ve
102 .1243
136 .984
(3.7)
= 0.7455
Ve
= V - Vi
(3.8)
= (2.5+1) x 40.321 0.08313
= 141.04
Le
= L - li
(3.9)
= (2.5+1) x 40.321 + 66.59 3.23
= 204.48
211
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Calculation of the slope for bottom operating line,
Le '
Ve '
141 .04
204 .48
(3.10)
= 1.45
Xb
flow .LK
flow ( LK + HK )
1
(1 +62 .47 )
at bottom,
(3.11)
at top,
(3.12)
at feed,
(3.13)
= 0.016
Xd
flow .LK
flow ( LK + HK )
Xd
33 .86
(33 .86 +1)
= 0.9713
Xf
Xf
flow .LK
flow ( LK + HK )
34 .86
(34 .86 +63 .44 )
= 0.3546
For vapor liquid equilibrium curve, we use the equation of
.x
[1 + ( 1) x]
6.391 x
[1 + ( 5.391 ) x]
from LK component
(3.14)
Table 3.7 MTBE Equilibrium Curve
y=
x
6.391 x
1 + 5.391 x
y
212
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0
0.41527398
0.61508201
0.73257407
0.80992987
0.86471539
0.9055511
0.93716326
0.96235974
0.9829137
1.0000
So from the data as above, the McCabe-Thiele diagram was constructed to determine
the number of plates. The top operating line and the bottom operating line were
determined first before the number of plates required could be calculated.
And from the graph plotted, the number of stages needed for the MTBE
distillation column is 11 with the feed point location is at stage number six from bottom.
213
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
McCabe-Thiele Diagram
1
0.9
0.8
Top
Operating
Line
0.7
0.6
Equilibrium
Curve
0.5
Line for
Number
Stages
0.4
0.3
Bottom
Operating Line
0.2
0.1
0
0
0.2
0.4
0.6
0.8
Xb = 0.016
Xf = 0.35
Xd = 0.97
At bottom
At feed
At top
214
the
of
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 3.2 McCabe-Thiele Diagram
From the graph plotted, the number of plate can be determined by calculating the stage
plotting at graph and the number of stages needed is 11 stages. The feeding stage
also can be determined from the graph, feed at stage 6 from bottom.
Calculation for minimum reflux ratio, Rmin,
To get the value of minimum reflux ratio, use the Underwood equation was used,
(J. Douglas, 1988),
Rmin
=
1
X D.L K X D.H K
X F .H K X F .H K
(3.15)
1
1.0
33 .86
6.391
6.391 1 63 .44
63 .44
= 0.0803
Optimum reflux ratio is 0.0803 x 1.5 = 0.1204
Where,
XD.LK
= Light key component at top flow
XD.HK
= Heavy key component at top flow
XF.LK
= Light key component at feed flow
XF.HK
= Heavy key component at feed flow
In the calculation, the optimum reflux ratio as 2.5 was used (based on Coulson
& Richardson, Chemical Engineering, Volume 6, and J.M Coulson, J.F Richardson,
Chemical Engineering, Volume Two, Third Edition) as 0.1204 is too low for the
calculation, based on statement from R. K. Sinnot, Coulson & Richardson, Chemical
Engineering, Volume 6, Butterworth Heinemann, 2001, page 495 At low reflux
ratios the calculated number of stages will be very dependent on the accuracy of the
vapor-liquid equilibrium data available. If the data are suspect a higher than normal
ratio should be selected to give more confidence in the design.
215
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.3.2
DETERMINATION OF THE NUMBER OF PLATES USING FENSKES
EQUATION
The Fenskes equation (1932) can be used to estimate the minimum stages required at
total reflux. The derivation of the equation is for binary system and applies equally to
multicomponents system. But in the design only the calculation of plates using
McCabe-Thiele method as the design plate number was taken into consideration.
Nmin
Nmin
x x
log LK HK
x HK d x LK b
log LK
33 .86 62 .44
log
1.000 1.000
log 6.392
4.13
5 stages
(3.16)
Normally after using the Fenskes Equation, the value of Nmin is given by the equation
below to get the number of stages, NT,
NT
2 (Nmin)
2 (5)
10 stages
To get the real number of stages, the efficiency of the process must be considered,
and the efficiency is calculated based on the equation by OConnells (J. Douglas,
1988),
Eo
0.5
( ) 0.25
0.5
( 0.224 x 6.392 ) 0.25
0.457
(3.17)
216
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.3.3
NT
Eo
=
11
0.457
24.07
24 plates
THE PHYSICAL PROPERTIES
The properties consider in this design are liquid flow rate, LW, vapor flow rate, VW, liquid
surface tension, , liquid density, l and vapor density, v. This physical properties
evaluated at the system temperature by using HYSIS generated data or by manual
calculations the from mass and energy balance data. The useful properties data are
from HYSIS, mass and energy balance data is given as below:
Liquid flow rate, LW
38747.97 kg/hr (10.7633 kg/s)
Vapor flow rate, VW
15251.95 kg/hr (4.2367 kg/s)
Liquid surface tension,
0.0351 N/m
Liquid density, l
746.74 kg/m3
Vapor density, v
3.8402 kg/m3
Data evaluated are at system temperatures and pressures.
3.3.4
DETERMINATION OF COLUMN DIAMETER
The principal factor that determines the columns diameter is the vapour flow-rate. The
vapour velocity must be below that which would cause excessive liquid entrainment or
a high-pressure drop. The equation below which is based on the well-known Souders
and Brown equation, Lowenstein (1961), Coulson & Richardson, Chemical
Engineering, Volume 6, page 556, can be used to estimate the maximum allowable
superficial vapour velocity, and hence the column area and diameter,
217
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
( L v)
u = ( 0.171 l2t + 0.27 lt 0.047 )
1/ 2
(3.18)
2
( 746 .74 3.8402
=
0.171 ( 0.9 ) +0.27 ( 0.9) 0.047
3.8402
1/ 2
= 0.7997 m/s
Where,
= maximum allowable vapour velocity, based on the gross (total)
column cross-sectional area, m/s,
= plate spacing, m (range 0.5 1.5).
Based on the equation below, the column diameter could be determined,
Dc
Dc
Dc
4 vw
vu v
(3.19)
4(0.6244 )
(3.8402 )( 0.7997 )
0.51 m
0.51 x 1.5
0.765 m
For safety reason, the approximate diameter was increased 50% more than the
calculated value, as it deals with vapour, which is in high pressure.
218
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.3.5
DETERMINATION OF PLATE SPACING
The overall height of the column will depend on the plate spacing. Plate spacings from
0.15 m (6 in.) to 1m (36 in.) are normally used. The spacing chosen will depend on the
column diameter and the operating conditions. Close spacing is used with smalldiameter columns, and where head room is restricted, as it will be when a column is
installed in a building. In the MTBE distillation column, the plate spacing was assumed
0.9 m as it is in the range of 0.5 m to 1 m recommended by Coulson and Richardson,
Chemical Engineering, Volume 6.
3.3.6
LIQUID FLOW ARRANGEMENTS
Before deciding liquid flow arrangement, maximum volumetric liquid rate were
determined by using equation below,
VL
Lw
(3.20)
VL
38747 .97
746 .74
VL
51.89 m3/hr
0.0144 m3/s
Based on the values of volumetric flow rate and column diameter, Dc. Figure
11.28 from Coulson & Richardson, Chemical Engineering, Volume 6, page 568.
Therefore, types of liquid flow could be considered as single pass.
3.3.7
PLATE LAYOUT
The value of downcomer area, active area, hole area, hole size, and weir height were
determined based on above value calculated, trial plate layout column area determined
by using the equation below,
219
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Column area, Ac
Where Um
Um
Uv (0.9)
4.181
0.7997 (0.9)
5.81 m2
Velocity at below plate,
(3.21)
Down comer area were found by assume 20% of column area and using equation
below,
Down comer Area Ad
0.2 Ac
0.2(5.81 m2)
1.162 m2
Net area and active area were determined by using equations below,
Net Area, An
Active area, Aa
Ac - Ad
5.181 -1.162
4.02 m2
Ac - 2Ad
5.181 - 2(1.162)
2.857 m2
Hole Area, AH are determine with trial value of 10% active area by equation below,
Hole Area, Ah
0.10(Aa)
0.10(2.857)
0.2857 m2
Weir Length, lw was calculated by referring Figure 11.31 from Coulson Richardson,
Chemical Engineering, Volume 6, page 572 which was determined based on the
value of the ratio of Ad/Ac to get the ratio of lw/ Dc .
The weir height determined and other dimensions are as below:
220
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.3.8
Weir Height, hw
50 mm (standard)
Hole diameter, dh
5 mm (standard)
Plate Thickness
5 mm (standard)
Weir Length, Iw
612 mm (80% x 765)
ENTRAINMENT EVALUATION
The entrainment checking can be done by determine actual flooding percentage, Uv by
using equation below,
Uv
Um
Ac
4.181
5.181
0.807 m/s
(3.22)
Liquid flow rate were determine by using below equation by using liquid vapor flow
factor.
FLV
0.5
Lw
Vw
38747 .97 3.8402
15251 .95
746 .74
0.182
(3.23)
0.5
Where FLV is liquid vapor factor.
Based on value of FLV and assumption made for initial tray spacing (0.9m) by
referring to Figure 11.27 from Coulson & Richardson, Chemical Engineering, Volume
6, page 567, the data were used to determine the constant, K1 for the estimation of
flooding velocity. Before that, correction factor are used as equation below:
0.2
K1 =
k1 0.02
(3.24)
221
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
0 .2
0.0351
0.1
0.02
0.112
And flooding velocity, Uf determine by equation below (correlation given by Fair,
Coulson & Richardson, Chemical Engineering, Volume 6, page 567,
Uf
l v
K1 v
l v
0.112 v 0.5
746 .74 3.8402
3.8402
0.112
1.56 m/s
(3.25)
0 .5
Actual % of flooding =
Uv
100
Uf
0.807
100
1.560
51.8%
(3.26)
Fractional entrainment is calculated based on this percentage and FLV by
referring to Figure 11.29 from Coulson & Richardson, Chemical Engineering, Volume
6, page 570, if unsatisfied, recalculation were done based on chosen diameter and
plate spacing acceptable to determine the lowest value. However, fractional
entrainment = 0.02, is below the initial guest of 0.1 and entrainment is acceptable.
3.3.9
WEEPING RATE EVALUATION
222
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Weir liquid crest were determined by using values of maximum liquid flow rate
and minimum flow rate based on the process condition and also assumption of
turndown percentage based on the liquid characteristic. Each weir liquid crest value
was determined by using equations as follow, the Francis weir formula (see also
Volume 1, Chapter 5),
Max how
Min how
2/3
Lw max
750
l (lw )
750
13.49 mm liquid
Lw min
750
l (lw )
10 .7633
750
(746 .74 )( 0.88 )
48.37 mm liquid
(3.27)
2/3
1.5860
(746 .74 )( 0.88 )
2/3
(3.28)
2/3
Where,
Iw
Weir length, 0.88 (standard)
how
weir crest
Lw
liquid flow rate
At minimum liquid flow rate, the value was determined by adding weir height H w and
weir crest, how the constant, K2 where it is found based on the value by referring to
Figure 11.30 from Coulson & Richardson, Chemical Engineering, Volume 6, page
571.
Minimum vapor velocity Uh, were determined by using the equation as below,
Uh
K 2 0.90 ( 25 .4 d h )
v 0.5
=
(3.29)
28 .60 0.90 (25 .4 0.005 )
(3.8402 ) 0.5
2.931 m/s
(3.3.10, 3.3.11 and 3.3.12: Please refer to the Appendix)
223
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.3.13 NUMBER OF HOLES
Area of hole,
AH
=
=
Number of Holes
3.3.14
d h
4
(3.30)
(0.05 ) 2
4
1.9634 x 10-3 m2
Ah
AH
0.2857
1.9634 10 3
145.49
146 units (at every sieve plate).
(3.31)
COLUMN SIZE
The column height will be calculated based on the equation given below. The equation
determines the height of the column without taking the skirt or any support into
consideration. Its determination is based on condition in the column.
Column Height
(No stage 1) (tray spacing)
+(Tray spacing x 2)
+(No stage-1) (Thickness of Plate)
(11 -1)(0.9)+(0.9)(2) + (11-1)(0.005)
10.85 m
12.00 m (including 10% safety factor)
The overall height from the calculation is 10.85 m, but in a real construction it
will be added slightly more (about 10%) because of vapor and liquid area at top and
bottom column. The space for vapor and liquid are required if uncertain condition occur
224
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
in the column, such as over flooding, over vapor pressure or upset in reaction situation.
The calculated result is tabulated in the Table 5.8 as below.
Table 3.8
Provisional Plate Design Specification
Item
Column Diameter, Dc
No of Plates
Plate Spacing
Plate Thickness
Total Column Height, Ht
Plate Pressure Drop, ht
Plate Material
Down Comer Area, Ad
Down Comer Material
Column Area, Ac
Net Area, An
Active Area, Aa
Hole Area, Ah
Number of Holes
Weir Length
Weir Height (standard)
Resident Time
Value
0.765 m
11 units
0.9 m
5 mm*
12.00 m
192.81 mm liquid*
SS 304
0.8348 m2
SS 304
5.181 m2
4.020 m2
2.857 m2
0.2857 m2
146 units
0.612 m
0.05 m
13.37 seconds*
* For the determination of these values, they are shown in the Appendix section.
3.4
MECHANICAL DESIGN
In the mechanical design, the temperature and pressure are important properties in
evaluating the thickness and the stress of material. Therefore, the safety factor also is
225
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
also added as precaution and determined by certain consideration such as corrosion
factor, location and process characteristic.
The safety factor is usually 10% above the operating pressure and as for this
MTBE distillation column, the operating pressure is 450 kPa. The chosen safety factor
is based on the process characteristics of the system. The design temperature is
related to the operating temperature. Based on the calculated result, the temperature
at the top of the column is 53.3oC and the temperature at the bottom of the column is
107oC.
Design Pressure, Pi
Design Temperature, T
3.4.1
0.450 N/mm2 x 110%
0.495 N/mm2
117.70 C (10% more than design temp.)
MATERIAL OF CONSTRUCTION
The material used in the construction of the distillation column is stainless steel
(18Cr/8Ni, 304) as the material is suitable in high temperature and less corrosive. For
this material, the design stress at 150 C is obtained from Table 13.2, page 809
Coulson & Richardson, Chemical Engineering, Volume 6.
3.4.2
Design stress, f
130 N/mm2
Diameter vessel, Di
860 mm
Tensile strength,
510 N/mm2
VESSEL THICKNESS
The minimum thickness of column required and other designs are calculated based on
equation below (Coulson & Richardson, Chemical Engineering, Volume 6, page 812):
226
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Pi Di
2Jf Pi
(0.495 )( 765 )
2(1)(145 ) (0.495 )
1.31 mm
(3.32)
Based on Table 13.4, Coulson & Richardson, Chemical Engineering, Volume 6,
page 811, this minimum thickness should be added 5 mm to withstand its own weight
and any incidental loads.
e
Assumed
1.31 mm + 5 mm
6.31 mm
7.00 mm
Where, Pi
Design pressure
Di
Column diameter
Design Stress
Joint factor (assumed = 1)
From Table 13.4, Coulson and Richardson, Chemical Engineering, Volume 6,
page 811, for diameter 1 m to 2 m the minimum thickness should not be less than 7
mm (including 2 mm of corrosion allowance). For vessel diameter around 0.5 m to 1 m,
a much thicker wall will be needed at the column base to withstand the wind and dead
weight loads.
A much thicker wall is needed at the column base to withstand the wind and
dead weight loads. As a first trial, divide the column into five sections, with the
thickness increasing by 2 mm per section. Try 7, 9, 11, 13 and 15 mm. The average
wall thickness is 11 mm.
3.4.3
HEADS AND CLOSURE
227
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Torispherical head had been chosen because of operating pressure below 10 bars and
suitable for liquid vapor phase process in inconsistent high pressure. The calculations
as below is considered,
Crown radius, Rc
= Di
= 0.765 m
Knuckle radius, Rk
= 6% Rc
= 0.046 m
Minimum Thickness =
Pi R c C s
2Jf + Pi (C s 0.2)
= 0.2137mm
(The method of calculations is shown in the Appendix section)
3.4.4
TOTAL COLUMN WEIGHT
Total Weight, Tw
Total weight is the summation of the weight of dead weight, the weight of plates and
the weight of insulation. The calculations for the dead weight, the weight of plates and
the weight of the insulation are shown in the Appendix.
Total weight, Wt
3.4.5
W v + Wp + WI
(28.46 + 68.53 + 10.63) kN
107.62 kN
WIND LOADS
The wind load is calculated based on location and the weather of surrounding.
Therefore, the value of wind speed is assumed as below and wind load is calculated
shown in the appendix. The wind load for the MTBE column is 62.91kN (methods of
calculation in shown in the appendix.
3.4.6 STIFFNESS RING (Please refer to the Appendix)
Table 3.9
Summarized Results of Mechanical Design
228
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Operating pressure, Po
0.45 N/mm2
Design Pressure, Pi
0.495 N/mm2
Safety factor
0.15
Design Temperature, TD
88.78 oC
Operating Temperature, To
80.71 oC
Heads and Closure
Types
Torispherical head.
Crown Radius, Rc = Di
0.765 m
Knuckle Radius, Rk = 6% Rc
0.046 m
Joint Factor, J
1.00
Cs
1.77
Minimum thickness head, e
0.2317 mm
Column Weight
Dead weight of vessel, Wv
28.46 kN
Weight of a plate, Wp
6.23 kN
Weight of 11 plates,Wp
68.53 kN
Weight of insulation, WI
10.63 kN
Total weight
107.62 kN
Win speed, Uw
160 km/hr
Wind pressure. Fw
1068.8 N/m2
Bending Moment (Mx)
62.91 kN
Stiffness Ring
Critical buckling pressure for ring, Pc
15 x 106 N/m2
229
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.5
VESSEL SUPPORT DESIGN (SKIRT DESIGN)
Type of Support
Straight cylindrical skirt
90
Material of Construction
Carbon steel
Design Stress, fs
135 N/mm2 at ambient temp. 20C
Skirt Height, Hv
2.5 m (standard)
Youngs Modulus
200, 000 N/mm2
Approximate Weight
8.418 kN
Total Weight
36.88 kN
230
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
(The method of calculations for other parameters in the vessel support design in shown
in the Appendix section)
Table 3.10
Design Specification of the Support Skirt
Support Data
Type of Support
Straight cylindrical skirt
Material of Construction
Carbon steel
Design Stress, at T 20C (ambient)
135 N/mm2
Skirt Height
2.50 m
Youngs Modulus
200000 N/mm2
746.74 kg/m3
Approximate Weight, Wapprox
8.418 kN
Total Weight
107.62 kN
Wind Load, Fw
1068.8 N/m2
Skirt Thickness, ts
15 mm
REFERENCES
J. M. Coulson, J. F. Richardson, Chemical Engineering, Volume Two, Third
Edition, The Pergamon Press, 1977.
R. K Sinnot, Coulson & Richardsons Chemical Engineering, Chemical
Engineering Design, Volume Six, Butterworth Heinemann, 1999.
Robert H. Perry, Don W. green, Perrys Chemical Engineers Handbook,
Seventh Edition, McGraw-Hill, 1998.
James, M. Douglas, Conceptual Design of Chemical Processes, McGraw-Hill
Book Company, 1988.
Martyn S. Ray and David, W. Johnston, Chemical Engineering, Design Project:
A Case Study Approach, Gordon and Breach Science Publishers, 1989.
Carl R. Branan, Rules of Thumb for Chemical Engineers, Gulf Publishing
Company, 1994.
231
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Billet, R., Distillation Engineering, Heydon Publishing, 1979.
King, C. J., Separation Processes, Second Edition, McGraw-Hill, 1992.
Kister, H. Z., Distillation Design, McGraw-Hill, 1992.
Lockett, M. J., Distillation Tray Fundamentals, Cambridge University Press,
1986.
Normans, W. S., Absorption, Distillation and Cooling Towers, Longmans, 1961.
Oliver, E. D., Diffusional Separation Procesess, John-Wiley, 1966.
Robinson, C.S., and Gilliland, E.R., Elements of Fractional Distillation, McGrawHill, 1950.
Smith, R., Chemical Process Design, McGraw-Hill, 1995.
Van Winkle, M., Distillation, McGraw-Hill, 1967.
Micheal J. Barber, Handbook of Hose, Pipes, Couplings and Fittings, First
Edition, The Trade & Technical Press Limited, 1985.
Louis Gary Lamit, Piping Systems: Drafting and Design, Prentice-Hall, Inc.,
1981.
David H. F. Liu, Bela. G. Liptak, Wastewater Treatment, Lewis Publishers, 2000.
SECTION 4
DESIGN OF LIQUID-LIQUID EXTRACTION COLUMN
4.1
INTRODUCTION
Liquid-liquid extraction has become an important separation technique in modern
process technology. This is has resulted in the rapid development of a great variety of
232
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
extractor types, in the evaluation of which the chemical engineer must primarily depend
on manufacturers literature.
To the design, only three components that are considered, - methanol, water
and isobutene- this is because of for most system containing more than four
components, the display of equilibrium data and the computation of stages is very
difficult. In such cases, the requirements are best obtained in the laboratory without
detail study of the equalibria. (Treybal, Mass Transfer Operations, 1987).
Beside that for multicomponent separations also, special computer programs
for these multistage operations embodying heat and material balances and
sophisticated phase equilibrium relations are best left to professionals. Most such work
is done by service organizations that specialize in chemical engineering process
calculations or by specialize in chemical engineering organizations. (Stanley M. Walas,
Chemical Process Equipment, 1988).
Sieve tray (perforated plate) Column were choose for the extraction of these
components. These multistage, countercurrent columns are very effective, both with
respect to liquid-handling capacity and with respect to extraction efficiency, particularly
for system of low interfacial tension, which do not require mechanical agitation for good
dispersion.
4.2
CHEMICAL DESIGN OF LIQUID LIQUID EXTRACTION COLUMN
4.2.1
Choice of Solvents
There is usually a wide choice of liquids to be used as solvent for extraction operations.
It is unlikely that any particular liquid will exhibit all the properties considered desirable
for extraction, and some compromise is usually necessary. The following factors need
to be considered when selecting a suitable solvent for a given extraction affinity for
solute, partition ratio, density, miscibility, safety and cost. Based on the factors that
need to be considered water was choosing as a solvent in this system.
4.2.2
Estimation or Gather the Physical Properties
233
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Most of the design parameter used were refer to mass balance, energy balance data
and generated by chemical Engineering Simulation Software, HYSIS.
The properties data as state below:
Flowrate at the dispersed phase, QD
1254.92 ft3/hr
Flowrate at the continuous phase, QC
1.785 ft3/hr
Density at the dispersed phase, D
24.10 lb/ ft3
Density at the continuous phase C
41.45 lb/ft3.
The data was evaluated at system temperature and pressure.
4.2.3
Determination of Number of Stages
To determine the theoretical stages required, by assuming the minimum solvent to feed
ratio required to remove all the minimum component, so that is the extraction factor, =
1(Schweitzer, Separation Handbook). Equation 4.1 was used. The value for X f =
0.0195 kg CH3OH/ kg water, Ys = 1.57 x 10-6 kg CH3OH/ kg water was compute from
the mass balance at this system.
Number of theoretical stage, Nf = Xf Ys/m
Xr Ys/m
Nf = 0.0195 (1.57 x 10-6 / 0.001)
(1.57 x 10-6 / 0.001)
-1
(4.1)
-1
= 10.42 stages
= 10 stages
Where,
Xf
= weight solute /weight feed solvent in the feed phase
Ys
= weight solute / weight extraction solvent in extract
Xr
= weight solute /weight feed solvent in the raffinate phase
= slope at the equilibrium line dY/dX
234
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The number of mass transfer unit, Nor is identical to the number of the theoretical
stages when extraction factor, = 1.
Nor
= Xf Ys/m
-1
(4.2)
Xr Ys/m
Nor
= 10 units
By assuming column efficiency, E is 80%, the number of real stages, N was
determining by using equation 4.3.
N = (Nf -1)/ E
(4.3)
= (10 1)/ 0.8
= 11 stages
4.2.4
Sizing of Sieve Tray
The sieve tray sizing was base on the manufacturers literature. Usually the tray
spacing is from (6 to 24) in, and perforation diameter, do usually from (0.32 to 0.64) cm
or (1/8 to 1/4) in diameter. By take 2 ft tray spacing, Zt, 0.25 in holes on 0.75 in
triangular spacing. The downcomer area is found with equation 4.4.
h
= 4.5 Vd2 C / 2gc
h = 2
(4.4)
= 4.5 Vd2 C / 2gc
4.5(41.45)
2(4.18 x 108) 17.35
Vd
= 12471 ft/hr
Ad
= QD/ Vd
Vd2
(4.5)
= 1254.92 / 12471
= 0.1006 ft2
235
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Dd
Where,
= 4.2948 in (Downcomer diameter)
Vd
= Velocity of the dowmcomer
= Density at the continuous phase
gc
= gravitational constant
To determine the total holes area in a tray used equation 4.6 and set the velocity
through the holes, Uh are kept below 0.8 ft/sec or 2880ft/ hr to avoid formation of very
small droplet.
Total hole area, AHT
= QD/ Uh
(4.6)
= (1254.92 / 2880)
= 0.4357 ft2
To find the tray area, by using ratio of the tray area to hole area as state below:
Tray area, AT
(/4) (do) 2
Hole area, AH
=
2.21( ds/ do) 2
2.21 (0.75/0.25)2
19.89
19.89(0.4357)
8.666 ft2
3.32 ft
do
perforation diameter
ds
triangular spacing
Tray Area, AT
Tray Diameter, DT
Where,
4.2.5
0.866 (ds) 2
Number of Holes
Hole area, AH
= (/4) (do) 2
(4.7)
236
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
= (/4) (0.25) 2
=0.0245 in2
Number of hole, NH
= AHT/AH
(4.8)
2
= 0.4357ft / 0.0245 in
= 2560 units
4.2.6
Column Parameter
Number of tray, NT required and the tower high, HT is determining by using equation
4.9 and 4.10 respectively. The efficiency of the tray is base on assumption of the
column efficiency.
Number of trays, NT
= Nf / ET
(4.9)
= 10 / 0.8
= 13 trays
Column Height, CT
= Zt x NT
(4.10)
= 2 (13)
= 26 ft + 3 ft (including 1.5 ft at each end)
= 29 ft
Column diameter same with the tray diameter, so
Column diameter, DC
= 3.32 ft.
Column area, AC
= 8.67 ft2
Net area and active are were determined by using equation 4.11 and 4.12 respectively.
Net area, AN
= AC - Ad
(4.11)
= 8.67 0.1739
= 28.4961ft 2
Active area, Aa
AC - 2Ad
(4.12)
= 8.67 (2 x 0.1739)
= 8.322 ft2
Where,
Ad = downcomer area
237
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The height equivalent to theoretical stages, (HETS) and height of transfer unit, Hor are
calculated by using equation 4.13 and 4.14 respectively.
HETS
Hor
4.2.7
CH / Nf
(4.13)
29 / 10
2.9
CH / Nor
2.9
(4.14)
Weeping Evaluation
By analogy with distillation. Weir length, lw is calculated by referring to Figure 11.31
from Coulson and Richardson Vol.6 page 572, which determined the value is base on
the ratio of Ad/AC to get the ratio of lw/DC. The weir height determine from standard form
as follows:
Weir height
= 50 mm
Hole diameter
= 5 mm
Plate thickness
= 5 mm
Weir crest were determined by using value of maximum flowrate and minimum
flowrate based on process condition. Each weir crest value determine by using
equation 4.15 and equation 4.16 respectively.
Max how
Min how
2/3
750
Lw max / D( lw)
750
3.8106/ (380.048 x 1.2705)
29.73 mm liquid
750
Lw min / D( lw)
750
0.4512/ (380.048 x 1.2705)
(4.15)
2/3
2/3
(4.16)
2/3
238
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
7.17 mm liquid
At minimum rate hw + how = 50 + 7.17 =57.17 mm
Where,
lw
= weir length
how = weir crest
Lw
= liquid flowrate
Orifice coefficient, Co was referring from figure 11.34 Coulson and Richardson Vol. 6
pg 576 and by assuming.
1.
Plate thickness : hole diameter
=1
2.
Ah/Ap
=5
Plate pressure drop, h
h
51 (Uh / Co)2 (C/D)
(4.17)
51 (0.2438/0.805) (640/380.048)
7.88 mm liquid
(12.5 x 103) / D
Residual head, hr
hr
(4.18)
12.5 x 10 / 380.048
32.89 mm liquid
Total plate pressure drop, ht
ht
h + (hw + how) + hr
7.88 + 57.17 + 32.89
97.94 mm liquid
(4.19)
Plate pressure drop, Pt
Pt =
9.81 x 10-3 ht D
(4.20)
9.81 x 10-3 (97.94) (380.048)
365.147 Pa (N/m2)
Table 4.1: Provisional Plate Design Specification
Column Diameter
1011
mm
239
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Number of Trays
Tray Spacing
Plate Thickness
Total Column Height
Plate Pressure Drop
Plate Material
Downcomer Area
Column Area
Net Area
Active Area
Hole Area
Number of Holes
Weir Length
Weir Height (standard)
Number of manhole
Manhole Diameter (BS 470:1984)
4.3
13
0.6
5
9
7.88
SS304
9.3 x 10-3
0.805
2.647
0.773
1.58 x 10-5
2560
1.2705
50
2
700
trays
m
mm
m
mm liquid
m2
m2
m2
m2
m2
units
m
mm
mm
MECHANICAL DESIGN OF LIQUID LIQUID EXTRACTION COLUMN
In mechanical design, the temperature and the pressure are important properties in
evaluate the thickness and the stress of material. Therefore, the safety factor also need
as precaution and determined by certain consideration such as corrosion factor,
location and process characteristic.
Based on Hysis data, the operating pressure is 2.75 kPa and the safety factor is
10% above operating pressure. The design temperature related to the operating
temperature. The temperature of column operated in 400C at top of column and 270C at
the bottom of the column. The design pressure and design temperature of the system
as follows:
Design Pressure, Pi
Design Temperature, T
4.3.1
0.275 N/mm2 x 1.1
0.3025 N/mm2
500C
Material Construction
The material used is stainless steel (18Cr/8Ni, 304). Design stress at 500C is gain from
table 13.2, pg 809 Coulson & Richardson Vol.6.
240
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.3.2
Design stress, f
165 N/mm2
Diameter Vessel, Di
1011 mm
Tensile Strength,
510 N/mm2
Vessel Thickness
The thickness of column is calculated based on the equation below:
e
PiDi
(4.21)
2f Pi
=
0.3025(1011)
2(165) 0.3025
0.9276 mm
Add corrosion allowance 4mm, so the thickness:
e
4.9276 mm
5 mm
From Coulson & Richardson, value for vessel diameter (m), 1 m, the minimum
wall thickness required should not be less than 5mm including corrosion allowance. A
much thicker wall will be needed at the column base to withstand the wind and dead
weight loads. As a first trial, divide the column into five sections (courses) with
thickness increasing by 2mm per section. Try 10,12,14,16, and 18mm. The average is
14 mm.
4.3.3
Design of Domed Ends
Standard torispherical head are the most commonly used end closure for vessel up to
operating pressure of 15 bar. Torispherical head had been choose because of
operating pressure below 10 bar.
Crown Radius, RC
Di
1.011m
Knuckle Radius, Rk
6%RC =
0.061 m
241
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
A head of this size would be formed by pressing: no joint, so J = one. Stress
concentration factor for torispherical heads, Cs:
Cs
(3 + (RC / Rk))
(3 + (1.011/0.061))
3.053
(4.22)
Therefore the minimum thickness:
e
PiRCCs
(4.23)
2fJ + Pi (Cs 0.2)
=
0.3025(1011) (3.053)
(2 x 165) + (0.3025(3.053 -0.2))
=
4.3.4
2.821 mm
Column Weight
4.3.4.1
Dead Weight of Vessel, Wv
Wv
240 Cv Dm(Hv + 0.8Dm) t
Where;Cv
a factor take 1.15, vessel with plates
Dm
mean diameter, m
(Di + t)
Hv
height or length between tangent line
wall thickness, m
(4.24)
To get a rough estimate of the weight of this vessel by using the average thickness 14
mm.
Dm
1.011 + 0.014
1.025 m
240(1.15) (1.025) (9 +0.8(1.025)) 14
38933.69N
Hence,
Wv
242
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
=
4.3.4.2
389 kN
Weight of plate, Wp
From Nelson (1963) pg 833 in Coulson & Richardson Vol. 6 rough guide to weight of
fittings, take contacting plates, steel including typical liquid loading, 1.2 kN/m2 plate
areas. The total of weight of plate determine by multiply the value with number of tray
design.
Tray area, AT
0.805 m2
Weight of plate
1.2 x AT
1.2(0.805)
0.966 kN
Weight of 13 trays, Wp
4.3.4.3
0.966(13)
12.558 kN
(4.25)
Weight of Insulation, Wi
The insulating material is mineral wool;
Density of mineral wool
130 kg/m3
Thickness of insulation, ti
75 mm
Volume of insulation, Vi
DiHv x ti
(1.0110) (9) (75 x 10-3)
2.144m3
Vig
2.144(130) (9.81)
2734.11 N
Weight of Insulation, Wi
(4.26)
(4.27)
Double this value to allow fittings, so weight of insulation, Wi = 5.468 kN
4.3.4.4
Total weight, W
243
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.3.4.5
Wv + Wp + Wi
389 + 12.56 + 5.47
407.03 kN
(4.28)
Wind Loading
Take dynamic wind pressure, Pw as 1280 N/m2.
Mean diameter, including insulation, Deff:
=
1.011 + 2(0.014 + 0.075)
2.791 m
Loading (per linear meter) Fw;
Fw
PwDeff
(4.29)
1280 (2.791)
3572.48 N/m
Bending moment at bottom tangent line. MX
MX
FwX2
(4.30)
2
=
3572.48 (9)2
2
Where; X
144685 Nm
Distance measured from the free end
The calculated value as the result tabulated in table 4.2. The value requires
determining in strength and suitability of column while in construction and operation. It
also required the safety consideration operation. The operating procedure of the
column should base on this value.
4.3.5
Analysis of Stress
At bottom tangent line,
244
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.3.5.1
Longitudinal and Circumferential Pressure Stress;
h
PiDi
(4.31)
2t
=
0.3025 (1011)
2 (14)
10.92 N/mm2
PiDi
(4.32)
4t
=
0.3025 (1011)
4 (14)
=
4.3.5.2
5.46 N/mm2
Dead Weight Stress
L
(4.33)
(Di + t) t
=
407030
(1011 + 14) 14
=
4.3.5.3
9.03 N/mm2
Bending Stress
b
MX / IV ((Di/2) + t)
Where;MX
Total bending moment
Second bending moment
/ 60 (Do4 Di4)
1011 + 2(14)
1039 mm
/ 60 (10394 10114)
6.32 x 109 mm4
IV
(4.34)
Which;
Do
IV
Therefore,
245
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
144685000
((1011 / 2) + 14)
6.32 x 109
=
11.89N/mm2
The result longitudinal stress, Z is:
Z
L + W b
Z (upwind)
L - W + b
5.46 11.15 + 11.89
6.2 N/mm2
L - W - b
5.46 11.15 11.89
-17.58 N/mm2
Z(downwind)
4.3.5.4
Elastic Stability (Buckling)
Critical buckling stress, C:
C
2 x 104 (t / Do)
(4.35)
2 x 10 (14 / 1039)
269.48 N/mm2
The maximum compressive stress will occurs when the vessel is not under pressure:
W + b
11.15 + 11.89
23.04N/mm2
This value is below critical buckling stress, so design is satisfactory.
Stresses analysis is tabulate in the table 4.3.
4.3.6
4.3.6.1
Vessel Supports Design
Skirt Supports
At ambient temperature.
246
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Type of support
Straight cylindrical skirt (S = 900)
Material construction :
Plain carbon steel
Design stress
135 N/mm2
Youngs Modulus
200000 N/mm2
Skirt height, Hs
3m
The maximum dead weight load on the skirt will occur when the vessel is full of the
mixture.
Approximate weight, Wapprox
Total weight
( / 4) Di2 HVLg
(4.36)
( / 4) (1.011 ) (9) (380.048) (9.81)
26936.56 N
26.9 kN
W + Wapprox
407.03 + 26.9
433.93kN
Bending moment at base of skirt, MS:
MS
Fw
(HV + HS)2
(4.37)
2
2
3.572 (12 / 2)
257.184 kNm
As a first trial, take the skirt thickness as the same as that of the bottom section of the
vessel, ts = 14 mm.
Bending stresses in skirt, bs
Where; Ms
= 4Ms / ( (Ds + ts) tsDs)
Maximum bending moment at the base of the skirt.
ts
Skirt thickness
Ds
Inside diameter of the skirt at the base
(4.38)
So,
247
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
bs
4 (257184 x 103) / (1011+14) (14) (1011)
22.5710 N/mm2
Dead weight stress in the skirt, Ws:
Ws(test)
Ws(operating)
W / ( (Ds + ts) ts)
26900 / (1011+14) (14)
0.60 N/mm2
407030 / (1011+14) (14)
9.03 N/mm2
(4.39)
Thus, the resulting stress in the skirt, s:
Max s (compressive) =
Ws(test) + bs
0.60 + 22.57
23.17 N/mm2
bs - Ws(operating)
22.57 9.03
13.54 N/mm2
Max s (tensile)
(4.40)
(4.41)
Take the joint factor, J as 0.85.
Criteria for design
s (tensile)
> fs J sin
13.54
> 0.85 (135 sin 900)
13.54
> 114.75
248
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
s (compressive)
> 0.125 E (ts/DS) sin
23.17
> 0.125 (200000) (14/1011) sin 900
23.17
> 346.19
Both criteria are satisfied; add 2mm for corrosion gives a design thickness of 16 mm.
4.3.6.2
Base Ring and Anchor Bolt
Approximate pitch circle diameter, say 2.2 m.
Circumferences of bolt circle = 2200
of bolt required, at minimum recommended bolt spacing:
=
2200 / 600
11.5
Closet multiple of 4
12 bolts
Take bolt design stress
125 N/mm2
Bending moment at skirt, MS =
301.834 kNm
Total weight vessel, W
502.53 kN
Area of bolt, Ab
1
Nbfb
Where:Nb
4MS - W
(4.42)
Db
Number of bolts
fb
Maximum allowable bolt stress
MS
Bending moment at the base
Weight of the vessel
249
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Db
Bolt Circle diameter
Therefore,
Ab
4 (257184)
12 (125)
=
Bolt root diameter
- 407030
2.2
40.35 mm2
=
(40.38 x 4) /
7.17 mm
Total compressive load on the base ring per unit length.
Fb
4MS
DS2
=
(4.43)
DS
4 (257184) +
(1.0112)
407030
(1.011)
448.5 x 103 N/m
By taking the bearing pressure as 5 N/mm2. The minimum width of the base ring, Lb:
Lb
Fb
Fc
1
103
448.5 x 103
(4.44)
5000
=
89.7 mm
Actual width can be calculated from this minimum width.
Use M24 bolts (BS 4190:1967) root area = 353 mm2 (Figure 13.30 Coulson &
Richardson Vol. 6)
250
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Actual width required =
Lr + ts + 50mm
150 + 14 + 50
214 mm
Actual bearing pressure on concrete foundation:
fc
Fb
actual width
448500
214000
2.10 N/mm2
Actual minimum base thickness:
tb
Where: fr
Lr (3 fc / fr)
150 ((3 x 2.1) / 140)
25.98 mm
(4.45)
= Allowable design stress in the ring material, typically 140 N/mm2
The design specifications of support are summarized in the table 4.4.
4.3.7
Piping Sizing
The optimum diameter for carbon steel pipe:
d, optimum =
293 G 0.53 -0.37
Where:G
Flowrate (kg/s)
Density (kg/m3)
Pipe thickness, t
P d, optimum
(4.46)
(4.47)
251
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
20 + P
Where:P
Internal pressure, bar
Design stress at working temperature, N/mm2
The piping sizing for this system are shown in table 4.5
Table 4.2 : Summary of the Mechanical Design
Design Pressure
Operating Pressure
2.75 kPa
Operating Temperature
40
0.3025 N/mm2
Design Pressure
Design Temperature
50
Safety Factor
0.10
Design of Domed Ends
Types
Crown Radius
Knuckle Radius
Joint Factor
Stress Concentration Factor
Minimum Thickness
Column Weight
Dead Weight of Vessel
Weight of Plate (per plate)
Weight of Insulation
Total Weight
Wind Pressure
Loading
Bending Moment
Torispherical head
1.011 m
0.061 m
1
3.053
2.821 mm
389
0.966
2734.11
407.03
1280
3572.48
144.685
kN
kN
N
kN
N/m2
N/m
kNm
252
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 4.3: Stress Analysis for Liquid-Liquid Extraction Column
Longitudinal Pressure Stress
Circumferential Pressure Stress
Dead Weight Stress
Bending Stress
Z (upwind)
Z(downwind)
Critical Buckling Stress
10.92 N/mm2
5.46 N/mm2
9.03 N/mm2
11.89
6.2
-17.58
269.48
N/mm2
N/mm2
N/mm2
N/mm2
Table 4.4: Design Specification of the Support Skirt
Types of Support
Material Construction
Design Stress
Skirt Height
Young Modulus
L
Approximate Weight
Total weight
Bending Moment at Skirt
Skirt Thickness
Bending Stress in Skirt
ws (test)
ws (operating)
Maximum s (compressive)
Maximum s (tensile)
Straight cylindrical skirt
90
Plain Carbon steel
135
3
200000
380.048
26.9
433.93
257.184
14
22.57
0.60
9.03
23.17
13.54
N/mm2
m
N/mm2
kN
kN
kN
kNm
m
N/mm2
N/mm2
N/mm2
N/mm2
N/mm2
253
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 4.5: Piping Sizing for Liquid-liquid Extraction Column
Feed Pipe Sizing
Flowrate, G
Density,
Internal Pressure, P
Design Stress,
Diameter Optimum, d optimum
Corrosion Allowance
Pipe Thickness, t
Solvent Pipe Sizing
Flowrate, G
Density,
Diameter Optimum, d optimum
Extract Pipe Sizing
Flowrate, G
Density,
Diameter Optimum, d optimum
Raffinate Pipe Sizing
Flowrate, G
Density,
Diameter Optimum, d optimum
4.4
13873.67
567.0445
2.75
165
55
4
4.05
kg/hr
kg/hr
bar
N/mm2
mm
mm
mm
1469.1 kg/hr
998 kg/hr
15 mm
1624.37 kg/hr
992.8161 kg/hr
15 mm
15096.74 kg/hr
564.7173 kg/hr
30 mm
PROCESS CONTROL AND INSTRUMENTATION OF THE LIQUID-LIQUID
EXTRACTION COLUMN
Control systems are very important in any of the chemical industries. It is essential for
a process to meet the design specification and products purity that imposed by the
designer or by external constrains such as government regulations and standards.
254
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Process parameters control is to compensate for the process changes with the
existence of external disturbances. Normally, an overall control strategy is design to
meet the following objectives:
1. Ensure stable plant operation conductive to power optimization
2. Maintain product quality according to specification
3. Operate the process and machinery in such a way as to estimate or minimize
the possibility of activating last resort safety measures such as relief valve and
surge system, thus ensuring safe plant operation.
Compensate for perturbations cause by external factors such as ambient and
cooling water temperature variations. Provide an intelligent man-machine interface
capable of presenting process and control system information and interactive format.
Typically, there are two types of control systems- feedback control and feed
forward control. In this case, the feedforward control is applied; in feedforward control
its take corrective action before they upset the process. In this system also, solvent use
is lighter than the material being extract, the two input indicated are of course
interchanged. Both inputs are on flow control. The light phase is removing from the
tower on LC (level control) or at the top of level maintain with an internal weir. The
bottom stream is removed on interfacial level control (ILC). The relative elevations of
feed and solvent input nozzles depend on the nature of the extraction process. The
temperature of an extraction process ordinarily is controlled by regulating the
temperature of the feed stream.
REFERENCES
Buford D.Smith, 1963. Design of Equilibrium Stage Processes, United State of
America. McGraw-Hill.
Robert C.Reid,Prausnitz & Poling,1987.The properties of Gases and Liquid, United
State of America. McGraw-Hill.
255
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Erneat J.Henley & J.D Seader, 1968.Equilibrium-Stages Separation Operations in
Chemical Engineering. Canada. John Wiley & Sons
Philip A.Schweitzer,1988. Handbook of Separations Techniques for Chemical
Engineers,2nd Edition. United State of America. McGraw-Hill.
J.D Seader & Erneat J.Henley, 1998.Separation Process Principles, United State of
America. John Wiley & Sons
R.K.Sinnott, 1999.Chemical Engineering Design, Coulson & Richardson Chemical
Engineering .3rd Edition. Volume 6 .Britain. Butterworth Heinemann
Robert H. Perry, Don W. Green, 1998 Perrys Chemical Engineers Handbook, Seventh
Edition, McGraw-Hill.
J.R Backhurst & J.H Harker.1987.Chemical Engineering Design, Coulson &
Richardson Chemical Engineering .3rd Edition. Volume 2 .United Kingdom.
Pergamon Press.
Stanley M. Walas. 1988. Chemical Process Equipment Selection and Design. United
State of America. Butterworths Series in Chemical Engineering.
Robert E.Treybal.1988. Mass-Transfer Operations,3rd Edition. Singapore, McGraw-Hill
International Series.
K.H Reissinger & Jurgen Schroter. 1980. Selections Criteria for Liquid-liquid
Extractors. Chemical Engineering Magazine, November: 274-256.
P.J Bailes,C.Hanson & M.A Hughes.1976. .Liquid-liquid Extraction: The process, the
equipment. Chemical Engineering Magazine, January 217-231.
Ariffin Marzuki & Nurul Izzi, 2004. PETRONAS Research Centre, Bangi. Interview,19
January.
SECTION 5
256
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
DESIGN OF HEAT EXCHANGER
INTRODUCTION
Shell and tube heat exchangers are the most versatile type of heat exchangers. They
are used in process industries, in conventional and nuclear power stations as
condensers, in steam generators in pressurized water reactor power plants, in feed
water heaters and in some air conditioning and refrigeration systems. They are also
proposed for many alternative energy applications including ocean, thermal and
geothermal. Shell and tube heat exchangers provide relatively large ratios of heat
transfer area to volume and weight and they can be easily cleaned.
Shell and tube heat exchangers offer great flexibility to meet almost any service
requirement. The reliable design methods and shop facilities are available for their
successful design and construction. Shell and tube heat exchangers can be designed
for high pressures relative to the environment and high pressure differences between
the fluid streams.
Shell and tube heat exchangers are built of round tubes mounted in a cylindrical
shell with the tubes parallel to the shell. One fluid flows inside the tubes, while the other
fluid flows across and along the axis of the exchanger. The major components of this
exchanger are tubes (tube bundle), shell, front-end head, baffles and tube sheets.
Shell types-various front and rear head types and shell types have been standardized
by Tubular Exchanger manufacturers Association (TEMA).
The E-shell is the most common due to its cheapness and simplicity. In this
shell, the shell fluid enters at one end of the shell and leaves at the other end that is
there is one pass on the shell side. The tubes may have a single or multiple passes
and are supported by transverse baffles. This shell is the most common for singlephase shell fluid applications. With a single-tube pass, a nominal counter flow can be
obtained. The design of a shell and tube heat exchanger is an iterative process
because heat transfer coefficients and pressure drop depend on many geometric
factors, including shell and tube diameters, tube length, tube layout, baffle type and
257
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
spacing and the numbers of tube and shell passes, all of which are initially unknown
and are determined as part of the design process.
In production of MTBE, heat exchanger is very important equipment. Heat
exchanger is used to increase or to decrease the mixture to the desired temperature. In
order to make the process of production of MTBE taking place in the system, it is
important to make the system at the correct environment. The heat exchanger that we
used here is the shell and tube exchanger. Shell and tube heat exchanger is the most
common type of heat exchanger used in the industry. This is because it has many
advantages. The advantages are: 1.
It provided a large transfer area in a small space.
2.
Good mechanical layout: a good shape for pressure operation.
3.
Used well-established fabrication techniques.
4.
It can be constructed from a wide range of materials.
5.
It can be clean easily.
6.
Well-established design procedures.
7.
Single phases, condensation or boiling can be accommodated in either
the tubes or the shell, in vertical or horizontal positions.
8.
Pressure range and pressure drop are virtually unlimited and can be
adjusted independently for the two fluids.
9.
Thermal stresses can be accommodated inexpensively.
10.
A great variety of materials of construction can be used and may be
different for the shell and tubes.
DESIGNING THE HEATER
In the production of MTBE, a heater is the most important heat exchanger in the
system. Therefore this chapter is going to describes details for this heater and the
design of this piece of equipment.
258
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Heater is used in the process to heated the raw material of MTBE consist of
isobutane and a bit of normal butane. Isobutane and normal butane from the storage is
in liquid form. The heating process is because to produce the isobutane and normal
butane in the gases form before this material is feed to the Snamprogetti Fluidized Bed
Reactor. Here the mixtures of this component initially are in the liquefied gas form at
temperature of -15 oC. It will be heated in the Heater (E-100) and Heater (E101) until it
converted into gas form at temperature of 250 oC using steam. For this heat exchanger
we use the counter current process. The temperature difference between liquid and
gas phase is quit big, so we need two heat exchanger in series. The shell and tube
heat exchanger is the floating head type.
T = 250OC
T = 350OC
STEAM IN
T = -15 OC
STREAM 2
STEAM IN
STREAM 3
STREAM 4
T = 250 OC
T = 117 C
E100
E101
STEAM OUT
T = 120 OC
STEAM OUT
T = 250OC
Figure 5.1 : Heat Exchanger In Series For The Heating Process
5.1
CHEMICAL DESIGN OF HEAT EXCHANGER
The chemical engineering design for the heat exchanger is also known as thermal. The
design requires the calculation of the heat transfer area required. From this value,
design features of the unit such as the tube and shell size, tube counts and layout is
determined. In addition, then pressure loss of the fluids across the unit is also
calculated by determined the pumping capacity required. The calculation of the design
259
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
is base on the first heat exchanger (E100). The chemical design is based on Bells and
Kern method. Bells method accounts for the major bypass and leakage streams. Kern
method was based on experimental work on commercial exchangers with standard
tolerances and will give a reasonably satisfactory prediction of heat-transfer coefficient
for standard design.
5.1.1
Physical Properties Of The Stream
Table 5.1:
Properties of Raw material (Isobutane and N-butane) and
Steam for (E100)
Component
Raw material (Isobutane
Steam
Temperature inlet, oC
Temperature outlet, oC
Specific heat, j/kg oC
Thermal conductivity, W/mK
Density, kg/m3
Viscosity, kg/ms
Feed flowrate, kg/s
and normal butane)
t1 = -15
t2 = 117
2155
0.07
485
1.30005 x 10-4
10.9314
T1 = 250
T2 = 120
2010
0.0306575
0.49375
1.55263 x 10-5
1.7649
5.1.2 The Calculation Of Tm
To determine the mean temperature, Tm
Tm = Ft Tlm
(5.1)
Where
Tm = true temperature difference
260
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Ft = temperature correction factor
Before Tm can be obtained, logarithmic mean temperature Tlm must be calculated
Using the equation:
Tlm =
(T1 - t 2 ) - (T2 - t1 )
(T - t )
ln 1 2
(T2 - t1 )
(5.2)
Where
Tlm = log mean temperature difference
T1
= inlet shell-side fluid temperature
T2
= outlet shell-side fluid temperature
t1
= inlet tube-side temperature
t2
= outlet tube-side temperature
Tlm =
( 250 - 117 ) - (120 + 15 )
(250 - 117)
ln
(120 - (-15))
Tlm = 134.00
the temperature correction factor can be obtain by using figure 12.19 (Coulson &
Richardsons Chemical Engineering)
261
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
R=
T1 - T2
t 2 - t1
R=
(250 - 120)
(117 - (-15))
(5.3)
R = 0.98
S=
t 2 - t1
T1 - t1
S=
(117 - (-15))
(250 - (-15))
(5.4)
S = 0.50
Using figure 12.19, (Coulson & Richardsons Chemical Engineering)
Ft = 0.82
Substitute the above value into equation (1.1) below :
Tm = Ft Tlm
Tm = ( 0.82 )(134 .00
Tm = 109.88
C)
From table 12.1 (Coulson & Richardsons Chemical Engineering), we take overall
Coefficient, U = 300 W/m2 0 C
Duty for this heat exchanger is obtain from the energy balance. The duty is,
Q = 3109542.883 W
262
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Then we need to calculate the surface area using equation below.
A=
Q
UTm
A=
3109542.88 3
(300 )(109 .88 )
(5.5)
A = 94.33 m2
5.1.3
Number Of Tubes Calculation
From table 12.3 (Coulson & Richardsons Chemical Engineering), we take standard
pipe of:
Inside diameter, di
= 16 mm
Outside diameter, do
= 20 mm
Length of pipe is assumed as 16 ft.
Length, L
= 4.88 m
Area of the pipe can be calculated using equation below
area = L D
(5.6)
area = (4.88)(3.142)(0.02)
area = 0.3067 m2
Using the area needed from the duty and area for each tube, the number of
Tube, Nt that we get is,
Nt =
Nt =
A
a
(5.7)
94.33
0.3067
Nt = 307.56
Therefore the number of tube, Nt = 308 tubes
263
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
5.1.4
Bundle And Shell Diameter Calculation
The tubes in heat exchanger are usually arranged in an equilateral triangular, square
or rotated pattern. The triangular and rotated square patterns give higher heat transfer
rates. Here we use the triangular pattern.
The triangular pitch of 1.25 is chosen as the tube arrangement.
Bundle diameter
Db = do (Nt / K1 )1 / n1
(5.8)
From table 12.4 (Coulson & Richardsons Chemical Engineering), for 1.25 triangular
pitch, number of passes = 2, then we can obtain
K1 = 0.249
n1 = 2.207
Db = do (Nt / K1 )1 / n1
Db = 0.02 (308 / 0.249 )1 / 2.207
Db = 0.50m
Assume using pull-through floating head type.
From figure 12.10 (Coulson & Richardsons Chemical Engineering), for bundle
diameter 0.33, bundle clearance is 93 mm.
Shell diameter, Ds
Ds = 0.50 + 0.093
= 0.60m
5.1.5 Tube Side Coefficient, hi
264
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Mean temperature of the tube
t mean =
t1 + t 2
2
t mean =
- 15 + 117
2
(5.9)
t mean = 51 o C
cross sectional area = d12/ 4
(5.10)
2
= (3.142)(0.016 ) / 4
= 0.0002 m2
Tube / pass = 308 / 2
(5.11)
= 154 tube / pass
Total flow rate area = (cross sectional area)(tube / pass)
(5.12)
= (0.0002)(154)
= 0.0308 m2
Steam mass velocity, Gt = (steam flow rate)/(total flow rate area)
(5.13)
= (1.7649)/(0.0308)
= 57.30 kg / sm2
Steam linear velocity, u1 = (Gt)/(steam density)
(5.14)
= (57.30)/(0.49375)
= 116.05 kg / ms
Ratio of L / di = 4.88 / 0.016
(5.15)
= 305
Reynolds number, Re
265
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Re =
di
Re =
(0.49375) (116 .05 ) (0.016)
1.55263 x 10 - 5
(5.16)
Re = 59047.87
Heat transfer factor figure 12.31 (Coulson & Richardsons Chemical Engineering),
Jh = 4.1 x 10-3
Prandtl number,
Pr =
Pr =
Cp
(5.17)
kf
(2.010 x 10 3 )(1.55263 x 10 - 5 )
0.0306575
Pr = 1.0180
Tube side coefficient, hi can be calculated using equation below.
jhk f Re Pr 0.33
hi =
di
hi =
(4.1 x 10 - 3 )(0.030657 5)(59047.8 7)(1.0180)
0.016
(5.18)
0.33
hi = 466.62 W / mo C
266
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
5.1.6 Shell Side Coefficient, hs
Take baffle spacing as 1/3 from the shell diameter, so Baffle spacing:
lB
= Ds / 3
(5.19)
= 0.60 / 3
= 0.20 m
Tube pitch, pt
= 1.25 do
(5.20)
= (1.25)(0.02)
= 0.025 m
Flow area, As
As =
As =
(Pt - do )(D s )(IB )
Pt
(5.21)
(0.025 - 0.02)(0.60 )(0.20)
0.025
A s = 0.0240 m2
Mass velocity, Gs
Gs =
Ws
As
Gs =
10.9314
0.0240
(5.22)
Gs = 455.48 kg / m 2 s
Shell side velocity
267
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
us =
Gs
us =
455.48
485
(5.23)
us = 0.9391 m / s
Shell side equivalent diameter for triangular pitch arrangement
de =
1.10 2
(p t - 0.917d o 2 )
do
de =
1.10
(0.025 2 - 0.917(0.02 )2 )
0.02
(5.24)
de = 0.0142 m
Calculate the Reynolds number
Re =
Re =
Gs de
Isobutane
(5.25)
(455.48)(0 .0142)
0.00013000 5
Re = 49750.52
Prandtl number
Pr =
Pr =
CpIsobutane Isobutane
kf
(5.26)
(2155)(0.0 00130005)
0.07
Pr = 4.0023
268
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Choose baffle cut of 25%, from figure 12.30 (Coulson & Richardsons Chemical
Engineering), we can obtained
Jf
= 2.70 x 10-2
Assumed that the viscosity correction is negligible
hs =
k f jf Re Pr 1 / 3
de
hs =
(0.07)(2.7 0 x 10 - 2 )(49750.52 )(4.0023 1/3 )
0.0142
hs = 10513.35 W / m2
(5.27)
5.1.7 Overall Heat Transfer Coefficient, Uo
Material of construction = carbon Steel
Thermal conductivity of the tube wall
Kw
= 38 W/moC
Assumed dirt coefficient as
hid = 8500 W/m2 oC
hod = 8500 W/m2 oC
1
1
1
d ln(d o /di )
1
( do /di ) + 1 (do /di )
=
+
+ o
+
Uo
hs hod
2k w
hid
hi
1
= 0.0039738 m2
Uo
Uo = 322.85 W / m2
5.1.8
C/W
(5.28)
Tube Side Pressure Drop
269
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Reynolds number , Re = 59047.87
From figure 12.24 (Coulson & Richardsons Chemical Engineering),
jf = 3.10 x 10-3
Neglect the viscosity correction term
Pt = Np [ 8 jf (L/d i )(/ W )- m + 2.5 ]
ii 2
2
Pt = 2[ 8(3.10 x 10 - 3 )(4.88/0.0 16) + 2.5 ]
(5.29)
( 0.49375 )(116 .05 )2
2
Pt = 66921.86 N / m2
Pt = 66.92 kPa
5.1.9
Shell Side Pressure Drop
Reynolds number
Re = 49750.52
From figure 12.30 (Coulson & Richardsons Chemical Engineering),
Jf = 2.70 x 10-2
Shell side pressure drop can be calculated using equation below
P = 8j f ( Dd / de ) ( L/I B ) ( s / 2) ( / w ) -0.14
(5.30)
P = 47.63 kPa
Table 5.2: Summary Of Chemical Design For Heat Exchanger In Series
270
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Heat Exchanger
Temperature range, (o C)
Feed flow rate, (kg/hr)
Steam flow rate, (kg/hr)
Log mean temperature
E100
Shell side
250 150
Tube side
-15 117
39353
6353.64
134.00
difference, , Tm (o C)
True temperature
109.88
difference, Tm (o C)
Overall coefficient, U (W/m2 300
o
C)
Duty, Q (w)
Surface are, A (m2)
Length of tube or shell, L
Tube diameter di (mm)
Tube diameter do (mm)
Area of pipe, a (m2)
Number of tube, Nt
Bundle diameter, Db(m)
Shell diameter, Ds(m)
Baffles spacing, IB(m)
Tube side coefficient, hi
3109542.883
94.33
4.88m or 16 ft
16
20
0.3067
308
0.50
0.60
0.2000
466.62
(W/m2 o C)
Shell side coefficient,
10513.35
hs(W/m2 o C)
Overall heat transfer
322.85
coefficent, Uo (W/m2 o C)
Tube side pressure drop,
66.92
P (kPa)
Shell side pressure drop,
47.63
P (kPa)
5.2
MECHANICAL DESIGN OF HEAT EXCHANGER
The mechanical engineering design of heat exchanger determines the physical
elements that make up the unit as well as their respective dimensions. This design
follows the procedures specified by the Tubular Heat Exchanger association (TEMA)
Mechanical Standards. It is applicable to shell and tube exchangers with internal
diameter not exceeding 60 in. (1524mm), a maximum design pressure of 3000psi (204
bar) or a maximum product of nominal diameter (in) and design pressure (psi) of
271
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
60000. In addition, the design also complies with the American Society of mechanical
Engineers (ASME) Boiler and Pressure Vessel Code, Section VIII, Division 1.
5.2.1
Design Pressure
For the design of tube and shell parts, a safety factor of 10% is included to determine
the design pressures.
Operating pressure = 1.1 bar
10% above the pressure for safety
Pi = 1.1 x 10
= 11 bar
= 1.1 N / mm2
5.2.2
Design Temperature
For the shell side and tube side the operating temperature is at 250 oC, so:
Shell-side design temperature = 1.1x 250 oC
= 275 oC
Adding 2 oC for uncertainties in temperature prediction
TD
= 275 + 2
= 277 oC
5.2.3
Material Of Construction
For the heat exchanger, the material used for construction is carbon steel. This
selection of material is depending on the economic factor and also level of
corrosiveness of the fluid used. Since the properties of the fluid both in the tube and
also in the shell are not corrosive fluid, therefore the carbon steel is used because it is
more economics compared to stainless steel.
5.2.4
Exchanger Type
For the heater design, pull through floating head exchanger is chosen as the
exchanger type. Internal floating head is versatile than other type and also suitable for
272
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
high temperature different between shell and tubes. The tube for internal floating head
also can be rod from end to ends and the bundle are easier to clean.
5.2.5
Minimum Thickness Of Cylindrical Section Of The Shell
The minimum thickness of the cylindrical section of the shell to stand the pressure can
be obtained from the calculation below.
e=
PiDi
2jf - Pi
(5.30)
Where,
Pi = design pressure
Di = shell diameter
F = design stress (from table 13.2, Coulson & Richardsons Chemical Engineering)
e=
(1.1)(600)
2(1)(85) - (1.1)
e = 3.91 mm
adding the corrosion allowance = 2 mm
e = 3.91 + 2
e = 5.91 mm
Take the round number of the thickness
e = 6.00 mm
5.2.6
Longitudinal Stress
h =
h =
PiDi
2t
(5.31)
(1.1)(600)
2(6)
h = 55.00 / mm 2
273
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
5.2.7
Circumferential Stress
h =
PiDi
4t
(5.32)
h = (1.1)(600) / 4(6)
h = 27.50 N / mm 2
5.2.8
Minimum Thickness Of Tube Wall
Minimum thickness of the tube wall can be calculated using the equation
e=
(5.30):
PiDi
2jf - Pi
e=
(1.1)(600)
2(1)(85) - (1.1)
e = 3.91 mm
adding the corrosion allowance = 2 mm
e = 3.91 + 2
e = 5.91 mm
5.2.9
Minimum Thickness Of Head And Closure
The minimum thickness of the torispherical head can be calculated by ,
e=
PiR c Cs
2jf + Pi (Cs - 0.2)
(5.33)
Rc = crown radius
Rk = knuckle radius
Cs = stress concentration factor for torispherical head
274
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Cs = 1/4 3 + R c / Rk
(5.34)
Rc = Ds = 600 mm
Rk = 0.06(500) = 36 mm
Cs = 1.77
e=
(1.1)(600) (1.77)
2(1)(85) + 1.1(1.77 - 0.2)
e = 6.80 mm
Adding corrosion allowance
e = 6.80 + 2
= 8.80 mm
5.2.10 Minimum Thickness Of The Channel Cover
e = (C p )(D e )(Pi /f) 1/2
(5.35)
where
Cp = a design constant, depend on the edge constraint (0.45)
De = nominal plate diameter
f
= design stress
e = (0.45)(600)(1.1/85)1/2
= 30.72 mm
Adding corrosion allowance = 2 mm
E = 30.72 + 2
= 32.72 33 mm
5.2.11 Design Load
Dead weight of vessel
275
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
W v = C v mDm g(H v + 0.8D m )t x 10 -3
(5.36)
where
Wv = total weight of the shell
Cv = 1.08 for vessels with only few internal fitting
Wv = (1.08) (7700)(0.602)(9.81)(4.88+0.8(0.602))(2 x 10-3)
= 1654.45 N
Weight of tubes
W t = Nt (do2 - d12 )Lmg
W t = 308 (0.02
(5.37)
- 0.016 2 )(4.88)(77 00)(9.81)
W t = 51362.08 N
Weight of insulation
Material used = mineral wool insulation
Insulation thickness = 50 mm = 0.05 m
Density = 130 kg / m3
Approximate volume of insulation
V = H v [ (r + r1 )2 - r 3 ]
(5.38)
V = (4.88) [ (0.60 + 0.05) - (0.50)
2
V = 2.64 m3
W t = V g
(5.39)
W t = (2.64)(130 )(9.81)
W t = 3366.79 N
Total weight of heat exchanger
WT = Wv + Wt + Wi
(5.40)
276
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
WT = 1654.45 + 51362.08 + 3366.79
= 56383.32 N
= 56.38 kN
5.2.12 Pipe Size Selection For The Nozzle
pipe size for isobutane inlet
material of construction
= carbon steel
density inlet isobutane,
= 600 kg / m3
flow rate isobutane at inlet, Gisobutane = 10.9314 kg / s
Diameter pipe for isobutane inlet, Disobutane
Disobutane (in) = 293G0.53
-0.37
(5.41)
Disobutane (in) = 293(10.9314) 0.53(600)-0.37
Disobutane (in) = 97.60 mm
Pipe size for isobutane at outlet,
material of construction
= carbon steel
density outlet isobutane,
= 370 kg / m3
flow rate isobutane at outlet, Gisobutane = 10.9314 kg / s
Diameter pipe for isobutane outlet, Disobutane
Disobutane (out) = 293G0.53
-0.37
Disobutane (out) = 293(10.9314) 0.53(370)-0.37
Disobutane (out) = 116.72 mm
Diameter pipe for steam at inlet stream
277
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
material of construction
= carbon steel
density inlet steam,
= 0.5654 kg / m3
flow rate steam at inlet, Gsteam
= 1.7649 kg / s
Diameter of pipe
Dsteam (in) = 293G0.53
-0.37
Dsteam (in) = 293(1.7649) 0.53(0.5654)-0.37
Dsteam (in) = 488.94 mm
Diameter pipe for steam at outlet stream
material of construction
density outlet steam,
= carbon steel
= 0.4221 kg / m3
flow rate steam at outlet, Gsteam = 1.7649 kg / s
Diameter of pipe
Dsteam (out) = 293G0.53
-0.37
Dsteam (out) = 293(1.7649) 0.53(0.4221)-0.37
Dsteam (out) = 544.79 mm
278
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 5.2 : Steel pipe nozzle
Table 5.3 : by taking D = 100 mm, the selected tube nozzle is :
Nominal pipe
size, inch
4
Outside
diameter, inch
4.5 (114.30)
Schedule no.
4OST
Wall thickness,
inch
0.237
(6.02 mm)
Inside
diameter, inch
4.026
(102.26 mm)
Table 5.4 : by taking D = 500 mm, the selected tube nozzle is :
Nominal pipe
size, inch
20
Outside
diameter, inch
20 (508)
Schedule no.
4OST
Wall thickness,
inch
0.375
(9.53 mm)
Inside
diameter, inch
19.250
(488.95 mm)
(From Perry R.H and Green, Don (1984), Perrys Chemical Engineers Handbook, 7th
Edition, McGraw-Hill Book)
5.2.13 Standard Flanges
279
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Flanges used in this design are chosen from the standard flanges. Here standard
flanges are adapted from the British standard (BS 4504), nominal pressure 10 bar.
Figure 5.3 Standard Flange
Standard flange for inlet isobutane
Diameter isobutane inlet pipe = 97.60 mm
Used standard o.d pipe
= 114.3 mm
Table 5.5 : Standard Flange for Inlet isobutane
nom.
size
100
pipe
o.d
d1
114.3
210
Flange
b
16
hi
Raised face Bolting
Drilling
d4
f
No.
d2
45
148
M16
18
d3
170
130
Neck
h2
10
Standard flange for outlet isobutane
Diameter isobutane outlet pipe
= 116.72 mm
Used standard o.d pipe
= 114.3 mm
280
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 5.6 : Standard Flange for Outlet isobutane
nom.
size
100
pipe
o.d
d1
114.3
210
Flange
b
16
hi
45
Raised face Bolting
Drilling
d4
f
No.
d2
148
M16
18
d3
170
130
Neck
h2
10
Standard flange for inlet steam
Diameter steam inlet pipe
= 488.94 mm
Used standard o.d pipe
= 508 mm
Table 5.7 : Standard Flange for Inlet Steam
nom.
size
pipe
o.d
d1
500
508
Flange
D
b
645
24
hi
68
Raised face Bolting
Drilling
d4
f
No.
d2
570
M20
20
22
Neck
h2
d3
600
538
15
12
Standard flange for outlet steam
Diameter steam outlet pipe
= 544.79 mm
Used standard o.d pipe
= 508 mm
Table 5.8 : Standard Flange for Outlet Steam
nom.
size
pipe
o.d
d1
500
508
Flange
D
b
645
24
hi
68
Raised face Bolting
Drilling
d4
f
No.
d2
570
M20
20
22
Neck
h2
d3
600
538
5.2.14 Design of Saddles
Table 5.9: Using Ds = 600mm, the standard steel saddles for vessels up
to 1.2m :
281
15
12
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Vessel
Maximum
diamete
weight
r (m)
0.9
(kN)
56.38
Dimension (m)
C
E
J
t2
0.63 0.15 0.81 0.34 0.275 0.095 10
t1
mm
Bolt diameter
20
5.2.15 Baffles
Type : transverse baffle
Baffle diameter for plate shell is given as, Ds = the nominal diameter of the shell for
plate shell. So baffle diameter = 0.600 m = 23.63 = 600 mm
Diameter of tube holes in baffles, Dh
Dh = outer diameter of the tube
= (0.16 + 1/32) x 20.2
= 3.86 mm
Number of baffle segmental, Nb
Nb = length tube / inside diameter shell
= 4880 / 600
= 8.13 8 baffles
Vent and drain -A drain and vent connection shall be provided on the shell side
Table 5.10: Summary Of Mechanical Design For Heat Exchanger In Series
Heat Exchanger
E100
282
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Design pressure, bar
11
design temperature, oC
277
Material Of Construction
Tube side: Carbon steel
Shell side : Carbon steel
6.0
Minimum Thickness Of
Cylindrical Section Of The
Shell, mm
Longitudinal Stress,
55.0
N/mm2
Circumferential Stress
27.5
N/mm2
Minimum Thickness Of
Tube Wall, mm
Minimum Thickness Of
Head And Closure,
5.91
8.80
Minimum Thickness Of
The Channel Cover,mm
33.0
Design Load, kN
Diameter pipe for
isobutene inlet and outlet,
mm
Diameter pipe for steam
inlet and outlet, mm
Vessel diameter, m
Types of baffles
Number of baffle
segmental, Nb
56.38
100
500
0.9
transverse
8
REFERENCES
D. Brian Spalding, J.Taborek, Heat Exchanger Design Handbook, Volume 1
- Heat Exchanger Theory, Hemisphere Publishing Corporation, Washington, New
York, London, 1983.
283
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
D. Brian Spalding, J.Taborek, Heat Exchanger Design Handbook, Volume 2
-
Fluid Mechanics and Heat Transfer, Hemisphere Publishing Corporation,
Washington, New York, London, 1983.
J. M. Chenoweth, D. Chisholm, R. C. Cowie, D. Harris, A. Illingworth, J. F.
Lancaster, M. Morris, I. Murray, C. North, C. Ruiz, E. A. D. Sauders, K. V. Shipes, J.
Dennis Usher, R. L. Webb, Heat Exchanger Design Handbook, Volume 4 Mechanical Design of Heat Exchangers, Hemisphere Publishing Corporation 1983,
Washington, New York, London, 1983.
D. K. Edwards, P. E. Liley, R. N. Maddox, Robert Matavosian, S. F. Pugh, M.
Schunk, K. Schwier, Z. P. Shulman, Heat Exchanger Design Handbook, Volume 5
- Physical Properties, Hemisphere Publishing Corporation 1983, Washington, New
York, London, 1983.
E. A. D. Saunders, B. Sc. C. Eng., M. I. Mech. E. Heat Exchangers,
Selection, Design and Construction, Longman Scientific and Technical, 1998.
Yokell, Stanley, A working Guide to Shell and Tube Heat Exchangers,
McGraw-Hill Publishing Company, 1990.
Sadik Kakac, Hongtan Cin, Heat Exchangers, Selection, Rating, and
Thermal Design, CRC Press, Boca Raton, Boston London New York, Washington,
D.C, 1998.
Gupta, J. P., Working With Heat Exchangers: question and answers,
Hemisphere Publishing Corporation, A member of the Taylor and Francis Group, 1990.
Warren D. Seider, J.D. Seider, Daniel R. Lewin, Process Design Principles,
Synthesis, Analysis and Evaluation, 1997.
Stanley M. Wales, Chemical Process Equipment, Selection and Design ,
Butterworths Series in Chemical Engineering, 1990.
Frank P. Incropera, David P. Dewitt, Fundamentals of Heat and mass
Transfer, 5th Edition, John Wiley & Sons, Inc, 2002.
Holman, J.P, Heat Transfer, 7th Edition, McGraw-Hill, Inc, 1992.
Kern. D.Q, Process Heat Transfer, International Editions, McGraw-Hill, Inc,
1965.
Perry R.H and Green, Don, Perrys Chemical Engineers Handbook, 7th
Edition, McGraw-Hill Book Company, Singapore, 1984.
Bhattacharya,
B.B,
Introduction
to
Chemical
Equipment
Design,
Mechanical Aspects, Indian Institute of Technology, 1976.
284
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Holman, J.P. Heat Transfer, 7th Edition, McGraw-Hill, Inc, 1992.
Kern. D. Q Process Heat Transfer, International Editions, McGraw-Hill, Inc,
1965.
Perry R.H and Green, Don, Perrys Chemical Engineers Handbook, 7th
Edition, McGraw-Hill Book Company, Singapore, 1984.
Sinnott, R. K., Coulson & Richardson, Chemical Engineering Volume 6,
Chemical Engineering Design, Butterworth Heinemann, 1999.
Carl R. Branon, Rules Of Thumb For Chemical Engineers, Gulf Publishing
Company, 1994.
Perry R.H and Green, Don, Perrys Chemical Engineers Handbook, 7th
Edition, McGraw-Hill Book Company, Singapore, 1984.
Sinnott, R. K., Coulson & Richardson, Chemical Engineering Volume 6,
Chemical Engineering Design, Butterworth Heinemann, 1999.
Peters, max Stone, Plant Design and Economics for Chemical Engineers,
2nd Edition, McGraw-Hill Chemical Engineering Series, 1968.
CHAPTER 2
285
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
PROCESS CONTROL AND INSTRUMENTATION
2.1
INTRODUCTION
All processes are subject to disturbances that tend to change operating conditions,
compositions and physical properties of the streams. In order to minimize those ill
effects that could result from such disturbances, chemical plants are implemented with
substantial amounts of instrumentation and automatic control equipment. In critical
cases and in especially large plants, moreover, the instrumentation is computer
monitors for convenient, safety and optimization.
A chemical plant is an arrangement of processing unit. The plant overall
objective is to convert the raw materials into desired product using available sources of
energy in the most economical way. All operating unit should be monitored. Methods of
limiting hazard levels by control features include sensoring control on limits and various
aspects of sequential and continuous monitoring.
In control situations, the demand for speed of response may not be realizable
with an overly elaborate mathematical system. Moreover, in practice, not all
disturbances are measurable and the process characteristics are not known exactly.
Accordingly, feedforward control is supplemented in most instances with feedback. In a
well-designed system, typically, 90% of the corrective action is provided by feed
forward and 10% by feedback with the result that the integrated error is reduces by a
factor 10%. The main types of instrument used for chemical process plants are flow
controller, temperature controller, pressure controller and level controller.
2.2
OBJECTIVES OF CONTROLL
The most important objectives of the designer when specifying control and
instrumentation schemes are:
1.
Safe Plant Operation
286
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
To keep the process variables within known safety operating limits and
within allowable limits.
To detect dangerous situations as they develop and to provide alarms
and automatic shut down system.
To provide interlocks and alarms to prevent unsafe operating
procedures.
2.
Production Specification
To achieve the design product output
To produce the desired quality of final product
To keep the product composition within the specified quality
standards.
3.
Economics
To operate at the lowest production cost, commensurate with the other
objectives.
The operation of the plant must conform to the market condition, which
is availability of raw materials and demand of the final product.
4.
Environmental Regulations
Variable controlled must not exceed the allowable limits set by various
federal and state laws.
5.
Operational Constraint
Various type of equipments used in chemical plant have constrains
inherent to their operation. Such constraints should be satisfied
throughout the operation of plant.
Control systems are needed to satisfy all these operational constrains.
In a typical chemical processing plant these objectives are achieve by combination
of automatic control, manual monitoring and laboratory analysis.
2.3
CONTROL SYSTEM DESIGN SHEET
2.3.1
Heat Exchanger (E-100)
287
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 2.1: Control Scheme for the Heat Exchanger
Table 2.1: Parameter at Heat Exchanger
Intention: To Heat Up the reactant Before Entering Reactor
Objective : To heat up the reactants to 250 oC
Objective
Measurable
Disturbances
Action
Variable
1. To control
Temperature at Change in
Control temperature
temperature of outlet stream
flow rate of the heating by V3 at
outlet stream
S4
feed
steam inlet S3
2.3.2
Set Point
E-100
Temperature
250oC
Catalytic Cracking Fluidized Bed Reactor (op-100)
288
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 2.2: Control Scheme for Catalytic Cracking Fluidized Bed Reactor
Table 2.2: Parameter at Catalytic Cracking Fluidized Bed Reactor
Intention: To Separate Vapor and Liquid from Stream Leaving Heat Exchanger
Set Point
Objective
Measurable
Disturbances
Action
1. To control flow
inside phase
reactor
2. To control solid
flow to relate
speed and flow rate
3. To control
pressure between
reactor and
regenerator
4. To control
temperature
in reactor
2.3.3
Variable
Flow of gas
in phase to
reactor
Solid in and
out in the
reactor and
regenerator
flow rate of
feed into reactor
and regenerator
Temperature in
reactor and
regenerator
Change in flow
of gas in phase
of reactor
Change of solid
flow rate moving
to reactor and
regenerator
Change of feed
flow rate
Control flowrate of the
Input stream S4 by
controlling V3
Control solid in and
solid out in the
reactor and
regenerator
Control pressure by
opening or closing
valve by adjusting V5
Stream S4
Change of
temperature
In reactor and
regenerator
Control temperature
by opening or closing
valve at the air feed
V4 and product V6
180oC
95679.8kg
2.89 bar
Control at Compressor (C-101)
S7
289
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
S8
Figure 2.3: Control Scheme for the Compressor
Table 2.3: Parameter at Compressor
Objective
To maintain
pressure inside
compressor
2.3.4
Intention: To maintain the pressure inside the compressor
Objective : To keep pressure maintain at bar
Measurable
Disturbances
Action
Variable
Pressure
Power or duty
Control pressure
inside
of the
by adjusting V8 at
compressor
compressor
steam inlet S7
Set Point
C-101
pressure
bar
Control at Condenser
290
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 2.4: Control Scheme for the Condenser
Table 2.4: Parameter at Condenser
Intention: To maintain the pressure inside the compressor
Objective : To keep pressure maintain at bar
Objective
Measurable
Disturbances
Action
Variable
1. To control
Temperature at Change in
Control temperature
temperature of outlet stream
flow rate of the heating by V9 at
outlet stream
S9
feed
steam inlet S8
Set Point
E-100
Temperature
50oC
2.3.5 Separator (V-100)
291
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 2.5: Control Scheme for the Separator
Table 2.5: Parameter at Separator
Intention: To Separate Vapor and Liquid from Stream Leaving Heat Exchanger
Objective
Measurable
Disturbances
Action
Set Point
Variable
1. To control level
Level of liquid Change in level
Control level by
Stream
inside phase
in phase
of liquid in phase V10 at inlet stream
S9 of
separator
separator
separator
S9
liquid height
1. To control
Maintain
Change in
Control pressure by
Pressure
pressure
pressure
pressure
opening and closing 0.5 bar
phase separator in phase
of liquid in phase valve by V28 at
separator
separator
stream S10
1. To control
Flow rate at
Change in the
Control Flow at the
Flow into
Flow rate
the bottom of
flowrate of
bottom by V27 at
The feed
inside phase
the separator the separator
stream S11
At stream
separator
S11
292
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
2.3.6 Fixed Bed Reactor (R-101)
V29
V13
Figure 2.6: Control Scheme for the Fixed Bed Reactor
Table 2.6: Parameter at Fixed Bed Reactor
Objective
Intention: To React Isobutene with Methanol to Produce MTBE
Measurable
Disturbances
Action
Set Point
To regulate
Variable
Cooling
Reactant feed
Control temperature
Set the
reactor
water make
temperature and
by V13
temperature
temperature
To maintain
up rate
Pressure in
composition
Pressure feed to
Control the pressure
at 53.3 oC
Pressure at
constant
the fed of
the reactor
in the reactor by
1 bar
pressure at the
stream S15
control of V29
feed stream
2.3.7
Distillation Column (T-101)
293
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 2.7: Control Scheme for the MTBE Distillation Column
Table 2.7: Parameter at MTBE Distillation Column
Intention: To Separate MTBE from Mixture Leaving Reactor
Objective
Measurable
Disturbances
Action
Variable
1. To control level Level of liquid Change of level Control level by
inside column
in column
of column
Adjusting V17
valve at raffinate outlet
Stream S19
2. To control
Temperature Change of
Control temperature by
temperature
inside column temperature
Control V16
inside column
in column
3. To control
Level in drum Change of level Control level by V15
level in drum
in drum
to maintain the
Product output
2.3.8
Set Point
Stream S19
at
15251.9kg/hr
61.2oC
Stream
S17 at
38747.97kg/hr
Liquid-Liquid Extraction Column (T-100)
294
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 2.8: Control Scheme for the Liquid-liquid Extraction Column
Table 2.8: Parameter at Liquid-liquid Extraction Column
Objective
To control flow
at stream S20
To
control
flow
at
Intention: To Extract Methanol and Water from Hydrocarbon
Measurable
Disturbances
Action
Variable
flow of liquid
Change of level
Control flow by adjust
in column
of column
Valve V19 at bottom
outlet stream
Flow of liquid in Change of flow of Control at V20 to keep
stream S21
To control level at the
column
Level of
raffinate
Set Point
Stream
S20 at
40.32kg/hr
Flow inlet
at
liquid extraction
Change of level in
the flow inlet maintain
Maintain the level by
stream S21
Level output at
going out of the
the column
control V21 at stream
raffinate section
Product of S24
To control the density
column
Density level
Change of the
S24
The bottom stream
S24
Interfacial level
intermediate between
change between
removed by control
at bottom
methanol and water
water and
between two
V22
product at
methanol
phase
2.3.9
liquid
interfacial of level
stream S26
Distillation Column (T-102)
295
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Figure 2.9: Control Scheme for the Distillation Column
Table 2.9: Parameter at Distillation Column
Objective
1. To control level
inside column
2. To control
temperature
inside column
3. To control
level in drum
Intention: To Separate Methanol and water
Measurable
Disturbances
Action
Variable
Level of liquid Change of level Control level by
in column
of column
opening and closing
valve V25 at bottom
outlet stream
Temperature Change of
Control temperature by
inside column temperature
opening or closing
in column
Valve 24 at top outlet
stream
Level in drum Change of level Control level by V23
in drum
opening or closing
valve at reflux stream
Set Point
Stream
S28 : at
63.4453kg/hr
62.17oC
Stream
S27
296
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
2.3.10 Mixer (MIX-100)
Figure 2.10: Control Scheme for the Mixer
Table 2.10: Parameter at Mixer
Intention: To Mix Methanol from Recycle and Make Up Methanol
Measurable
Disturbances
Action
Set Point
Variable
1. To control the
Flowrate of
Change of
Control flowrate
M - 101
flowrate
the reactant
flowrate to the
bypass valve
Stream S14
reactor
V12
at 2.88 m3/hr
Objective
297
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
2.3.11 Expander (EX-100)
Figure 2.11: Control Scheme for the Expander
Table 2.11: Parameter for Expander
Intention: To Expand the Pressure Leaving the Reactor
Measurable
Disturbances
Action
Variable
1. To expand the
Pressure
Change of
Control pressure by
pressure
inside the
pressure
adjusting valve V14
expander
Objective
Set Point
Pressure
0.45
38
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
Figure 2.13 : PFD Diagram Before Control
39
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
Legend
TC
Process Flow
PC
Pressure Control
Control Flow
FC
Flow Control
LC
ILC -
Temperature Control
TI
Temperature Indicator
DPC -
Interfacial Level Control
Level control
Differential Pressure Control
Figure 2.13 : Process Control P and ID Diagram
40
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
41
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
REFERENCES
Luyben, W. l., (1990), Process Modelling simulation and Control for Chemical
Engineers, 2nd Edition, McGraw-Hill Chemical Engineering Series
Ogunnaike, B. A. and Ray, W. H. (1994), Process Dynamics, Modeling and
Control, New York, Pxford.
R.K.Sinnott, 1999.Chemical Engineering Design, Coulson & Richardson Chemical
Engineering .3rd Edition. Volume 6 .Britain. Butterworth Heinemann
J.R Backhurst & J.H Harker.1987.Chemical Engineering Design, Coulson &
Richardson Chemical Engineering .3rd Edition. Volume 2 .United Kingdom.
Pergamon Press.
Stanley M. Walas. 1988. Chemical Process Equipment Selection and Design.
United State of America. Butterworths Series in Chemical Engineering.
Chemical Engineering Progress, February 2001, American Institute of Chemical
Engineering.
42
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
CHAPTER 3
SAFETY CONSIDERATION
3.1 INTRODUCTION
Any organization has a legal and moral obligation to safe guard the health and
welfare of its employees and the public. Safety is also good business: the good
management practices needed to ensure safe operation would also ensure efficient
operation.
All manufacturing processes there are additional, special, hazard associated
with the chemical used and the process condition. The designer must be aware of
these hazards and ensure through the application of sound engineering practices
that the risks are reduced to acceptable levels.
Safety and loss prevention in process design can be considered under the
following broad headings :( Coulson and Richardsons Volume 6)
1. Identification and assessment of the hazards
2. Control of the hazards; for example by containment of flammable and toxic
materials
3. Control the process. Prevention of hazardous deviation in the process
variables, (pressure, temperature, flow) by provision of automatic control
system interlocks, alarms, trips, together with good operating practices and
management.
4. Limitation of the loss. The damage and injury caused if an incident occurs,
pressure relief, plant layout, provision of fire-fighting equipment.
In Malaysia, The Occupational Safety and Health Act, 1994 is a tool which
provided a new legal and administrative as a driving force to promote, encourage
and stimulate the high quality standards of health and safety at work place. Both
parties such as employers and employees must give their support and cooperate to
43
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
comply the law and not misuse safety in order to increasing the promotion of safety
awareness and effective safety organization and performance in companies.
3.2 HAZARD AND OPERABILITY STUDY
HAZOP stands for hazard and operability studies. This is asset of formal hazard
identification and elimination procedures designed to identify hazard to people,
process plants and the environment. The techniques aim to stimulate in a
systematic way the imagination of designers and people who operate plants or
equipment so they can identify potential hazard. In effect, HAZOP studies make the
assumption that hazard or operating problem can arise when there is a deviation
from the design or operating intention. Corrective actions can then be made before
a real accident occurs.
The primary goal in performing a HAZOP study is to identify, not analyse or
quantify, the hazard process. The end product of a study is a list of concerns and
recommendation for prevention of the problem, not an analysis of the occurrence,
frequency, overall effects, and the definite solution. If HAZOP is started too late in a
project, it can lose effectiveness because:
1. There may be a tendency not to challenge an already existing design.
2. Changes may come too late, possibly requiring redesign of the process.
3. There may be loss of operability and design decision data used to generate
the design.
HAZOP is a formal procedure that offers a great potential to improve the
safety, reliability and operability of process plants by recognizing and eliminating
potential problems at the design stage. It is not limited to the design stage,
however. It can be applied anywhere that a design intention. (Perrys Handbook,
1998)
When using the operability study technique to vet a process design, the
action to be taken to deal with a potential hazard will often be modification to the
control system and instrumentation, the inclusion of additional alarms, trips or
interlock. If major hazard are identified, major design changes may be necessary,
alternatives processes, material and equipment. In order to have a safe process
successfully producing to specification to the required product, a sound control
system is necessary but not sufficient. (Coulson & Richardsons, 1999).
44
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
The objectives of HAZOP study are:
1. To identify areas of the design that may posses a significant hazard
potential
2. To identify and study features of the design that influences the probability of
a hazardous incident occurring.
3. To familiarize the study team with the design information available.
4. To ensure that a systematic study is made of the areas of significant hazard
potential.
5. To identify design information not currently available to the team.
6. To provide a mechanism for feedback to the client of the study teams
detailed comments. (Sydney Lipton and Jeremiah Lynch, 1994)
The advantages of HAZOP study to the design application:
Early identification of problems areas when conceptual design stage.
Identifies need for emergency procedures to mitigate.
Provide essential information for safety case, such as on the hazards
identified and effectiveness of safety systems.
Through examination of hazard and operability problems when applied at
detailed stage.
Meets legislative requirements.
Identifies need for commissioning, operating and maintenance procedures
for safe and reliable operations.
45
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
Table 3.1: A HAZOP study contains the following important features:
Features :
Intention
Guide words
Deviations
Causes
Consequences
Action
Meaning :
Defines how the part or process is expected to
operate.
Simple word used to qualify the intention in order to
guide and stimulate creative thinking.
Departures from the intention discovered by
systematic application of guide words.
Reasons that deviations might occur.
Results of deviations if they occur.
Prevention, mitigation and control
-
Prevent causes.
Mitigate the consequences.
Control action such as provide alarms to indicate
things getting out of control: define control actions
to get back into control.
The MTBE Plant HAZOP Study is included at Appendix: Safety.
3.3
PLANT START UP AND SHUT DOWN PROCEDURE
Safe procedures must be well known for the start up and shut down of plant and
deviations from normal operating conditions. Whenever process conditions are
changed, opportunities are presented for hazardous situations to arise. Building up
the process consistency may reduce the investigating breakdown and malfunction,
availability, the design cycle, operability, flexibility including blending and recycling
experience and known how personnel.
The start-up and shutdown of the plant must proceed safely and easily, yet
be flexible enough to be carried out in several ways. The operating limits of the
plant must not exceed and dangerous mixtures must not be formed because of
abnormal states of concentration, temperature, phase, reactant, catalyst and
products.
During start-up, the catalyst in the reactor should be activated and
sufficiently warm for reaction to begin when the flow of reactant is started.
Contaminants often enter the system at this stage. Materials are added by
operations such as purging, drying and flushing. Water and other materials may
46
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
condense in unplanned location causing process levels to get out of control and
other problems. The ignition of fuel to heat the high temperature fluid during start-up
must be designed to accomplish this safely Some typical errors that could occur
during start-up of the plant include :
1. Wrong routing, involving failure to ensure that correct valves are closed.
This is especially crucial in the adsorption section where three different
processes are occurring at any one time.
2. Drain valves are left open resulting in loss of material and possibly
endangering the lives of workers.
3. Valves left closed resulting in over pressure in the vessel.
4. Failure to complete purging cycle before admission of fuel air mixture.
5. Backflow of material from high pressure to low pressure system.
6. Setting of wrong valves for operating parameters such as jacket
temperature in the reactor and reflux in the distillation column.
3.3.1
Normal Start up and Shut down the Plant
The study of the plant start up and shut down must include investigation of the
operating limits, transient operating conditions, process dynamics, contamination
and added material, emissions, hot standby and emergency shut down with plant
protection control systems and alarms.
3.3.1.1 Operating Limits
The operating limits of the plant are imposing by mechanical, electrical, civil and
process design. Where necessary, it has to be introduce additional equipment,
sampling points, instrumentation and lines, and identify their use on the engineering
line diagram,
3.3.1.2 Transient Operating and Process Dynamic
The transient operating conditions must be studied to safe time and operating cast.
The process dynamics that to be investigated includes excessive heat transfer
47
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
when more or less floe in either energy exchanging streams through activations and
warming of catalyst when the flow of reactant starts to entrance of contamination
and others
3.3.1.3 Added Materials
Materials are added by operations may not be tolerated in part of the system or
clean material may be needed for the start up of the plant. Residues or unwanted
products such as out of specification may be discharged or hold in tank for further
treatment.
3.3.1.4 Hot Standby
Time is save during restart up if plant are kept partially working such as when other
unit operation are ceased to function temporarily, the converter can be left in hot
standby conditions.
3.3.1.5 Emergency Shut Down
Special plant protection known as process trip system or emergency shut down
system is design to affect the emergency shut down through the push button by
operator or from automatic activation of a relay when necessary. The trip systems
should be reliable and operate when required to avoid a nuisance shut down of
plant.
3.3.2
Start up and Shut down Procedure for the Main Equipment
3.3.2.1 Reactor
1. It is recommended that the internal reactor vessel measurements (ID, Bed
Depth not the overall vessel height, etc.) be verified, so that product
loading is consistent with the "Estimated Performance Sheet" (EPS).
2. Prior to any loading, it is necessary to make an internal and external
inspection of the reactor vessel. In other words, there should not be any
pipes or hollow devices in the vessel, which could allow the gas to travel
without contacting the product.
48
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
3. There are two vessel types of bed supports that can be used; (1) has a
support grid permanently installed about 2 inches below the throat of the
lower manway; or (2) uses a level bed of washed gravel, ceramic balls, or
pawl rings. The bed support must be leveled.
4. Close and secure the bottom manway.
5. Through the top manway, load the remaining feed to level as stated in the
EPS. During the latter stages of loading level off the cone of the filled
product bed and continue loading until finished.
6. Close and secure the top manway.
Upon operational start-up, record the required measurements temperature,
pressure, and flow rate - from each bed (if applicable). This data should be kept on
some routine basis (daily, weekly bi-weekly, etc.) so that any problems that might
develop can be identified and corrected.
3.3.2.2 Distillation Column
Start-Up Procedure
1. Turn the switch box indicator to Distillation Control setting.
2. Switch the column power source lever to the "on" position. Turn the Reboiler
Heater Control knob clockwise. This prevents over heating of the reboiler.
3. Turn on the cold water supply ( CWS )valve until the computer stops telling
the user to increase the volume of the CWS valve.
4.
Adjust the Reflux Control to the desired setting.
5.
Assure all computer settings are as desired.
6. Allow the tray temperatures to reach a steady-state value.
7. Turn on the feed and reboiler pumps as applicable. The pump settings can
be adjusted on the computer.
Caution: DO NOT ALLOW THE WATER LEVEL TO FALL BELOW THE
CALROD HEATERS.
49
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
This will result in damage to the heaters. If the heaters are exposed turn off the
column, this allows the vapor in the column to condense and return to the
reboiler. If the liquid level is still below the heaters, more liquid must be added to
the reboiler.
Shut-Down Procedure
1. Turn the Reboiler Heater Control knob to zero.
2. Turn off the pumps (feed and reboiler).
3. Turn off the CWS valve when the temperature of the distillate is below the
boiling point of the light component of the mixture.
4.
Press the stop button.
5. Shut off the computer, by selecting the "Shut-down" option from the Special
menu.
3.3.2.3 Liquid-Liquid Extraction Column
Start Up Procedure
1. Check to see that all the drainage valves are closed.
2. Check to be sure the top water vent valve is open.
3. When the liquid level in the column reaches the top right nozzle(water in
nozzle), turn the water flowrate down to the desired setpoint. Turn on and
set the feed flowrate to the desired setpoint by adjusting the pump speed,
and close the top water vent.
4. Allow the interface to form between the top mesh and the top left nozzle.
5. Small adjustments should be made in order to keep the interface constant.
Shut down procedure
1. Turn off all inlet flowrates on the right control panel.
2. Shut off the stirrer on the right control panel.
50
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
3. Open the top water in vent valve.
4. Open the bottom centre black valve to drain the column.
3.3.2.4 Heat Exchanger
Start up Procedure
When putting a heat exchanger in operation, open the vent connections and
slowly start to circulate the cold medium only. Be sure that the entire cold side
of the exchanger is completely flooded before closing its vents. The hot medium
should then be gradually introduced until all passages are filled with fluid. Then
close the hot side vents and slowly bring the unit up to its operating
temperature.
Shut down Procedure
When heat exchanger is required to be shutdown, the hot fluid should be turned
off first. If it is necessary to stop the circulation of the cold fluid, the hot medium
should also be stopped by by-passing the heat exchanger.
3.4
EMERGENCY RESPONSE PLAN (ERP)
It is expected that every person who working in this plant will act responsibly in any
Department of Emergency. ERP procedures were state in Appendix: Safety. In most cases,
the observer of an emergency is faced with decision to leave the scene to summon help or
stay and provide help. The basic rule is as follows.
Unless we are sure that we are not putting ourselves in any danger and we know we can
make a difference, summon help.
3.4.1
Emergency Response Procedures
General Procedures
51
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
When alarm system is activated whether by break glass or by smoke detector, the
alarm that is siren sound will be triggered throughout plant. The following general
procedures should be followed if there is no immediate emergency in your area.
Do not panic and stay alert:
Stay calm and be alert and ready to response to any emergency according
to the
Plant Emergency Organization Programmed. The shift manager should go
to the security guard to identify the location of the alarm.
Access the situation around your area :
Look around and ensure that your immediate area is not in danger or affected by
the emergency. Stop all contractors from working immediately.
Wait for instruction from Supervisor/Shift Manager :
Do no evacuate from ones post unless one is immediate danger or when instructed
by ones supervisor or the shift manager.
Avoid using the telephone :
Do not use the telephone unless it is absolutely necessary, as the telephone lines
must be reserved for calling emergency services. All incidents resulting in
injuries, property damage and/ or productions loss shall be investigated and
reported for within 24 hours. Corrective action to prevent the recurrence of
the incident shall be initiated 48 hours.
3.4.2
Evacuation Procedures:
Exit building via the stairways :
It takes time to the person familiarize with evacuation routes in advance.
The maps showing the location of all emergency exits and extinguishers are
posted on all floors.
Assist the injured when possible :
Do not move the seriously injured unless there is danger of further injury. If it
is necessary to leave someone in the building, try to leave him in a relatively
secure place (example, the stairwell is one of the safer places to be in fire).
After someone has been evacuated the building, find the proper officials and
report the location and condition of persons who need assistance.
Designed persons are responsible for clearing the production floors and
offices :
52
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
Efforts to clear the production floor and offices should be limited to 5
minutes. As the areas are cleared, all doors should be closed.
Once outside the building :
Keep at least 50 feet away from the building to avoid danger from falling
glass, example Evacuate to the Emergency Assembly Area .
Do not re enter the building until safety officer or fire personnel have been
determined that it is safe.
3.4.3
Fires:
If the fire alarm sounds or a fire broke out in the plant, turn off any electrical
equipment that been operating and evacuate the building immediately.
Close all doors to help prevent fires from spreading. Exit via stairwells.
Call 994 to give location and extent of fire and notify the management to
report the fire :
State if there are any special circumstances, such as the presence of
dangerous chemicals.
Dont attempt to fight a fire unless we have been trained in fire extinguisher
use and the fire is very small :
When fighting a fire, always position ourselves between the exit and the fire
to ensure an escape route. IF THE FIRE CANNOT BE CONTAINED, GET
OUT QUICKLY!
3.4.4
Explosion, Line Rupture or Serious Leak
Do the following if possible.
1. Turn the Emergency Stop switch to Off position
2. Isolate the effected area of the limit. Block in high-pressure source
as required accomplishing this.
3. Complete the shut down
3.4.5
Other Emergencies :
Injuries :
53
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
For life threatening emergencies, CALL 991 for medical aid and for
transportation to hospital and at same time notify the management as listed
on the Notification Lists. For less serious injuries or illness, first aid can be
obtained at the nearest clinic. Report all injuries to the management.
Equipment failures :
Any equipment failures, which may harm or injured the workers, should be
reported to the Production Supervisors or the management for further action
to be taken.
3.5
PLANT LAYOUT
The process units and ancillary buildings should be laid out to give the most
economical flow of material and personnel around the site. Hazardous process
must be located at a safe distance from other buildings. Consideration must be also
being given to the future expansion of the site. The ancillary buildings and services
required on site, in addition to the main processing units (building) will include:
(Coulson and Richardsons 1999)
1. Storage for raw materials and products; tank farms and warehouses
2. Maintenance workshop
3. Stores, for maintenance and operating supplies
4. Laboratories for process control
5. Fire stations and other emergency services
6. Utilities: steam boilers, compressed air, power generation, refrigeration,
transformer stations.
7. Effluent disposal plant
8. Offices for general administration
9. Canteens and other amenity buildings, such as medical centres
10. Car parks
Normally, the process units will be sited first and arranged to give a smooth
flow of materials through various processing steps, from raw material to final
product storage. It is normally spaced at least 30 apart and for hazardous
processes, the greater spacing may be needed. Then, the principal ancillary
buildings to be located and arranged in order to minimize the time spent by the
personnel in travelling between the buildings. The administration offices and
54
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
laboratory should be located well away from potentially hazardous processes since
a many people be in here. The control rooms normally are located adjacent to the
process units but if it with the potentially hazardous processes has to be sited at
safer distance.
Besides that, the layout of the plant roads, pipe alleys and drains also must
be considered to locate the main process units. Easy access roads will be needed
to each building for construction and for operation and maintenance. The utilities
buildings should be sited to give most economical run of pipes to and from the
process units. Finally, the main storage areas should be placed between loading
and unloading facilities and the process units they serve. Storage tanks containing
hazardous materials should be sited at least 70 m (200 ft) from the site boundary.
There are 7 principal factors to be considered :
1. Economic considerations: construction and operating costs.
2. The process requirements.
3. Convenience of operation.
4. Convenience of maintenance.
5. Safety.
6. Future expansion.
7. Modular construction.
The MTBE site layout have shown in Figure 3.1.
55
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
Figure 3.1 Methyl tert-Butyl Ether (MTBE) Plan Layout
U
56
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
Figure 3.2: Methyl tert-Butyl Ether (MTBE) Plant Evacuation Routes
Legand
Evacuation Routes
57
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
Figure 3.3 PID before HAZOP
58
PRODUCTION OF 300,000 METRIC TON MTBE PER YEAR
Figure 3.4 PID after HAZOP
59
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
REFERENCES
Clarles A.Wentz, 1998. Safety, Health and Environmental Protection. United State of
America:McGraw-Hill.
R.K.Sinnot, 1999. Chemical Engineering Design. Coulson & Richardsons Chemical
Engineering 3rd Edition. Volume 6. Britain; Butterworth Heinemann.
Robert H. Perry, Don W. Green, 1998 Perrys Chemical Engineers Handbook, Seventh
Edition, McGraw-Hill.
http://www.sulfatreat.com/Documents/HTML/St/startup.html
http://www.amberjet.com/IP/start_up.htm
http://www.sulfatreat.com/Documents/PDF/SulfaTreat/ST-Start_Up_Procedure.pdf
http://chem.engr.utc.edu/Webres/435F/Proc.htm
http://www.eng.buffalo.edu/Courses/ce428/Distillation/procedure.htm
http://users.rowan.edu/~savelski/uol/liqliq.html
http://pharmaflo.com/heatexch/
60
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CHAPTER 4
ECONOMIC EVALUATION
INTRODUCTION
Economic evaluation is very important for a proposed plant. We have to be able to
estimate and decide between alternative designs and for project evaluation. Chemical
plants are built to make a profit an estimate of the investment required and the cost of
production are needed before the profitability of a project can be assessed. The total
investment needed for a project is the sum of the fixed and working capital. Fixed
capital is the total cost of the plant ready for start up. It is the cost paid to the
contractors. Working capital is the additional investment needed, over and above the
fixed capital, to start the plant up and operate it to the point when income is earned.
Most of the working capital is recovered at the end of the project.
61
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.1.1 The Specification Of Plant
In this chapter, the costing of equipment which has been designed will be
estimated and the feasibility of MTBE production will be evaluated by
profitability analysis to make sure the project is economically attractive.
There are some general assumptions to this chapter;
i.
The plant life span is fifteen years.
ii.
The currency exchange rate of US dollar to Ringgit Malaysia is fixed at 3.8 as
fixed by Malaysian Government.
iii.
The price of raw materials, catalyst and product is fixed for the whole period of
operation.
Price of raw material
: Isobutane - RM 0.8094 / kg
: Methanol - RM 0.988 / kg
Price of product
: MTBE - RM 1.320 / kg
: TBA - RM 1.035 / kg
: DME - RM 0.655 / kg
62
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.1.2
Revenue From Sales
i.
MTBE
37878.76 kg/h =
300000 tonne
year
Price: RM 1.320/kg
=
37878 .76 kg 24 hr 365 day
RM 1.320
0.90
hr
day
year
kg
=
ii.
TBA
RM 394199710/yr
639.663 kg/h
Price: RM 1.035/kg
639 .663 kg 24 hr 365 day
RM 1.035
0.90
hr
day
year
kg
=
iii.
DME
RM 5219612/yr
1210.9868 kg/h
Price: RM 0.655/kg
1210 .9868 kg 24 hr 365 day
RM 0.655
0.90
hr
day
year
kg
=
iv.
Isobutane
RM 6253560/yr
13718.4558 kg/h
Price: RM 0.8094/kg
=
13718 .4558 kg 24 hr 365 day
RM 0.8094
0.90
hr
day
year
kg
RM
87541714/yr
v.
n-butane
157.412 kg/h
Price: RM 0.35/kg
Total revenue
157 .412 kg 24 hr 365 day
RM 0.35
0.90
hr
day
year
kg
RM 434363/yr
RM 493648959/yr
63
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.2 COST ESTIMATION
4.2.1 Capital cost estimation
Capital cost estimates are essentially paper and pencil studies. The cost of making
an estimate indicates the personnel hours required in order to complete the estimate.
The capital needed to supply the necessary manufacturing and plant facilities is called
the fixed capital investment (FC), while the additional investment needed for the plant
operation (for plant start-up and operation to the point when income is earned) form the
working capital (WC).
Capital cost estimates for chemical process plants are often based on an estimate of
the purchase cost of the major equipment items required for the process, the other
costs being estimated as factors of the equipment cost. The accuracy of this type of
estimate will depend on what stage the design has reached at the time estimate is
made and on the reliability of the data available on equipment cost.
The cost of the purchased equipment is used as the basis of the factorial method of cost
estimation and must be determined as accurately as possible. It should preferably be
based on recent prices paid for similar equipment.
Calculation of total module cost and gross roots cost (based on table 4.2)
CTC = CFC + CWC + CL
Where,
CTC
total capital cost
CFC
fixed capital cost
CWC
working capital cost
CL
cost of land & other non-depreciable costs
FP
Pressure factor to account for high pressure
FM
Material factor to account for material of constructions
CP
Purchase cost for base condition
FBM
Bare module cost factor
64
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CBM
Bare module equipment cost for base condition
CBM
Bare module equipment cost for actual condition
Total Module Cost, CTM
Grass Root Cost, CGR
1.18 (CBM )
1.18 (3223841)
RM 3804132 x 3.8
RM 14455703
CTM + 0.35 ( CBM)
=
=
14455703 + 0.35 (12659343) x 3.8
RM 31292629
Since, Grass Root Cost (CGR) is:
CGR
CFC + CL
Area for 1 lot of land = 200000 m2
The price of land is RM 60 per m2
CL
RM 12000000
CFC
CGR - CL
RM 31292629 - RM 12000000
RM 19292629
Working capital is the additional investment needed over and above the fixed capital to
start the plant up and operate it to the point when income is earned.
Working capital cost = 5% of fixed capital costs
CWC
5% fixed capital cost (CFC)
(Coulson & Richardson,
RM 964631
1990)
Thus,
Total capital cost (CTC)
CFC + CL + CWC
RM 19292629+ RM 12000000+ RM 964631
RM 32257260
65
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.2.2
Manufacturing Cost Estimation
The cost of associated with the day to day operation of a chemical plant must be
estimated before the economic feasibility of a proposed process can be assessed.
The equation below is used to evaluate the cost of manufacture:
Cost of manufacture (COM) =
Direct Manufacturing Cost (DMC) +
Fixed Manufacturing Cost (FMC) +
General Expenses (GE)
COM = 0.304FCI + 2.73COL + 1.23(CUT + CWT + CRM)
The cost of manufacturing (COM) can be determined when the following costs are
known or can be estimated:
1. Fixed Capital Investment (FCI): (CTM or CGR)
2. Cost of Operating Labor (COL)
3. Cost of Utilities (CUT)
4. Cost of Waste Treatment (CWT)
5. Cost of Raw Material (CRM)
4.2.2.1 Cost of Operating Labor (COL)
Table 4.1 Labor Cost
Equipment type
Heat exchangers
Reactor
Vessels
Pumps
Compressor
No of
Operators per shift per
Operator per
equipment
6
2
1
5
2
equipment
0.1
0.5
0.0
0.0
0.15
shift
0.6
1.0
0.0
0.0
0.3
66
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Towers
Waste Treatment
3
1
0.35
0.5
1.05
0.5
3.45
Since, a single operators works on the average 48 weeks (3 weeks time off for
vacation and sick leave) a year, five 8-hour shifts a week.
1 operator
48 week
5 shift
Year
week
240 shift
Year
Working days for MTBE plant is 7920 hour = 330 days
Operating shift per Year
330 days
3 operating
Year
days
990 operating shift
Year
Working days for MTBE plant is 7920 hour
So, the number operator needed
990 operating shift
Year
240 shift
Year
4.125 operators
Thus,
Operating Labor
4.125 operators x 3.45 operator per shift
14.23 operator
15 operator
A mechanical engineers maximum wages per year (MIDA 2002) RM 60,000.00
Thus,
Labor Cost (1996)
15 x RM 60,000.00
RM 900,000.00
67
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.2.2.2 Cost of Utilities (CUT)
Yearly costs
flowrate x costs x period x steam factor
Since, assuming the plants operating days per year = 330 days
So,
Steam factor (SF)
no . of day ' s plant operates per year
no . of days per year
330
= 0.90
365
1. Heater (E-100)
Duty
3109542
J s = 11 .16 G J hr
From table 8.5 (W.R Wan Daud, Princip Reka bentuk Proses Kimia, 2002, page
285) cost of heater RM 19.6 / GJ.
Thus ,
Yearly cost
= (Q) (C steam) (t)
= 11 .16
GJ
19 .6
hr
day
RM
24
365
0.90
hr
GJ
day
yr
= RM 1724515
2. Heater (E-101)
Duty
3423874
J s = 12 .24 G J hr
From table 8.5 (W.R Wan Daud, Princip Reka bentuk Proses Kimia, 2002, page
285) cost of cooling water RM 0.6 / GJ.
Thus ,
Yearly cost
= (Q) (C steam) (t)
= 12 .24
GJ
19 .6
hr
day
RM
24
365
0.90
hr
GJ
day
yr
= RM1891403
68
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3. Compressor (C-100)
Power (shaft)
137.73kW
Effeciency of drives, dr
92.28% (refer to table 3.7) Appendix
Electric Power, Pr
Power output
dr
137 .73
0.9228
149.25 kW
Yearly cost
149 .25 kW
0.228
hr
day
24
365
0.90
kWh
day
yr
= RM 268285
4. Cooler 1 (E-102)
Duty
= 4014590
KJ s = 14 .4 GJ hr
From table 8.5 (W.R Wan Daud, Princip Reka bentuk Proses Kimia, 2002, page
285) cost of cooling water RM 0.6 / GJ.
Thus ,
Yearly cost
= (Q) (C steam) (t)
= 14 .4
GJ
0.6
hr
day
RM
24
365
0.90
hr
GJ
day
yr
= RM 68118
5. Cooler 2 (E-103)
Duty
= 4204290
J s = 15 .12 G J hr
From table 8.5 (W.R Wan Daud, Prinsip Reka bentuk Proses Kimia, 2002, page
285) cost of cooling water RM 0.6 / GJ.
Thus ,
Yearly cost
= (Q) (C steam) (t)
= 15 .12
GJ
0.6
hr
day
RM
24
365
0.90
hr
GJ
day
yr
= RM71524
69
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
6. Cooler 3 (E-104)
Duty
J s = 1.8 G J hr
503240
From table 8.5 (W.R Wan Daud, Princip Reka bentuk Proses Kimia, 2002, page
285) cost of cooling water RM 0.6 / GJ.
Thus ,
Yearly cost
= (Q) (C steam) (t)
GJ
0.6
hr
day
= 1.8 hr RM GJ 24 day 365 yr 0.90
= RM 8514
7. Cooler 4 (E-105)
Duty
J s = 0.72 G J hr
240320
From table 8.5 (W.R Wan Daud, Princip Reka Bentuk Proses Kimia, 2002,
page 285) cost of cooling water RM 0.6 / GJ.
Thus ,
Yearly cost
= (Q) (C steam) (t)
= 0.72
GJ
0.6
hr
day
RM
24
365
0.90
hr
GJ
day
yr
= RM 3406
8. Pump 1(P100)
Power (shaft)
327.94kW
Effeciency of drives, dr
93.71% (refer to table 3.7) Appendix
Electric Power, Pr
Power output
dr
Yearly cost
349 .95 kW
327 .94
0.9371
349.95kW
0.228
hr
day
24
365
0.90
kWh
day
yr
= RM 629053
70
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
9. Pump 2(P101)
Power (shaft)
79.19kW
Effeciency of drives, dr
91.58% (refer to table 3.7) Appendix
Electric Power, Pr
Power output
dr
79 .19
0.9158
86.47kW
Yearly cost
= 86 .47 kW
0.228
hr
day
24
365
0.90
kWh
day
yr
= RM 155434
10. Pump 3(P102)
Power (shaft)
22.85kW
Effeciency of drives, dr
86.93% (refer to table 3.7) Appendix
Electric Power, Pr
Power output
dr
Yearly cost
22 .85
0.8693
26.29kW
= 26 .29 kW
0.228
hr
day
24
365
0.90
kWh
day
yr
= RM 47258
11. Pump 4(P103)
Power (shaft)
685.64kW
Effeciency of drives, dr
95.37% (refer to table 3.7) Appendix
Electric Power, Pr
Power output
dr
685 .64
0.9537
718.93kW
71
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Yearly cost
718 .93 kW
0.228
hr
day
24
365
0.90
kWh
day
yr
= RM 1292314
12. Pump 5(P104)
Power (shaft)
322.75kW
Effeciency of drives, dr
93.67% (refer to table 3.7) Appendix
Electric Power, Pr
Power output
dr
Yearly cost
344 .56 kW
322 .75
0.9367
344.56kW
0.228
hr
day
24
365
0.90
kWh
day
yr
= RM 619366
Total cost of utilities (CUT)
= RM 6779190
4.2.2.3Cost of Raw Material (CRM)
1.
Isobutane
39353 kg/h
Price RM 0.8094/kg
2.
Methanol
39353 kg 24 hr 365 day
RM 0.8094
0.90
hr
day
year
kg
RM 251123677/yr
15462 kg/hr
Price RM 0.988/kg (Chemical week, June 2003)
15462 kg
24 hr
365 day
RM 0.988
0.90
hr
day
year
kg
RM 120439579/yr
72
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
3.
Catalyst (Alumina silica)
95679 kg
RM 9.5 1 tonne
tonne
1000 kg
RM909
RM 251123677/yr + RM 120439579/yr
RM 371563256/yr
Total cost of catalyst (for 3 year)
RM 909
Total cost
RM 371564165
Total cost of raw material
The estimation of total manufacturing cost (with catalyst):
COM = 0.304FCI + 2.73COL + 1.23(CUT + CWT + CRM)
COM = 0.304 (31292629) + 2.73 (900000) + 1.23 (6779190 + 371564165)
COM = RM 477332286/yr
The estimation of total manufacturing cost (without catalyst):
COM = 0.304FCI + 2.73COL + 1.23(CUT + CWT + CRM)
COM = 0.304 (31292629) + 2.73 (900000) + 1.23 (6779190 + 371563256)
COM = RM 477331168/yr
73
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 4.2: Estimation Cost Of Purchased Equipment
Equipment
Capacity/Size
Operating
Pressure
0.0 barg
Area =94.33m2
(floating head)
Material Of
Construction
Carbon
Steel
Carbon
Steel
Tube-CS
Shell-CS
C100
W=137.73kW
C101
W = 22.85kW
E 100
Heat
Exchanger
E 101
Heat
exchanger
E 102
Heat
Exchanger
E 103
Heat
Exchanger
E 104
Heat
Exchanger
E 105
Heat
Exchanger
FP
Tube- 9 barg
Shell- 9 barg
1.0
1.0
Area =95.71m2
(floating head)
Tube-CS
Shell-CS
Tube- 9 barg
Shell- 9 barg
Area =94.33m2
(floating head)
Tube-CS
Shell-CS
Area =94.33m2
(floating head)
FM
FBM
Cp
CBM
2.2
16000
264000
3.0
3536676
10610028
1.0
1.0
3.2
3.2
15000
15000
48000
1.0
1.0
1.0
1.0
3.2
3.2
16000
16000
51200
Tube- 9 barg
Shell- 9 barg
1.0
1.0
1.0
1.0
3.2
3.2
15000
15000
48000
Tube-CS
Shell-CS
Tube- 9 barg
Shell- 9 barg
1.0
1.0
1.0
1.0
3.2
3.2
15000
15000
48000
Area =94.33m2
(floating head)
Tube-CS
Shell-CS
Tube- 9 barg
Shell- 9 barg
1.0
1.0
1.0
1.0
3.2
3.2
15000
15000
48000
Area =94.33m2
(floating head)
Tube-CS
Shell-CS
Tube- 9 barg
Shell- 9 barg
1.0
1.0
1.0
1.0
3.2
3.2
15000
15000
48000
0.1 barg
Co BM
74
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 4.2: Estimation Cost Of Purchased Equipment
Equipment
Capacity/Size
P100
W = 327.94kW
P101
P102
W = 79.19kW
W = 22.85kW
Material Of
Construction
Cass Steel
Cass Steel
Cass Steel
Operating
Pressure
3.5 barg
0.1 barg
0.0 barg
P103
W = 685.64kW
Cass Steel
1.50 barg
P104
W = 322.75kW
Cass Steel
0.0 barg
R100
R101
T100
Sieve tray
T101
T102
Sieve tray
V100
Total
D = 6.512
H = 18m
D = 1.814m
H = 4m
D = 0.765 m
H = 10.85
11 trays
D = 1.01 m
H =9 m
D = 0.765 m
H = 10.85
11 trays
Stainless Steel
1.75 barg
Carbon Steel
1.0 barg
Stainless
Steel
Stainless
Steel
Stainless
Steel
Stainless Steel
3.5 barg
1.75 barg
3.5 barg
FP
FM
FBM
Cp
1.0
1.0
1.0
1.0
1.0
1.0
1.0
1.0
1.0
1.0
1.3
1.0
1.0
1.0
1.2
1.0
1.8
1.0
1.8
1.0
1.8
1.0
1.8
1.0
1.8
1.0
4.0
1.0
1.0
1.0
4.0
1.0
4.518
3.31
4.518
3.31
4.518
3.31
4.518
3.31
4.518
3.31
11.5
4.2
4.2
4.2
11
4
2.0
1.0
2.0
1.2
11
4
2.0
1.0
36881
1.2
1.0
1.2
1.0
4.0
1.0
4.0
1.0
CBM
Co BM
333257
244152
8571
77448
56740
1429
12912
9460
52276
472366
346067
36593
330654
121123
200000
840000
230000
19000
79800
79800
40000
440000
160000
294
9702
5821
17000
178000
68000
40000
440000
160000
294
9702
5821
7131
12659343
3223841
75
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.3
PROFITABILITY ANALYSIS
Profitability is used as the general term for the measure of the amount of profit
that can be obtained from a given situation. Profitability therefore, is the common
denominator for all business activities.
The feasibility of MTBE production in Malaysia is evaluated by profitability analysis.
The profitability of the project will be the largest factor that makes a project
economically attractive.
To this stage, almost all the design and cost information
required for the profitability analysis were obtained.
Based on the information
available, the best methods assessing the profitability of alternatives are based on
projections of the cash flows during the project file.
A proposed capital investment / project and its associated expenditures can be
recovered by revenue (or savings) over time in addition to a return on the capital that is
sufficiently attractive in view of the risks involved of the potential alternatives uses.
There are 5 common methods in performing engineering economic analysis:
1) Present Worth (PW)
2) Future Worth (FW)
3) Annual Worth (AW)
4) Internal Rate Of Return (IRR)
5) Benefits / Cost Ratio (B/C)
4.3.1
Discounted Cash Flow
The economic feasibility of this plant is evaluated using the Discounted Cash Flow
Analysis (DCF), which is the most frequently used method of economic evaluation in
the chemical industry. This method measures the profitability of the project taking into
account the time value of money. From this method, the internal rate of return (IRR) of
the project can be determined which indicates the feasibility of the project. The value of
IRR is calculated using the information obtained in the sections.
76
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 4.3: Annual Cash Flow Before Tax
Year
Gross Income
(RM)
0
0
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
15
Annual
Expenses (RM)
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
-477332286
-477331168
-477331168
-477332286
-477331168
-477331168
-477332286
-477331168
-477331168
-477332286
-477331168
-477331168
-477332286
-477331168
-477331168
Estimated salvage value
Investment &
Salvage Value
(RM)
3
-19292629
-964631
-12000000
1929262.9
10%CFC
0.1 x RM 19292629
RM 1929262.9
Before Tax
Cash Flow
(RM)
(4)= (1)+(2)+(3)
-19292629
-964631
-12000000
16316673
16317791
16317791
16316673
16317791
16317791
16316673
16317791
16317791
16316673
16317791
16317791
16316673
16317791
16317791
1929262.9
(Coulson & Richardson, 1990)
77
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 4.4 : Annual Cash Flow After Tax
Taxes rate at 38% is chosen referring to reference from MIDA
Year
Gross
Income
(RM)
1
0
0
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
15
15
Expenses
(RM)
2
Investment
& Salvage
Value (RM)
3
Depreciation
Cost Basis *
MACRS
Rates
4
Taxable
Income (RM)
Taxes (RM)
Taxes rates
= 38%
After Tax
Cash Flow
(RM)
(5)=(1)+(2)(4)
(6)=(5)*0.38
2756916.69
4724764.84
3374280.81
2409649.36
1722831.77
1720902.50
1722831.77
860451.25
0
0
0
0
0
0
0
13559756.32
11593026.16
12943510.19
13907023.64
14594959.23
14596888.49
14593841.23
15457339.75
16317791
16316673
16317791
16317791
16316673
16317791
16317791
1929262.9
5152707.4
4405349.94
4918533.871
5284668.982
5546084.508
5546817.627
5545659.668
5873789.104
6200760.58
6200335.74
6200760.58
6200760.58
6200335.74
6200760.58
6200760.58
733119.902
(7)=(1)+(2)+
(3)-(6)
-19292629
-964631
-12000000
11163965.6
11912441.06
11399257.13
11032004.02
10771706.49
10770973.37
10771013.33
10444001.9
10117030.42
10116337.26
10117030.42
10117030.42
10116337.26
10117030.42
10117030.42
1929262.9
12964631
-19292629
-964631
-12000000
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
493648959
-477332286
-477331168
-477331168
-477332286
-477331168
-477331168
-477332286
-477331168
-477331168
-477332286
-477331168
-477331168
-477332286
-477331168
-477331168
1929262.9
12964631
Cumulative
Cash Flow
(RM)
-32257260
-21093294.4
-9180853.34
2218403.789
13250407.81
24022114.3
34793087.67
45564101
56008102.9
66125133.32
76241470.58
86358501
96475531.42
106591868.7
116708899.1
126825929.5
128755192.4
141719823.4
78
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.3.2 Net Present Value
4.3.2.1 Present Worth
MARR is the rate set by an organization to designate the lowest level that makes an
investment acceptable. For a risky investment, MARR should be set higher.
However, for public purpose (government, public utility), MARR is lower. For this
proposed plant, MARR that has been selected is 15%.
By using present worth, we can determine either this proposed plant is
profitable and acceptable or not. Table 4.4 shows the value of present
worth by using 15%, therefore this proposed plant is profitable
because the value of PW is > 0.
PW when MARR = 15%
Table 4.5: Present Worth Value
Year
0
0
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
15
PW
After Tax Cash
Flow (RM)
-19292629
-964631
-12000000
11163965.6
11912441.06
11399257.13
11032004.02
10771706.49
10770973.37
10771013.33
10444001.9
10117030.42
10116337.26
10117030.42
10117030.42
10116337.26
10117030.42
10117030.42
13697750.9
MARR = 15%
0.86957
0.75614
0.65752
0.57175
0.49718
0.43233
0.37594
0.3269
0.28426
0.24718
0.21494
0.18691
0.16253
0.14133
0.12289
0.12289
Present Worth
(PW), (RM)
-19292629
-964631
-12000000
9707849.57
9007473.18
7495239.55
6307548.3
5355477.03
4656614.92
4049254.75
3414144.22
2875867.07
2500556.24
2174554.52
1890974.16
1644208.29
1429839.91
1243281.87
1683316.61
33178940.2
MARR = 15%
PW = RM 33178940.2
This project is attractive and acceptable because PW > 0
79
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
4.3.3 Cumulative Cash Flow Diagram For The Evaluation Of A New
Project
Table 4.6: After Tax Cumulative Cash Flow
Year
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
15
After Tax Cash
Flow (RM)
-32257260
11163965.6
11912441.06
11399257.13
11032004.02
10771706.49
10770973.37
10771013.33
10444001.9
10117030.42
10116337.26
10117030.42
10117030.42
10116337.26
10117030.42
10117030.42
13697750.9
Cumulative Cash
Flow
-32257260
-21093294.4
-9180853.34
2218403.789
13250407.81
24022114.3
34793087.67
45564101
56008102.9
66125133.32
76241470.58
86358501
96475531.42
106591868.7
116708899.1
126825929.5
140523680.4
Cumulative Cash Flow vs Year
150000000
125000000
100000000
Cumulative 75000000
Cash Flow 50000000
25000000
(RM)
0
-25000000 0
-50000000
10
12
14
16
Year
Figure 4.1: Cumulative Cash Flow (RM) Versus Year
4.3.4
Rate Of Return (ROR)
4.3.4.1 Internal Rate Of Return
80
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
In theory, the Minimum Attractive Rate of Return (MARR) is chosen higher than the
rate expected from the bank or some safe investment that involved minimal
investment risk. The MARR for after taxes is selected at 15%. (Analysis, and
Design of Chemical Processes)
IRR is a method that produces an annual rate of profit, or return, resulting from an
investment and compared with the MARR. In determining the internal rate of return,
trial and error has been done based on the MARR that mentioned before. By trial
and error, the IRR for this plant has been found as 34.94%.
Table 4.7: Present Value (RM) when i = 30% and i = 40%
Year
0
0
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
15
PW
After Tax
Cash Flow
(RM)
-19292629
-964631
-12000000
11163965.6
11912441.06
11399257.13
11032004.02
10771706.49
10770973.37
10771013.33
10444001.9
10117030.42
10116337.26
10117030.42
10117030.42
10116337.26
10117030.42
10117030.42
13697750.9
i = 30%
0.76923
0.59172
0.45517
0.35013
0.26933
0.20718
0.15937
0.12259
0.0943
0.07254
0.0558
0.04292
0.03302
0.0254
0.01954
0.01954
Present
Value (RM)
i = 40%
-19292629
-964631
-12000000
8587657.26
7048829.62
5188599.87
3862635.57
2901143.71
2231530.26
1716576.39
1280330.19
954035.969
733839.105
564530.297
434222.946
334041.456
256972.573
197686.774
267654.053
4303026.05
0.71129
0.5102
0.36443
0.26031
0.18593
0.13281
0.09486
0.06776
0.0484
0.03457
0.02469
0.01764
0.0126
0.009
0.00643
0.00643
Present
Value (RM)
-19292629
-964631
-12000000
7940817.092
6077727.429
4154231.275
2871740.966
2002783.388
1430492.974
1021738.325
707685.5685
489664.2723
349721.7791
249789.4811
178464.4166
127465.8495
91053.27378
65052.5056
88076.5383
-4410754.867
By interpolation, IRR value is 34.94% when P is at 0 value.
IRR (34.94%) > MARR (15%). This project is profitable and acceptable.
4.3.5 Sensitivity Analysis
A sensitivity analysis is a way of examining the effects of uncertainties in the
forecasts on the viability of a project.
AW = PW (A/P, 15%, 15)
81
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
= 33178940.2 (0.17102)
= RM 5674262.32
FW = PW (F/P, 15%, 15)
= 33178940.2 (8.13706)
= RM 269979027.1
Table 4.8: Future Worth (RM) when MARR = 15%
Year
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
15
After Tax Cash
Flow (RM)
-32257260
11163965.6
11912441.06
11399257.13
11032004.02
10771706.49
10770973.37
10771013.33
10444001.9
10117030.42
10116337.26
10117030.42
10117030.42
10116337.26
10117030.42
10117030.42
13697750.9
MARR = 15%
8.13706
7.07571
6.15279
5.35025
4.65239
4.04556
3.51788
3.05902
2.66002
2.31306
2.01136
1.74901
1.52088
1.3225
1.15
-
FW
4.3.6
Future Worth
(FW), (RM)
-262479260.1
78992983.04
73294748.23
60988875.45
51325185.17
43577584.92
37890991.81
32948745.2
27781253.92
23401298.38
20347596.11
17694787.37
15386789.23
13378856.03
11634584.98
10117030.42
13697750.9
269979801.1
Payback Period
The payback period analysis is a more simplistic method of calculating the
economic feasibility of a project. This method determines the period in number of
years required for the project to recover back the initial capital investment of the
plant. In using this method, the assumptions stated above such as below still
applies:
1) The plant life is taken as 15 years.
2) The annual net profit of the plant is taken as constant.
3) The class life or recovery period is 7 years.
4) The working capital is taken as 5% of the total fixed capital cost.
4.3.6.1
Simple Payback Period
Table 4.9 : Simple Payback Period
82
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Year
0
1
2
After Tax Cash Flow
-32257260
16316673
16317791
Cumulative Cash Flow
-32257260
-15940587
377204
By interpolation,
Table 4.10 : The Interpolation Simple Payback Period
Cumulative Cash
Flow
-15940587
0
377204
Year
1
1.97
2
Therefore, MTBE plant can have payback period with:
Payback period = (1 + 0.97) years
= 1 years 12 month
2 years
4.3.6.2
Discounted Payback Period
Normally, the interest in Malaysia standardized for chemical plant. By referring to
Hong Leong Bank, the interest is 15% and use as a basis for discounted payback
period.
Table 4.11 : Discounted Payback Period
Year
Annual Cash
Flow After Tax
0
1
2
3
-32257260
16316673
16317791
16317791
Cumulative
Cash Flow
Cumulative
Cash Flow
After Tax
-32257260
-15940587
-2013884.05
14001824.34
-18331675.05
-2315966.658
16102097.99
By interpolation,
Table 4.12 : The Interpolation Discounted Payback Period
Cumulative Cash
Flow
-2315966.658
0
16102097.99
Year
2
2.03
3
Therefore, MTBE plant can have payback period with:
= 2 + (0.03) years
83
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
= 2 year 1 month
4.4 CONCLUSION
Based on this chapter, the economic evaluation of MTBE plant are made
through study in all aspect including feasibility study, process synthesis and flow
sheeting and designed of major equipment.
From the cash flow analysis, the payback period for the MTBE plant is about 2
years. Furthermore, it should be stated that the present work is primarily illustrated
based on the method of engineering economic analysis of chemical processes.
PW is positive value so the project is attractive and acceptable same as when IRR
is bigger than MARR.
By estimate the value of PW, FW and AW by using relationship between PW and
FW the answer of FW same with estimate direct from CFAT.
REFERENCES
84
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Perry R.H and Green, Don, Perrys Chemical Engineers Handbook, 7th
Edition, McGraw-Hill Book Company, Singapore, 1984.
Sinnott, R. K., Coulson & Richardson, Chemical Engineering Volume 6,
Chemical Engineering Design, Butterworth Heinemann, 1999.
Peters, max Stone, Plant Design and Economics for Chemical
Engineers, 2nd Edition, McGraw-Hill Chemical Engineering Series, 1968.
85
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CHAPTER 5
PROCESS INTEGRATION AND PINCH TECHNOLOGY
5.1
INTRODUCTION
Process integration can lead to a substantial reduction in the energy requirements
of a process. One of the most successful and generally useful techniques is that
developed by Bodo Linnhoff and other workers: pinch technology. The term derives
from the fact that in a plot of the system temperatures versus the heat transferred, a
pinch usually occurs between the hot stream and cold stream curves. It has been
shown that the pinch represents a distinct thermodynamic break in the system and
that, for minimum energy requirements, heat should not be transferred across the
pinch, Linhoff and Townsend (1982) (Ref: Coulson & Richardsons vol. 6)
5.2
PINCH TECHNOLOGY
There are four streams to consider the problem of integrating the utilization of
energy. Two hot streams which require cooling and two cold streams that have to
be heated. Each streams starts from a source temperature Ts, and is to be heated
or cooled to a target temperature, Tt. The heat capacity of each stream is shown as
CP.
CP is given by:
CP = mCp
(5.1)
Where,
m = mass flow rate, kg/s
Cp = average specific heat capacity between Ts and Tt,
(KJ/kgoC)
Table 5.1:
Shows the process data for each stream.
86
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Heat
Stream number
Type
1
2
3
4
HOT
HOT
COLD
COLD
Heat capacity
Cp(KW/ oC)
3.2
1.3
2
4
Ts(oC)
Tt (oC)
180
150
20
75
50
30
140
135
Load
(KW)
416
156
240
240
The heat load shown in the table is the total heat required to heat or cools the
stream from the source to target temperature. The stream are shown
diagrammatically below,
Stream 1
180C
CP = 3.2 kW/C
50C
Stream 2
150C
CP = 1.3kW/C
30C
Stream 3
20C
CP = 2 kW/C
140C
Stream 4
75C
CP = 4 kW/C
135C
Figure 5.1:
5.3
Diagrammatically representation of process stream
THE PROBLEM TABLE METHOD
The problem table is a numerical method for determining the pinch temperature and
the minimum utility requirements. Firstly, it needs to convert the actual stream
temperature Tact into the interval temperatures Tint.
Hot streams Tint
Tact (Tmin/2)
Cold streams Tint
Tact (Tmin/2)
The minimum temperature difference taken from composite curve as, Tmin = 10 oC
87
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Hot a nd Cold Com posite curve s
350
Temperature
300
250
200
Hot Stre am s
150
Cold Stre am s
100
50
0
1
Enthalpy,1 0kW
Figure 5.2: Hot and cold streams composite curves
Table 5.2: Interval Temperature for Tmin = 10oC
Actual Temp. oC
Stream
Interval Temp. oC
180
50
175
45
150
30
145
25
20
140
25
145
10
75
135
80
140
The heat balance for the streams falling within each temperature interval:
For the nth interval:
Hn
(CPc - CPh) (Tn)
net heat required in the nth interval
(5.2)
Where, Hn
CPc =
sum of the heat capacities of all the cold streams in interval
CPh =
sum of the heat capacities of all the hot streams in the
interval
Tn
interval temperature difference=(Tn-1-Tn)
88
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
175
Interval 1:
145
1
Interval 2:
140
2
Interval 3:
80
Interval 4:
45
Interval 5:
25
Figure 5.3:
Table 5.3:
Intervals and streams
Ranked order of interval temperature
Rank,oC
Interval, Tn oC
Stream in interval
145
30
-1
140
4-(2+1)
80
60
(3+4)-(1+2)
45
35
3-(1+2)
25
20
(3-2)
175
Cascading the heat from one interval to the next implies that the temperature
difference is such that the heat can be transferred between the hot and cold
streams. A negative value in the column indicates the temperature gradient is in the
wrong direction and that the exchange is not thermodynamically possible.
Table 5.4:
Problem Table
CPc - CPh
Interval
Tint, C
o
n
T ,C
(Kw/oC)
Hn (KW)
surplus/deficit
89
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
175
1
145
30
-3.2
-96
140
0.5
2.5
80
60
2.1
126
45
35
-2.0
-87.5
25
20
1.0
14
Interval temperature
Rank oC
175oC
0kW
145oC
-96kW
96 kW
-96 kW
128.5 kW
140oC
2.5 kW
93.5 kW
2.5 kW
126 kW
80oC
126 kW
-32.5 kW
126 kW
0 kW
45oC
-87.5 kW
55 kW
-87.5 kW
87.5 kW
25oC
14 kW
-41 kW
14 kW
73.5 kW
32.5 kW
Figure 5.4 Heat Cascade
From figure 5.4: pinch occurs at interval temperature 80oC
At the pinch, hot stream
80 + 5
85 oC
80 5
75oC
(5.3)
Cold stream
(5.4)
5.4
THE HEAT EXCHANGER NETWORK
The grid representation of the stream is shown in Figure 5
CP
90
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
(KW/ oC)
o
85 C75 C
180oC
50 oC
3.2
1
150 oC
30 oC
1.3
2
140 oC
20 oC
2.0
3
135 oC
75 oC
4.0
Figure 5.5 Grid for 4 stream problem
For maximum energy recovery (minimum utility consumption) the best performance
is obtained if no cooling is used above the pinch. This means the hot streams
above the pinch should be brought it the pinch temperature solely by exchange with
the cold streams.
THE NETWORK DESIGN ABOVE THE PINCH
CP hot CP cold
Applying this condition at the pinch, stream 1 can be matched with stream 4, but not
with 3.
Matching streams 1 and 4 and transferring the full amount of heat required to bring
stream 1 to the pinch temperature gives;
Hex
CP (T pinch - Ts)
304 kW
(5.5)
This will also satisfy the heat load required to bring stream 4 to its target
temperature.
91
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Stream 2 can be matched with stream 3, whilst satisfying the heat capacity
restriction. Transferring the full amount to bring stream 3 to the pinch temperature:
Hex
CP (T pinch- Ts)
97.5 kW
The heat required to bring stream 3 to its target temperature, from the pinch
temperature, is:
H
2.0(140-75)
130 kW
So a heater will have to be included to provide the remaining heat load:
Hhot
130-97.5 kW
32.5 kW
(5.6)
THE NETWORK DESIGN BELOW THE PINCH
Stream 1 is matched with stream 3 transferring the full amount to bring stream 1 to
its target temperature; transferring:
Hex
3.2(85-50)
112 kW
Stream 3 requires more heat to bring it to pinch temperature; amount needed:
H
2.0(75-20)-85kW
25 kW
So transferring 25 kW will raise the temperature from the source temperature to:
20+ 25/2 =32.5 kW
and gives a stream temperature difference on the outlet side of the exchanger of:
85-32.5 = 52.5 kW
So the minimum temperature difference condition, 10oC will not be violet by this
match.
Stream 2 will need further cooling to bring its to its target temperature, so a cooler
must be included; cooling required.
Hcold =
=
1.3 (85-50)-25
73.5 kW
92
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The proposed network for maximum energy recovery is shown in Figure 5.5
Figure 5.6 Proposed heat exchanger network
5.5
MINIMUM NUMBER OF EXCHANGERS
The network shown in figure 5.5 was designed to give the maximum heat recovery,
and therefore give the minimum consumption, and cost of the hot and cold utilities.
In figure 5.5 it is clear that there is scope for reducing the number of exchangers.
Exchanger D can be deleted and the heat loads of the cooler and heater increased
to bring stream 2 and 3 to their target temperatures. Heat would across the pinch
and the consumption of the utilities would be increased
For complex networks a more general expression is needed to determine the
number of exchangers:
Zmin = N +L S
(5.7)
Where L= the number of internal loops present in the network
S= the number of independence branches (subsets) that exist in the
network
N= the number of streams including the utilities
93
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
In a final design, there are 3 exchangers, rather than 4 before the process
integration and pinch technology, with the minimum heating and cooling loads, 32.5
kW and 73.5 kW, respectively, match those predicted from the problem table,
compare with the loads for heating and cooling before process integration: 416 kW
and 240 kW.
94
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
CHAPTER 6
WASTE TREATMENT
6.1
INTRODUCTION
Pollution is something that should be taken into serious consideration in any
petrochemical plant especially the MTBE plant. Pollution, no matter what kind of the
pollution is has serious negative effects not only to human beings, but also to
animals, plants and to the environment. Therefore, it is the responsibility of each
individual to ensure that their activities are not harmful to the environment. This
includes activities and works involved in designing a plant. Waste from any
petrochemical plant should be treated according to the local and international
standards before being released to the environment.
In the MTBE plant, the wastes are only the in liquid form and gas form. The liquid
will be treated in the wastewater treatment plant. The hydrogen gas leaving the
reactor is sent to a gas cylinder which it is then sold to interested company at
market price. The treatment processes and systems employed by the MTBE plant
is the typical processes and systems based on Howard S. Peavy, Donald R. Rowe,
George Tchobanoglous; Environmental
Engineering, McGraw-Hill, 1985 and
David H. F. Liu, Bela G. Liptak, Wastewater Treatment, Lewis Publishers, 1999.
6.2
WASTEWATER TREATMENT
95
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Wastewater treatment is a must in any plant. The wastewater from plant
cannot be channeled into the sewage system without being treated. The
wastewater from the MTBE plant may contains a small amount of the feed and
product and a small quantity of by-products such as butene, TBA and dimethylether. According to the site location selected, the wastewater from the plant is to be
treated and should comply with Standard B which is shown Table 6.1.
In the design of the wastewater treatment plant for the production of MTBE,
the COD value used is based of the COD value of wastewater from other plants
that are in operation. The COD value of 3000 mg/l will be used for design purposes.
The design of the plant consists of preliminary, primary and secondary treatment
where each treatment unit chosen is based on the characteristics of the influent to
be treated. Before being treated, all the wastewater is channeled to the holding
tank. It is then ferried for screening and the next process is the primary settling
tank. The effluent leaving this tank is sent to the active sludge reactor. After that, it
will be sent to the secondary settling tank. Finally, the effluent undergoes
adsorption by activated carbon before leaving the wastewater treatment plant.
6.2.1 Denitrification Process (Chemical Treatment)
96
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
To remove methanol in the waste water, we use the denitrification process
in our plant waste treatment. In the denitrification process, nitrate is reduced to
nitrogen gas by the same facultative, heterotrophic bacteria involved in the
oxidization of carbonaceous material. For reduction to occur the dissolved oxygen
level must be at or near zero and a carbon supply must be available to the bacteria.
Because low carbon content is required for the previous nitrification step, carbon
must be added before denitrification can proceed. A small amount of primary
effluent, bypassed around secondary and nitrification reactors, can be used to
supply the carbon. However, the unnitrified compounds in this water will be
unaffected by the denitrification process and will appear in the effluent. When
essentially complete nitrogen removal is required, an external source of carbon
containing no nitrogen will be required. The most commonly used external carbon
source is methanol, CH3OH. When methanol is added, the denitrification reaction is:
NO3- +
5
CH3OH
6
1
5
7
N2 + CO2+ H2O +OH2
6
6
Theoretically, each milligram per liter of nitrate should require 1.9 mg/L of
methanol. Under treatment plant conditions, however about 3.0 mg/L of methanol is
required for each milligram per litre of nitrate, making this process an expensive
one.
97
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Table 6.1 Parameter Limits for Wastewater and Effluent Under the Environmental
Quality Act 1974.
PARAMETER
Temperature
pH
BOD5 at 20oC
COD
Suspended solid
Mercury
Cadmium
Chromium Hexavalent
Arsenic
Cyanide
Lead
Copper
Manganese
Nickel
Tin
Zinc
Boron
Iron
Phenol
Free Chlorine
Sulfide
Oil and grease
UNIT
o
C
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
mg/l
STANDARD A
40
6.0-9.0
20
50
50
0.005
0.01
0.05
0.05
0.05
0.10
0.20
0.20
0.20
0.20
1.0
1.0
1.0
0.001
1.0
0.5
None
STANDARD B
40
5.5-9.0
50
100
100
0.05
0.02
0.05
0.10
0.10
0.5
1.0
1.0
1.0
1.0
1.0
4.0
5.0
1.0
2.0
0.5
10
* Both standards could be used and acceptable, but only one is chosen, Standard B
for the wastewater treatment for the MTBE plant.
6.3
WASTEWATER TREATMENT PLANT DESIGN
The design of the wastewater treatment plant consists of the holding tank, the
design of the screening device, then the design of the settling tank, the design of
the primary and secondary sedimentation tanks, and lastly the design for the
activated sludge process.
98
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The next step is the sludge treatment which consists of the sludge
thickening by centrifugation, condensation by using heat treatment and lastly
dehydration by using vacuum filter.
Since the gas produced only hydrogen and no other gases, so the gas
needs no treatment. The hydrogen gas produced is stored to be sold to interested
companies like MOX Sdn. Bhd..
6.3.1
Design of Holding Tank
The purpose of the holding tank is to hold and accumulate the wastewater
before it undergoes further treatment. The design of the holding tank is as follows:
Wastewater flow rate
Volume of wastewater per day
m 3 24 hr
hr
day
1.5642
37 .54
37.54 m3
m3
day
By taking into consideration the depth of the holding tank as 3 m, and the ratio of
the length to the width as 3:1, the dimensions of the holding tank are as follows:
Depth of holding tank
3.0 m
Width of holding tank
3.0 m
Length of holding tank
9.0 m
Volume of holding tank
81.0 m3
This means that the holding time is about two days (81m3/37.54m3) and it is
acceptable.
6.3.2
Design of Screening Device
In the preliminary treatment of the wastewater, the treatment chosen is by
using screening. Fine screens made of woven-wire cloth are used. The screen is
used to remove small particles which might be in the wastewater. A screen with
small sized openings is chosen because the wastewater does not contain large
particles. The head loss is calculated by using the following equation:
99
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
1 Q
hL =
C( 2g ) A
(6.1)
Where hL
head loss, m
waste constant for screen
0.6 for clean screen (Metcalf & Eddy, 1979)
gravity, ms-2
Flow through screen, m3s-1
Effective opening area for submerged screen, m2
take as 9m2
Thus, h L =
1
0.6 2 9.81
2
4.345 x10 4
h L = 1.9799 10 10 m
6.3.3
Design of Settling Tank
For the wastewater treatment, sulfide precipitation treatment is used to
remove heavy metals from the wastewater. The precipitant used is sodium sulfide,
which will react with the metal ions and will form non-soluble sulfide metal.
However, extra care is required in this process to avoid sulfide poisoning. Pretreatment which involves the increasing of the pH of the wastewater is required to
minimize the release of hydrogen sulfide gas. The design of the settling tank is as
follows:
Take holding time
15 minutes (standard holding time)
Average flow rate
1.5642
Holding time
15 minutes
0.25 hr
Average flow rate
1.5642
0.391 m 3
Tank volume
m3
hr
Holding time
m3
0.25 hr
hr
100
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
By taking the depth as L=0.8m, and using a square tank,
Length of tank
V
L
0.391
0.8
=
6.3.4
0.70 m
Design of Primary and Secondary Sedimentation Tank
Primary sedimentation is a unit operation designed to concentrate and
remove suspended organic
solids
from the wastewater. The secondary
sedimentation tank is designed as a unit operation for the activated sludge process.
The design for the sedimentation tanks are as follows:
Take holding time
2 hr
Average flow rate
1.5642
Holding time
2 hr
Tank Volume
Average flow rate
1.5642
3.1284 m 3
m3
hr
Holding time
m3
2hr
hr
By taking the depth as L=2m,
Length of tank
6.3.5
V
L
3.1284
2
2.50 m
Design for Activated Sludge Process
The activated sludge process is a treatment process that involves the production of
a living or active microorganism which is used to stabilize the waste aerobically. A
completely mixed reactor is used in this process. This reactor is used as it is
suitable for general wastewater treatment and it has a high efficiency (85-95%) to
reduce high COD or BOD. This process involves a completely mixed reactor
followed by a secondary sedimentation tank. Part of the sludge from the secondary
101
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
sedimentation tank is recycled to the influent reactor. The design for the reactor is
as follows:
m3
hr
Average flow rate
1.5642
Holding time
3 hr
Tank Volume
Average flow rate
1.5642
4.693 m 3
Holding time
m3
3hr
hr
By taking the depth as L=2m,
Length of tank
6.3.6
V
L
4.69
2
1.53 m
Oxygen Demand and Aerator
COD (kg/day)
Influent COD
112 .62
wastewater flow rate
kg
m3
37
.
54
m3
day
kg
day
By assuming that 1kg COD requires 1kg of oxygen, thus 112.62 kg/day COD
requires 112.62 kg O2/day. The aerator used is a mechanical aerator with an
oxygen transfer rate of 1.8 kg O2/day.
Power needed
Oxygen needed per hour
O2 needed per hour / O2 transfer rate
112 .62
4.69
kg
1day
day
24 hr
kg
hr
102
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Thus, power needed
4.69 kg day
1.8 hr
kg
2.61 kW
An aerator unit provides 10kW of power. Thus, one unit is sufficient to provide
enough power for the activated sludge process.
6.4
SLUDGE TREATMENT
In this wastewater treatment plant, sludge is formed in the primary and
secondary sedimentation tank. This sludge needs to be treated and disposed. For
the MTBE plant, the following operations are used for the sludge treatment system:
i.
Sludge thickening by centrifugation
ii.
Condensation by using heat treatment
iii.
Dehydration by using vacuum filter
Wastes from the sludge treatment system, for example the filtrate from the
vacuum filter, are sent to the influent treatment plant to undergo further treatment.
Dehydrated sludge is sent to the sludge dump site. The flow chart for the sludge
treatment is shown in Figure 6.1. Although the treatment is expensive, it is
necessary for our plant.
103
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
S l udge P la nt
C EN TR IFU G ATIO N
TH IC K EN IN G
H EA T
TR E ATM EN T
T o influ ent plant
S l udge to dis pos al s ite
SL U D G E
STO R AG E
D ehy dr ated s lud ge
VAC U U M
FIL TE R
F iltrate to influen t pl an t
Figure 6.1 The Sludge Treatment System
6.5
WASTE TREATMENT PLANT LAYOUT
Generally, the layout of a waste treatment plant depends on the process
requirements. The treatment plant covers an area of 800m2. The plant layout is
shown in Figure 6.3. The waste treatment plant consists of the following units:
1.
1 holding tank
2.
1 primary sedimentation tank
3.
1 secondary sedimentation tank
4.
1 activated sludge reactor
5.
1 screening device
6.
1 power house
7.
4 pumps
8.
1 sludge store
9.
Units in the sludge treatment process
Table 6.2 Functions of Pumps in the Waste Treatment Plant
Pump
Function
104
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
To pump sludge from the sedimentation tank to the sludge
treatment tank.
To pump sludge from the secondary sedimentation tank to be
recycled to the activated sludge reactor.
To pump sludge from the secondary sedimentation tank to the
sludge treatment process.
To pump wastewater from the sludge treatment system to be
recycled to the influent plant.
U
LEG END
Pum p
P R IM A R Y
S E D IM E N T A T IO N T A N K
S lu d g e
f ro m
p r im a ry
s e d im e n t a t io n t a n k to b e
t re a t e d
S lu d g e
f ro m
s e c o n d a ry
s e d im e n t a t io n t a n k to b e
r e c y c le d
S lu d g e
f ro m
s e c o n d a ry
s e d im e n t a t io n t a n k to b e
t r e a te d
W a s t e w a te r f r o m s lu d g e
t r e a tm e n t s y s te m to in flu e n t
p la n t
F lo w
S lu d g e F lo w
S E T T L IN G T A N K
W a ste w a te r
F lo w
POW ER HOUSE
A E R A T IO N T A N K
S C R E E N IN G D E V IC E
2
S LU D G E TR EA TM E N T PR O C E SS
SECO NDARY
S E D IM E N T A T IO N T A N K
H O L D IN G T A N K
Figure 6.2 Waste Treatment Plant Layout
6.6
ABSORPTION TANK USING GRANULAR ACTIVATED CARBON
105
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
In the operation of absorption tank, granular activated carbon (GAC) is used
which has a diameter between 0.1-2.0 mm. The contact system for GAC consists of
cylindrical tanks, which contain a bed of the carbon material (refer the design
given). The water is passed through the bed with sufficient residence time allowed
for completion of the adsorption process. The system is operating in a fixed bed
mode. Fixed bed systems are batch operations that are taken off the line when the
adsorptive capacity of the carbon in used up.
Although fixed granular carbon beds can be cleaned in a place with
superheated steam, the most common practice is to remove the carbon for cleaning
in a furnace. The regeneration process is essentially the same as the original
activation process. The adsorbed organics are first burned at about 800 oC in the
absence of oxygen. An oxidizing agent, usually stream, is then applied at slightly
higher temperatures to remove the residue and reactivated carbon.
6.6.1 Analysis of the Absorption Process
The adsorption process takes place in the three steps, macrotransport,
microtransport and sorption. The quantities of adsorbate that can be taken up by an
adsorbent are function of both the characteristics and concentration of adsorbate
and the temperature. Generally, the amount of material absorbed is determined as
a function of the concentration at a constant temperature and the resulting function
is called an absorption isotherm. Equation that are often used to described the
experimental isotherm data where developed by Freundlich by Langmuir and by
Brunauer, Emmet and Teller (BET isotherm).
Freundlich Isotherm Equation:
x
= K f Ce1 n
m
Where x/m
= amount adsorbate absorbed per unit weight of absorbent (activated
carbon)
Ce
= equilibrium concentration of absorbed in solution after absorption
K f , n = empirical constant
106
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
The constants in the Freundlich isotherm can be determined by plotting (x/m)
versus C and making use of above equation rewritten as:
1
x
log = log K f + log Ce
n
m
6.6.2
Breakthrough Absorption Capacity
In the field, the breakthrough adsorption capacity, ( x m )b , of the GAC in a
full-scale column is some percentage of the theoretical absorption capacity found
from the isotherm. The
(x
m )b of a single column can be assumed to be
approximately 25 to 50 percent of the theoretical capacity ( x m ) o . Once ( x m )b is
known, time to breakthrough can be calculated by solving the following equation for
tb
X
C t
x
= b = Q Ci b b [8.34 lb Mgal .( mg L ) ]
2 Mc
m b M c
x
Where = field breakthrough adsorption capacity, lb/lb or g/g
m b
X b = mass of organic material absorbed in the GAC column at
breakthrough, lb or g
M c = mass of carbon in the column, lb or g
Q = flow rate, Mgal/d
Ci = influent organic concentration, mg/L
Cb = breakthrough organic concentration, mg/L
tb = time to breakthrough, day
Equation above was developed assuming that Ci is constant and that the effluent
concentration increases linearly with time from 0 to Cb value. The time breakthrough
can be calculated using the rearranging equation above. From the test data by
Freundlich adsorption isotherm plotted,
107
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
x
3.56
= 0.0015 Ce
m
x
= 0.0015(3.250)3.56
m 0
= 0.0996 mg/mg say 0.10 mg/mg = 0.10 Ib/Ib
Determination of breakthrough time,
tb =
( x / m) b M c
Q (Ci Cb / 2[8.34 Ib / Mgal .( mg / L)]
Following condition apply:
(x
m )b =50 percent of ( x m ) o =o.5 (0.10 lb/lb) = 0.050 lb/lb
Assuming from the data testing by Freundlich adsorption isotherm,
Surface area = 10 ft 2 = 0.929m2
M c = (10 ft 2 ) ( 5.0 ft ) 38 lb ft 3 = 1,900 lb
Q = 5.0 gal ft 2 . min 1440 min d 10 ft 2 = 72000 gal d = 0.072 Mgal d
Ci = 3.2 mg L
Cb = 0.75 mg L
the time to breakthrough is
tb =
( x / m) b M c
Q (Ci Cb / 2[8.34 Ib / Mgal .( mg / L)]
tb = 56 day
Therefore, results from our study based on Freundlich adsorption isotherm the
activated carbon in our design waste treatment column vessel can long lasting for
56 day. Therefore, our estimation is changing the activated carbon in the vessel
waste treatment in every 56 day to make sure the efficiency capacity of adsorption
carbon is in the maximum capacity.
REFERENCES
108
PRODUCTION OF 300,000 METRIC TON OF MTBE PER YEAR
Howard S. Peavy, Donald R. Rowe, George Tchobanoglous; Environmental
Engineering, McGraw-Hill, 1985.
David H. F. Liu, Bela G. Liptak, Wastewater Treatment, Lewis Publishers, 1999.
109
You might also like
- Senior ReportDocument113 pagesSenior ReportAnkit VermaNo ratings yet
- Project Group 9 CC01 PDFDocument24 pagesProject Group 9 CC01 PDFHuy NinhNo ratings yet
- Production of AcetaldehydeDocument80 pagesProduction of Acetaldehydeyinka omojesuNo ratings yet
- Project 6 - Ethylene Oxide PDFDocument13 pagesProject 6 - Ethylene Oxide PDFStephanie Hawkins100% (1)
- Lab 4 Cobalt LabDocument10 pagesLab 4 Cobalt LabadfsgsgsNo ratings yet
- Although This Process Is No Longer in Common UseDocument15 pagesAlthough This Process Is No Longer in Common Usedia_aldy100% (1)
- AcetoneDocument7 pagesAcetoneGeorgiana AndreeaNo ratings yet
- Phenol From CumeneDocument2 pagesPhenol From CumenealiNo ratings yet
- Ethylene Glycol Chemical Engineering Final Year ProjectDocument108 pagesEthylene Glycol Chemical Engineering Final Year Projectridzuwan rahimiNo ratings yet
- Manufacture of TrifluralinDocument49 pagesManufacture of TrifluralinAhmed Ali0% (1)
- Plant Design Project FinalDocument221 pagesPlant Design Project FinalYasser AshourNo ratings yet
- New Aspects of Spillover Effect in Catalysis: For Development of Highly Active CatalystsFrom EverandNew Aspects of Spillover Effect in Catalysis: For Development of Highly Active CatalystsNo ratings yet
- PRODUCTIONOFMALEICANHYDRIDEFROMOXIDATIONOFn BUTANE PDFDocument456 pagesPRODUCTIONOFMALEICANHYDRIDEFROMOXIDATIONOFn BUTANE PDFRitik Chaudhary100% (2)
- ReportDocument20 pagesReportCrazy HelloNo ratings yet
- Report 0Document19 pagesReport 0Joseph OrjiNo ratings yet
- LECTURE - 6: Ethylene Derivatives: Ethylene Oxide and Ethanol Amines 6.1 Ethylene OxideDocument7 pagesLECTURE - 6: Ethylene Derivatives: Ethylene Oxide and Ethanol Amines 6.1 Ethylene Oxideمحمود محمدNo ratings yet
- Formic Acid TechnologyDocument3 pagesFormic Acid Technologyatharnadim_osNo ratings yet
- Ethylene Oxide Kinetics and MechanismDocument10 pagesEthylene Oxide Kinetics and MechanismjohnNo ratings yet
- Production of Acrylonitrile by Ammoxidation of PropyleneDocument33 pagesProduction of Acrylonitrile by Ammoxidation of PropyleneJ José B VelasquezNo ratings yet
- Arrieta Ethylene GlycolDocument8 pagesArrieta Ethylene GlycolNguyen VietNo ratings yet
- Acrolein Project Final PDFDocument104 pagesAcrolein Project Final PDFPankaj RanaNo ratings yet
- Manufacture of Maleic Anhydride: Chemical EngineeringDocument63 pagesManufacture of Maleic Anhydride: Chemical EngineeringmgajenNo ratings yet
- It1.Introduction & History:-: 1.1 Introduction To Cumene:-StructureDocument12 pagesIt1.Introduction & History:-: 1.1 Introduction To Cumene:-StructureJaymin GoswamiNo ratings yet
- 14 Production of 1,3-Propanediol by Hydrogenolysis of Glycerol Catalyzed by PtWO3ZrO2Document4 pages14 Production of 1,3-Propanediol by Hydrogenolysis of Glycerol Catalyzed by PtWO3ZrO2ChauNo ratings yet
- التعديل النهائي محمد احمد علي عبدالله 22130011 PDFDocument115 pagesالتعديل النهائي محمد احمد علي عبدالله 22130011 PDFRojan Pradhan0% (1)
- CUMENEDocument24 pagesCUMENEhiteshNo ratings yet
- N-Butane To Maleic AnhydrideDocument6 pagesN-Butane To Maleic AnhydrideNomeacuerdo Yo MismoNo ratings yet
- 1.project FullDocument75 pages1.project FullKolliparaDeepakNo ratings yet
- Mek From N Butene PDFDocument111 pagesMek From N Butene PDFAlexis PulhinNo ratings yet
- FYDP Final Report G13 PDFDocument30 pagesFYDP Final Report G13 PDFJeanette Hong May Hurn0% (1)
- Vinyl Chloride Production: Name of StudentDocument12 pagesVinyl Chloride Production: Name of Studentعبدالمحسن علي ENo ratings yet
- CEPSA Good Reference For ZeoliteDocument29 pagesCEPSA Good Reference For Zeolitedie_1No ratings yet
- A Project Report Submitted By: in Partial Fulfilment For The Award of The DegreeDocument91 pagesA Project Report Submitted By: in Partial Fulfilment For The Award of The DegreeHari BharathiNo ratings yet
- Production of CyclohexaneDocument2 pagesProduction of Cyclohexanesushant kadamNo ratings yet
- PPD Final Report Group 9Document63 pagesPPD Final Report Group 9Ananda Subramani67% (3)
- Chemical Kinetics On Thermal Decompositions of CumeneDocument8 pagesChemical Kinetics On Thermal Decompositions of CumeneMario Alonso Velasquez FlorezNo ratings yet
- Production of MTBE Using Reactive DistilDocument4 pagesProduction of MTBE Using Reactive DistilIndraNo ratings yet
- Preliminary Design Chemical Plant LABDocument9 pagesPreliminary Design Chemical Plant LABgeorge cabreraNo ratings yet
- Mthanol ProductionDocument61 pagesMthanol Productionvv vvNo ratings yet
- Mtbe PDFDocument47 pagesMtbe PDFYayee LalainheavenNo ratings yet
- Brandstaedter Willi MichaelDocument202 pagesBrandstaedter Willi MichaelApril JuneNo ratings yet
- Reactor Design 7SONDocument53 pagesReactor Design 7SONYasemin KaradağNo ratings yet
- Design ReportDocument193 pagesDesign ReportNhut Nguyen100% (1)
- Production of Methyl Ethyl KetoneDocument89 pagesProduction of Methyl Ethyl KetonePablo HernandezNo ratings yet
- Plug FlowDocument17 pagesPlug FlowNurshahirahSapianNo ratings yet
- Production of Aniline by Reduction of Nitrobenzene: Group#2Document26 pagesProduction of Aniline by Reduction of Nitrobenzene: Group#2Arsal MaqboolNo ratings yet
- Butene-1: Trans-2-Butene, Isobutylene, and ButadieneDocument1 pageButene-1: Trans-2-Butene, Isobutylene, and ButadieneYESIKBMARTIN100% (1)
- Art:10 1134/S0965544111010038Document10 pagesArt:10 1134/S0965544111010038CátiaLuzNo ratings yet
- Ethylbenzene ProductionDocument30 pagesEthylbenzene ProductionUum LukmanNo ratings yet
- Final Project2Document135 pagesFinal Project2Mr NU KHANNo ratings yet
- Cumene Production Process DescriptionDocument1 pageCumene Production Process DescriptionAudrey Patrick KallaNo ratings yet
- Viewcontent11 PDFDocument54 pagesViewcontent11 PDFEr Mayur PatilNo ratings yet
- MEK in School SecondDocument13 pagesMEK in School Secondifiok100% (1)
- Adiabatic and Non-Isothermal Reactor DesignDocument35 pagesAdiabatic and Non-Isothermal Reactor DesignTesfaye Kassaw100% (1)
- Benzene Production Using Hydrodealkylation RouteDocument3 pagesBenzene Production Using Hydrodealkylation RouteCluisantony Jayco DizeNo ratings yet
- Hydrogenation of Nitrobenzene To AnilineDocument8 pagesHydrogenation of Nitrobenzene To AnilineYu HuiNo ratings yet
- Recent Advances in the Science and Technology of Zeolites and Related Materials: Proceedings of the 14th International Zeolite Conference, Cape Town, South Africa, 25-30th April 2004From EverandRecent Advances in the Science and Technology of Zeolites and Related Materials: Proceedings of the 14th International Zeolite Conference, Cape Town, South Africa, 25-30th April 2004No ratings yet
- International Thermodynamic Tables of the Fluid State: Propylene (Propene)From EverandInternational Thermodynamic Tables of the Fluid State: Propylene (Propene)No ratings yet
- Full Report With Cover Page PDFDocument91 pagesFull Report With Cover Page PDFFareez AizatNo ratings yet
- Metal MtrixDocument9 pagesMetal MtrixPrabhu chauhanNo ratings yet