Professional Documents
Culture Documents
Objectives Template50 PDF
Objectives Template50 PDF
Uploaded by
Peyman GrdOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Objectives Template50 PDF
Objectives Template50 PDF
Uploaded by
Peyman GrdCopyright:
Available Formats
2/17/2017 Objectives_template
Module3:
Lecture10:MaterialProperties:TheRoleofCrystalStructure
MoreaboutCrystalStructures
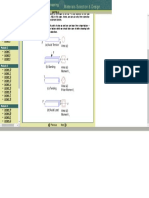
Note that each of the seven crystal systems that we have discussed may have several variants in
terms packing of atoms inside each geometrical configuration. For example, the cubic crystal system
canhavethreepackingvariationsasshownbelow:
SimpleCube BodyCentredCube FaceCentredCube
ThreevariantsoftheCubicSystem
Thenumberofatomsperunitcelldependsonthetypeofpacking.Forexample,theunitcellofsimple
cubic structure contains 8x1/8 = I atom per cell, whereas the BCC contains 8x1/8 +1 = 2 atoms per
unitcellandtheFCCcontains8x1/8+6x1/2=4atoms.
Knowingalltheseparametersyoucandeterminethetheoreticaldensityofamaterialwhich is defined
as:
Density=[(numberofatoms/unitcell)(atomicmass)]/[(Volumeofunitcell)(AvogadrosNumber)]
Forexample,IronhasBCCcells,
Hence,no.ofatomsperunitcell=2,atomicmass=55.84g/mol,Volumeofunitcell=a3=(2.866e
8)3=23.54e24
Hence,itsdensityshouldbe(2x55.84)/(23.54e24)(6.02e23)=7.88g/cc
http://nptel.ac.in/courses/112104122/lecture9/9_4.htm 1/1
You might also like
- Crystal LatticesDocument11 pagesCrystal LatticeschrischeelyNo ratings yet
- Chemistry Lecture Manual 2020 CHAPTER 03-3-2 Chemistry of Materials CrystalDocument6 pagesChemistry Lecture Manual 2020 CHAPTER 03-3-2 Chemistry of Materials CrystalPals TripNo ratings yet
- Objectives Template50Document1 pageObjectives Template50Peyman GrdNo ratings yet
- Objectives Template50 PDFDocument1 pageObjectives Template50 PDFPeyman GrdNo ratings yet
- ME 2203 Engineering Materials: Dr. Kazi Md. ShorowordiDocument23 pagesME 2203 Engineering Materials: Dr. Kazi Md. ShorowordiTahmim AlamNo ratings yet
- Lecture 1-4miller Indices APFDocument63 pagesLecture 1-4miller Indices APFAbdla DoskiNo ratings yet
- Structure of SolidsDocument18 pagesStructure of SolidsAfridi AnsariNo ratings yet
- Wk4 Atoms and MicroscopesDocument21 pagesWk4 Atoms and MicroscopesRobin MackrellNo ratings yet
- 02-Pet Eng Design - PTE - 470 - The Structure of Crystalline SolidsDocument15 pages02-Pet Eng Design - PTE - 470 - The Structure of Crystalline SolidsHassan KhalifeNo ratings yet
- Crystal StructureDocument47 pagesCrystal Structurenaz_qd358No ratings yet
- Lesson 4.0 Basic Concepts of Crystalline StructureDocument11 pagesLesson 4.0 Basic Concepts of Crystalline StructureyoureqtNo ratings yet
- Chapter 3: The Structure of Crystalline Solids: Course Objective..Document70 pagesChapter 3: The Structure of Crystalline Solids: Course Objective..venosyah devanNo ratings yet
- How Do Atoms Arrange Themselves To Form Solids?: Chapter Outline Types of SolidsDocument7 pagesHow Do Atoms Arrange Themselves To Form Solids?: Chapter Outline Types of SolidsAbhijith MadabhushiNo ratings yet
- Chapter 3: The Structure of Crystalline Solids: Issues To Address..Document48 pagesChapter 3: The Structure of Crystalline Solids: Issues To Address..Fatih KESKİNo ratings yet
- Microstructure - Crystalline and Amorphous Materials Live VersionDocument49 pagesMicrostructure - Crystalline and Amorphous Materials Live VersionbasitNo ratings yet
- Chapter 3Document26 pagesChapter 3Bala SubramanianNo ratings yet
- Structure of Crystalline 1-DikonversiDocument24 pagesStructure of Crystalline 1-DikonversiAndhika Setyo AdjieNo ratings yet
- Chapter 3 - Crystal Structures & PropertiesDocument29 pagesChapter 3 - Crystal Structures & PropertiesOmair SadiqNo ratings yet
- DocumentDocument60 pagesDocumentSyazwani ShahnunNo ratings yet
- How Do Atoms Arrange Themselves To Form Solids?: Simple CubicDocument29 pagesHow Do Atoms Arrange Themselves To Form Solids?: Simple CubicAbduljabbar Tudu IbrahimNo ratings yet
- CHEM 102 Last ChapterDocument10 pagesCHEM 102 Last ChapterTemitope OlawuyiNo ratings yet
- Solid State TheoryDocument28 pagesSolid State TheoryrahulNo ratings yet
- Course Objective... : You Will Learn AboutDocument70 pagesCourse Objective... : You Will Learn AboutThaneswaran BaluNo ratings yet
- The Structure of Crystalline Solids: Chapter 3Document76 pagesThe Structure of Crystalline Solids: Chapter 3ShakeelAhmadNo ratings yet
- Unit - 1 CrystallographyDocument12 pagesUnit - 1 Crystallographyjitendrarc1No ratings yet
- 9-18 Crystal StructuresDocument10 pages9-18 Crystal StructuresChamalNo ratings yet
- MME M1 Ktunotes - inDocument76 pagesMME M1 Ktunotes - inkannanNo ratings yet
- EPM - Mid - Lecture - 02 - Chapt - 1 CompressDocument24 pagesEPM - Mid - Lecture - 02 - Chapt - 1 CompressPartho Protim MondolNo ratings yet
- Crystal StructureDocument30 pagesCrystal StructureSujit Singh100% (1)
- 7 Crystalline & Solid StateDocument37 pages7 Crystalline & Solid StateNazmi LatifNo ratings yet
- UntitledDocument10 pagesUntitledAli KashifNo ratings yet
- Chap 1 - Crystalline & Noncrystalline StructureDocument55 pagesChap 1 - Crystalline & Noncrystalline StructureJordan ThongNo ratings yet
- 03 - Crystal Structures of MetalsDocument8 pages03 - Crystal Structures of MetalsJant Erbert GarbosoNo ratings yet
- PQT Chapter 3 The Structure of Crystalline SolidsDocument34 pagesPQT Chapter 3 The Structure of Crystalline SolidsDương Hữu PhươngNo ratings yet
- C2ADocument22 pagesC2AYong Meng HowNo ratings yet
- Crystal StructuresDocument87 pagesCrystal Structuresrogerio-camposNo ratings yet
- Solid StateDocument13 pagesSolid StatesachinkurhekarNo ratings yet
- Quarter 3 - Module 1C: Nature of CrystalsDocument14 pagesQuarter 3 - Module 1C: Nature of CrystalsNala Akut JasmienNo ratings yet
- Jupeb CHM 003 M2Document4 pagesJupeb CHM 003 M2olatejuomole78No ratings yet
- Structure of MaterialsDocument114 pagesStructure of Materialstharushisewwanndi43No ratings yet
- Part 2Document3 pagesPart 2Duy Do MinhNo ratings yet
- EE2317-Course-Structures of Crystalline SolidsDocument148 pagesEE2317-Course-Structures of Crystalline SolidsJOSEPH BENEDICT PRIMNo ratings yet
- Lec 2Document24 pagesLec 2ahmed emadNo ratings yet
- Chapter 3Document75 pagesChapter 3SAIF ULLAHNo ratings yet
- Chapter 3Document25 pagesChapter 3Masoud SalehNo ratings yet
- Solid State ChemistryDocument6 pagesSolid State ChemistryAravindan B BabuNo ratings yet
- MSE - ChapterDocument42 pagesMSE - ChapterFaisal MumtazNo ratings yet
- Chapter 3 (2022) (Rev 02) : The Structure of Crystalline SolidsDocument49 pagesChapter 3 (2022) (Rev 02) : The Structure of Crystalline SolidsLizzsel FranchesNo ratings yet
- Solid State Broad DescriptionDocument14 pagesSolid State Broad DescriptionbookregtestNo ratings yet
- BMT ManualDocument23 pagesBMT ManualAsheesh KumarNo ratings yet
- Lecture 2aDocument25 pagesLecture 2aAbdul AhadNo ratings yet
- Chapter Two, The Structure of Crystalline Solids PDFDocument23 pagesChapter Two, The Structure of Crystalline Solids PDFOmar Abu MahfouthNo ratings yet
- Crystal StructuresDocument65 pagesCrystal StructuresnaverfallNo ratings yet
- Solids: Xii Science Chemistry Notes Unit 1 Solid StateDocument3 pagesSolids: Xii Science Chemistry Notes Unit 1 Solid StateRaashiNo ratings yet
- Electrical Properties of Materials: Chap 1Document21 pagesElectrical Properties of Materials: Chap 1Nur Mohammad AkramNo ratings yet
- Chapter 3Document86 pagesChapter 3Jose L. Rosado100% (1)
- Material Teknik Part 1Document35 pagesMaterial Teknik Part 1MuhammadZakyMubarokNo ratings yet
- Cbse Test Paper-02 CLASS - XII CHEMISTRY (The Solid State)Document1 pageCbse Test Paper-02 CLASS - XII CHEMISTRY (The Solid State)Srivathson EswaranNo ratings yet
- Objectives Template13 PDFDocument1 pageObjectives Template13 PDFPeyman GrdNo ratings yet
- Objectives Template12 PDFDocument1 pageObjectives Template12 PDFPeyman GrdNo ratings yet
- Advantages of Using Aluminium in Automobiles: Module 1: Lecture 6: Case Study: Materials Selection For Vehicle BodyDocument1 pageAdvantages of Using Aluminium in Automobiles: Module 1: Lecture 6: Case Study: Materials Selection For Vehicle BodyPeyman GrdNo ratings yet
- Crystal Structure in Metals: Critical Resolved Shear StressDocument1 pageCrystal Structure in Metals: Critical Resolved Shear StressPeyman GrdNo ratings yet
- Surface Mechanical Property: Wear Due To Corrosion: Aerated WaterDocument1 pageSurface Mechanical Property: Wear Due To Corrosion: Aerated WaterPeyman GrdNo ratings yet
- Traditional Materials: Module 1: Lecture 2: Evolution of Engineering Materials Traditional MaterialsDocument1 pageTraditional Materials: Module 1: Lecture 2: Evolution of Engineering Materials Traditional MaterialsPeyman GrdNo ratings yet
- Module 5: Lecture 27: Multiple ObjectivesDocument1 pageModule 5: Lecture 27: Multiple ObjectivesPeyman GrdNo ratings yet
- How Do We Quantify The Availability of Materials?: Module 1: Lecture 4: Material Resource in Indian ContextDocument1 pageHow Do We Quantify The Availability of Materials?: Module 1: Lecture 4: Material Resource in Indian ContextPeyman GrdNo ratings yet
- Objectives Template5 PDFDocument1 pageObjectives Template5 PDFPeyman GrdNo ratings yet
- What Is The Significance of Materials in Design?Document1 pageWhat Is The Significance of Materials in Design?Peyman GrdNo ratings yet
- Module 1: Lecture 5: Classification of MaterialsDocument1 pageModule 1: Lecture 5: Classification of MaterialsPeyman GrdNo ratings yet
- Topics To Cover in This Lecture:: Module 1: Lecture 2: Evolution of Engineering Materials Traditional MaterialsDocument1 pageTopics To Cover in This Lecture:: Module 1: Lecture 2: Evolution of Engineering Materials Traditional MaterialsPeyman GrdNo ratings yet
- Objectives Template18Document1 pageObjectives Template18Peyman GrdNo ratings yet
- 26 3 PDFDocument1 page26 3 PDFPeyman GrdNo ratings yet
- Nptel T Bombay5 PDFDocument1 pageNptel T Bombay5 PDFPeyman GrdNo ratings yet
- Module 5: Lecture 26: Multiple Constraints in Material SelectionDocument1 pageModule 5: Lecture 26: Multiple Constraints in Material SelectionPeyman GrdNo ratings yet
- Definition of Engineering Properties in A CompositeDocument1 pageDefinition of Engineering Properties in A CompositePeyman GrdNo ratings yet
- Module 1Document1 pageModule 1Peyman GrdNo ratings yet
- Course Calendar 2012Document9 pagesCourse Calendar 2012Peyman GrdNo ratings yet
- Objectives Template85 PDFDocument1 pageObjectives Template85 PDFPeyman GrdNo ratings yet
- How To Include Shape Factor in MPI?: Module 6: Lecture 29: The Role of Shape Factors in Material SelectionDocument1 pageHow To Include Shape Factor in MPI?: Module 6: Lecture 29: The Role of Shape Factors in Material SelectionPeyman GrdNo ratings yet
- Basalt Fibre: Module 4: Lecture 20: Reinforcement Fibres For Composite MaterialsDocument1 pageBasalt Fibre: Module 4: Lecture 20: Reinforcement Fibres For Composite MaterialsPeyman GrdNo ratings yet
- Objectives Template63 PDFDocument1 pageObjectives Template63 PDFPeyman GrdNo ratings yet
- Objectives Template85Document1 pageObjectives Template85Peyman GrdNo ratings yet
- 51 PDFDocument1 page51 PDFPeyman GrdNo ratings yet
- Surface Mechanical Property: Wear Rate: Module 2: Lecture 8: Surface Properties of MaterialsDocument1 pageSurface Mechanical Property: Wear Rate: Module 2: Lecture 8: Surface Properties of MaterialsPeyman GrdNo ratings yet
- Carbon Fibre: Module 4: Lecture 20: Reinforcement Fibres For Composite MaterialsDocument1 pageCarbon Fibre: Module 4: Lecture 20: Reinforcement Fibres For Composite MaterialsPeyman GrdNo ratings yet
- What Is Involved in The Design of Mechanical Systems?: Module 1: Lecture 1: Materials and DesignDocument1 pageWhat Is Involved in The Design of Mechanical Systems?: Module 1: Lecture 1: Materials and DesignPeyman GrdNo ratings yet
- Typical DMTA Output: Module 3: Lecture 17: Phases and Microstructure of PolymersDocument2 pagesTypical DMTA Output: Module 3: Lecture 17: Phases and Microstructure of PolymersPeyman GrdNo ratings yet
- Objectives Template129Document1 pageObjectives Template129Peyman GrdNo ratings yet