Professional Documents
Culture Documents
CASE 1: Manipac Yao: Cmmatias (Cmed 2022)
Uploaded by
Charm MatiasOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CASE 1: Manipac Yao: Cmmatias (Cmed 2022)
Uploaded by
Charm MatiasCopyright:
Available Formats
CASE 1: Manipac Yao
1. The major cause his gastritis and gastric ulcer is the NSAID a non-selective COX-1 and COX-2 inhibitor.
2. Ibuprofen affects the synthesis of the two isoforms of PGH2 synthase which are COX-1 and COX-2.
COX-1 is essential for thromboxane formation in blood platelets, and for maintaining the integrity of the gastrointestinal
epithelium.
COX-2 is stimulated by growth factors, cytokines, and endotoxins.
- Inflammation is associated with the up-regulation of COX-2 and increased formation of prostaglandins. COX-2
levels increase in inflammatory disease states such as arthritis.
- Increased expression of COX-2 is seen in some cancer cells.
- It is also related to the expression of pain.
BIOCHEMICAL BASIS:
Cyclooxygenase is an enzyme which is essential to convert arachidonic acid to prostaglandins. Arachidonic acid is
converted to prostaglandin H2 in body with the help of enzyme cyclooxygenase. If the cyclooxygenase is inhibited by the
NSAID , there will be no formation of COX-1 and COX-2. COX-1 is needed to maintain the mucous secretion in the
stomach so that it will not be affected by the acid secretions. But since COX-1 is blocked therefore the mucous are not
secreted enough to help the stomach maintain its integrity therefore when the stomach pH is high the stomach will be
affected therefore ulcers and bleeding can occur, because the protective covering is lesser than is needed to protect it.
3. The patient can take COX-2 selective drugs like celecoxib, and etoricoxib.
CASE 2: Haya Lin
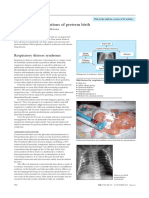
1. Respiratory distress syndrome (HYALINE MEMBRANE DISEASE) is one of the most common problems of
premature babies. It can cause babies to need extra oxygen and help breathing. The course of illness with
respiratory distress syndrome depends on the size and gestational age of the baby, the severity of the disease,
the presence of infection, whether or not the baby has patent ductus arteriosus, and whether or not the baby
needs mechanical help to breathe. (Children’s Hospital of Philadelphia)
Respiratory distress syndrome is caused by pulmonary surfactant deficiency in the lungs of neonates, most
commonly in those born at <37-week gestation. Risk increases with degree of prematurity. Symptoms and signs
include grunting respirations, use of accessory muscles, and nasal flaring appearing soon after birth.
Pulmonary surfactant is a mixture of phospholipids and lipoproteins secreted by type II pneumocytes. It diminishes the
surface tension of the water film that lines alveoli, thereby decreasing the tendency of alveoli to collapse and the work
required to inflate them. With surfactant deficiency, a greater pressure is needed to open the alveoli. Without adequate
airway pressure, the lungs become diffusely atelectatic, triggering inflammation and pulmonary edema. Because blood
passing through the atelectatic portions of lung is not oxygenated (forming a right-to-left intrapulmonary shunt), the
infant becomes hypoxemic. Lung compliance is decreased, thereby, increasing the work of breathing. In severe cases,
the diaphragm and intercostal muscles fatigue, and CO2 retention and respiratory acidosis develop.
CMMATIAS (CMED 2022)
2. Biochemical basis
Role of phosphatidylcholine in lung surfactant.
Major constituents of surfactant are dipalmitoylphosphatidylcholine (DPPC or dipalmitoyl lecithin),
phosphatidylglycerol, apoproteins and cholesterol.
These components normally contribute to a reduction in the surface tension within the air spaces (alveoli) of
the lung, preventing their collapse.
Lung maturity of fetus can be gauged by determining the ratio of DPPC to sphingomyelin or the
Lecithin:Sphingomyelin or L:S ratio in amniotic fluid.
L:S ratio value of 2 or more is evidence of lung maturity. An indication of a major shift from sphingomyelin to
DPPC synthesis that occurs in pneumocytes that occurs at about 32 wees of gestation.
3. SYMPTOMS
Rapid, labored, grunting respirations appearing immediately or within a few hours after delivery, with
suprasternal and substernal retractions and flaring of the nasal alae. As astelectasis and respiratory failure
progress, symptoms worsen, with cyanosis, lethargy, irregular breathing, and apnea.
Neonates weighing <1000g may have lungs so stiff that they are unable to initiate or sustain respirations in
the delivery room.
On examination, breath sounds are decreased. Peripheral pulses may be decreased with peripheral
extremity edema and decreased urine output.
DIAGNOSIS
Clinical presentation, including recognition of risk factors, ABGs showing hypoxemia and hypercapnia; and
chest x-ray. Chest x-ray shows diffuse atelectasis classically described as having a ground-glass appearance
with visible air bronchograms; appearance correlates loosely with clinical severity.
RDS can be anticipated prenatally using tests of fetal lung maturity, which are done on amniotic fluid obtained by
amniocentesis or collected from the vagina (if membranes have ruptured) and which can help determine the optimal
timing of delivery. These are indicated for elective deliveries before 39 wk when fetal heart tones, human chorionic
gonadotropin levels, and ultrasound measurements cannot confirm gestational age and for nonelective deliveries
between 34 wk and 36 wk.
Amniotic fluid tests include the
Lecithin/sphingomyelin ratio
Foam stability index test (the more surfactant in amniotic fluid, the greater the stability of the foam that forms
when the fluid is combined with ethanol and shaken)
Surfactant/albumin ratio
Risk of RDS is low when lecithin/sphingomyelin ratio is > 2, phosphatidyl glycerol is present, foam stability
index = 47, or surfactant/albumin ratio is > 55 mg/g.
4. PREVENTION
CMMATIAS (CMED 2022)
When a fetus must be delivered between 24 wk and 34 wk, giving the mother 2 doses of betamethasone 12
mg IM 24 h apart or 4 doses of dexamethasone 6 mg IV or IM q 12 h at least 48 h before delivery induces
fetal surfactant production and reduces the risk of RDS or decreases its severity.
Prophylactic intratracheal surfactant therapy given to neonates who are at high risk of developing RDS
(infants < 30 wk completed gestation especially in absence of antenatal corticosteroid exposure) has been
shown to decrease risk of neonatal death and certain forms of pulmonary morbidity (eg, pneumothorax).
TREATMENT
Intratracheal surfactant
Supplementary oxygen as needed
Mechanical ventilation as needed
Specific treatment of RDS is intratracheal surfactant therapy. This therapy requires endotracheal intubation, which
also may be necessary to achieve adequate ventilation and oxygenation. Less premature infants (those> 1 kg) and
those with lower oxygen requirements (fraction of inspired oxygen [FIO2] < 40 to 50%) may respond well to
supplemental oxygen alone or to treatment with nasal continuous positive airway pressure. A treatment strategy of
early (within 20 to 30 min after birth) surfactant therapy is associated with significant decrease in duration of
mechanical ventilation, lesser incidence of air-leak syndromes, and lower incidence of bronchopulmonary dysplasia.
Surfactant hastens recovery and decreases risk of pneumothorax, interstitial emphysema, intraventricular
hemorrhage, bronchopulmonary dysplasia, and neonatal mortality in the hospital and at 1 yr. However, neonates
who receive surfactant for established RDS have an increased risk of apnea of prematurity. Options for surfactant
replacement include
Beractant
Poractant alfa
Calfactant
Lucinactant
Beractant is a lipid bovine lung extract supplemented with proteins B and C, colfosceril palmitate, palmitic acid, and
tripalmitin; dose is 100 mg/kg q 6 h prn up to 4 doses.
Poractant alfa is a modified porcine-derived minced lung extract containing phospholipids, neutral lipids, fatty acids,
and surfactant-associated proteins B and C; dose is 200 mg/kg followed by up to 2 doses of 100 mg/kg 12 h apart
prn.
Calfactant is a calf lung extract containing phospholipids, neutral lipids, fatty acids, and surfactant-associated
proteins B and C; dose is 105 mg/kg q 12 h up to 3 doses prn.
Lucinactant is a synthetic surfactant with a pulmonary surfactant protein B analog, sinapultide (KL4) peptide,
phospholipids, and fatty acids; dose is 175 mg/kg q 6 h up to 4 doses.
Lung compliance can improve rapidly after therapy. The ventilator peak inspiratory pressure may need to be
lowered rapidly to reduce risk of a pulmonary air leak. Other ventilator parameters (eg, FIO2, rate) also may need to
be reduced.
CMMATIAS (CMED 2022)
You might also like
- 4 Respiratory Distress Syndrome in The NewbornDocument3 pages4 Respiratory Distress Syndrome in The NewbornChristine Danica BiteraNo ratings yet
- RDS Ppts 2021Document37 pagesRDS Ppts 2021Mohammad NajjarNo ratings yet
- Respiratory Distress Syndrome in NewbornDocument24 pagesRespiratory Distress Syndrome in NewbornDrRajneesh Shastri100% (1)
- Respiratory Distress Syndrome: NewbornDocument17 pagesRespiratory Distress Syndrome: NewbornLoloNo ratings yet
- Neonatal Acute Respiratory Distress Syndrome (RDS) ManagementDocument30 pagesNeonatal Acute Respiratory Distress Syndrome (RDS) ManagementSanjay Kumar Sanju100% (1)
- Intensive Care Nursery House Staff ManualDocument9 pagesIntensive Care Nursery House Staff ManualVenus MargaretteNo ratings yet
- Respiratory Distress SyndromeDocument30 pagesRespiratory Distress SyndromeDennis MiritiNo ratings yet
- Illness in The NewbornDocument34 pagesIllness in The NewbornVivian Jean TapayaNo ratings yet
- Idiopathic Respiratory Disease SyndromeDocument30 pagesIdiopathic Respiratory Disease SyndromeAllan-VonNo ratings yet
- Embryonic 8wks (3-7wks) Lung Hypoplasia, TEF Pseudoglandular 16 Wks CDH Canalicular 24 Wks Surfactant def-RDSDocument40 pagesEmbryonic 8wks (3-7wks) Lung Hypoplasia, TEF Pseudoglandular 16 Wks CDH Canalicular 24 Wks Surfactant def-RDSMischief ManagerNo ratings yet
- Surf Act Ant ReplacementDocument28 pagesSurf Act Ant Replacementapi-27504339100% (3)
- 5. respiratory distress sydrome year 5-2Document36 pages5. respiratory distress sydrome year 5-2chebetnaomi945No ratings yet
- Medication: Respiratory Distress SyndromeDocument3 pagesMedication: Respiratory Distress Syndromenoeh_olofernesNo ratings yet
- Prematures With Respiratory Distress Syndrome Do Not Have Increased The Chance of Patent Ductus Arteriosus Requiring Indomethacin Therapy ThereafterDocument58 pagesPrematures With Respiratory Distress Syndrome Do Not Have Increased The Chance of Patent Ductus Arteriosus Requiring Indomethacin Therapy ThereafterAndy SungNo ratings yet
- Respiratory Distress Syndrome (Iniego Carlo Jay)Document9 pagesRespiratory Distress Syndrome (Iniego Carlo Jay)Carlojay IniegoNo ratings yet
- Surfactantreplacementtherapy 160926164838Document33 pagesSurfactantreplacementtherapy 160926164838revathidadam55555No ratings yet
- Respiratory Distress in NewbornDocument7 pagesRespiratory Distress in NewbornChimot Ona MilanelloNo ratings yet
- Clinical Approach To Respiratory Distress in NewbornDocument29 pagesClinical Approach To Respiratory Distress in Newbornabhivnair100% (1)
- Eni Rahmawati, S.Kep., NS., M.KepDocument46 pagesEni Rahmawati, S.Kep., NS., M.KepLisa Qoriana RohmaniNo ratings yet
- RESPIRATORY DIS-WPS OfficeDocument12 pagesRESPIRATORY DIS-WPS OfficeGiselle EstoquiaNo ratings yet
- Infant Respiratory Distress SyndromeDocument39 pagesInfant Respiratory Distress SyndromeIoana MuntianuNo ratings yet
- 1) Respiratory Distress Syndrome (RDS) Hyaline Membrane Disease (HMD)Document9 pages1) Respiratory Distress Syndrome (RDS) Hyaline Membrane Disease (HMD)ُEssraa AdeelNo ratings yet
- Respiratory Distress Syndrome Ped 401 MLDocument23 pagesRespiratory Distress Syndrome Ped 401 MLWongani ZuluNo ratings yet
- Respiratory Distress Syndrome, Transient Tachypnea of The Newborn and Meconium Aspiration SyndromeDocument69 pagesRespiratory Distress Syndrome, Transient Tachypnea of The Newborn and Meconium Aspiration SyndromeKate PanoliNo ratings yet
- Extremely Low Birth Weight (ELBW) InfantDocument48 pagesExtremely Low Birth Weight (ELBW) InfanthannanyusofNo ratings yet
- Respiratory Diseases GuideDocument58 pagesRespiratory Diseases GuideSarahNo ratings yet
- Respiratory Distress Syndrome Hmd-Ttnb-Mas-Neonatal PneumoniaDocument65 pagesRespiratory Distress Syndrome Hmd-Ttnb-Mas-Neonatal PneumoniaBLOBLOBNo ratings yet
- Common Neonatal Disorders 3Document25 pagesCommon Neonatal Disorders 3Laishram Deeva ChanuNo ratings yet
- Respiratory Distress SyndromeDocument9 pagesRespiratory Distress SyndromeRamina HiponiaNo ratings yet
- Respiratory Distress Syndrome - MBCHBDocument23 pagesRespiratory Distress Syndrome - MBCHBMalueth AnguiNo ratings yet
- RD in Newborn by Yong Part 2Document37 pagesRD in Newborn by Yong Part 2semangat akreNo ratings yet
- Aspiration SyndromesDocument16 pagesAspiration SyndromesAswathy RCNo ratings yet
- The Preterm InfantDocument26 pagesThe Preterm Infantnursereview100% (7)
- Vol-2 Issue-1 Approach To Respiratory Distress in The Newborn 19Document13 pagesVol-2 Issue-1 Approach To Respiratory Distress in The Newborn 19salamredNo ratings yet
- NB Hyaline Membrane DiseaseDocument27 pagesNB Hyaline Membrane DiseaseDr.P.NatarajanNo ratings yet
- Transient Tachypnea of The Newborn: BackgroundDocument9 pagesTransient Tachypnea of The Newborn: BackgroundAnnisa YusufNo ratings yet
- Respiratory Distress SyndromeDocument9 pagesRespiratory Distress SyndromeIrawati Eka PutriNo ratings yet
- Paeds ProtocolDocument44 pagesPaeds ProtocolDesmond LingNo ratings yet
- Respiratory Distress SyndromeeDocument42 pagesRespiratory Distress SyndromeeRoman MamunNo ratings yet
- Newborn Respiratory Disorders PDFDocument10 pagesNewborn Respiratory Disorders PDFMax RodriguezNo ratings yet
- Treatment of AsthmaDocument6 pagesTreatment of AsthmaEdmond HubertNo ratings yet
- Respiratory Distress SyndromeDocument10 pagesRespiratory Distress SyndromeKomang Rendy KrisnadiNo ratings yet
- Newborn Tachypnea GuideDocument14 pagesNewborn Tachypnea GuideMarielaTessyGonzalesParedesNo ratings yet
- The Preterm InfantDocument26 pagesThe Preterm Infantmardsz100% (10)
- Modul Skills KKD4 Reproduksi 2018Document31 pagesModul Skills KKD4 Reproduksi 2018rizkiya novitaNo ratings yet
- Congenital HerniaDocument5 pagesCongenital HerniaBlazxy EyreNo ratings yet
- Complicaciones en El PreterminoDocument4 pagesComplicaciones en El PreterminonadisjaviNo ratings yet
- From The Journal of The American Academy of PediatricsDocument10 pagesFrom The Journal of The American Academy of PediatricsMichi MichNo ratings yet
- Targeting Oxygen Levels for Term and Preterm InfantsDocument15 pagesTargeting Oxygen Levels for Term and Preterm InfantsJulio Cesar Robles ZanelliNo ratings yet
- Diseases of The Newborn IDocument40 pagesDiseases of The Newborn InewazNo ratings yet
- COPD Management and PrognosisDocument21 pagesCOPD Management and PrognosisMudrekaNo ratings yet
- Management of Respiratory Distress Syndrome: An Update: Ricardo J Rodriguez MDDocument9 pagesManagement of Respiratory Distress Syndrome: An Update: Ricardo J Rodriguez MDIndah Afriani NasutionNo ratings yet
- Respiratory Distress in Neonates: Causes and ManagementDocument8 pagesRespiratory Distress in Neonates: Causes and ManagementMikey ZhitnitskyNo ratings yet
- Newborn Acute Conditions GuideDocument46 pagesNewborn Acute Conditions GuideCamille Joy BaliliNo ratings yet
- Acute Bronchiolitis: Zakaria Omar Elzwie Najwa Abdulallah Alfergany (Mub) NovemberDocument28 pagesAcute Bronchiolitis: Zakaria Omar Elzwie Najwa Abdulallah Alfergany (Mub) Novemberزكريا عمرNo ratings yet
- Surfactan Therapy For NeonatesDocument20 pagesSurfactan Therapy For Neonatesfarah1218No ratings yet
- Pediatric Anesthesia: A Guide for the Non-Pediatric Anesthesia ProviderFrom EverandPediatric Anesthesia: A Guide for the Non-Pediatric Anesthesia ProviderNo ratings yet
- The Lung: Development, Aging and the EnvironmentFrom EverandThe Lung: Development, Aging and the EnvironmentKent PinkertonNo ratings yet
- Respiratory Monitoring in Mechanical Ventilation: Techniques and ApplicationsFrom EverandRespiratory Monitoring in Mechanical Ventilation: Techniques and ApplicationsJian-Xin ZhouNo ratings yet
- CASE 1: Manipac Yao: Cmmatias (Cmed 2022)Document3 pagesCASE 1: Manipac Yao: Cmmatias (Cmed 2022)Charm MatiasNo ratings yet
- Chronic Kidney DiseaseDocument9 pagesChronic Kidney DiseaseCharm MatiasNo ratings yet
- Chronic Kidney DiseaseDocument9 pagesChronic Kidney DiseaseCharm MatiasNo ratings yet
- HETARDocument4 pagesHETARCharm MatiasNo ratings yet
- Ana PhyDocument9 pagesAna PhyCharm MatiasNo ratings yet
- Phar DoseDocument3 pagesPhar DoseCharm MatiasNo ratings yet
- Phar DoseDocument3 pagesPhar DoseCharm MatiasNo ratings yet
- Pharchem Lecture Chap 6Document12 pagesPharchem Lecture Chap 6Charm MatiasNo ratings yet
- Latinterms76 109Document1 pageLatinterms76 109Charm MatiasNo ratings yet
- Fetal Heart Rate MonitoringDocument13 pagesFetal Heart Rate MonitoringNinaSimone17No ratings yet
- Medical Astrology TableDocument9 pagesMedical Astrology TableBirdNo ratings yet
- LPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini DELHI 110085Document1 pageLPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini DELHI 110085Vinothkumar VKNo ratings yet
- Trionara 06 UM Pac T3 E211128Document133 pagesTrionara 06 UM Pac T3 E211128Rudi CanicelaNo ratings yet
- Review Cram SheetDocument57 pagesReview Cram Sheetjim j100% (1)
- History Taking and Physical ExaminationDocument45 pagesHistory Taking and Physical ExaminationUnyime SamuelNo ratings yet
- Sepmate ProtocolDocument2 pagesSepmate ProtocolZil PatelNo ratings yet
- GP41A6 SampleDocument13 pagesGP41A6 SampleAndy BogardNo ratings yet
- 1) The Case of Life-Threatening Immune Reaction: Acute Systemic AnaphilaxisDocument9 pages1) The Case of Life-Threatening Immune Reaction: Acute Systemic AnaphilaxisMariam TavdgiridzeNo ratings yet
- Forms of Isotonic Exercises: TM 1-B Group 2Document13 pagesForms of Isotonic Exercises: TM 1-B Group 2Vivien CabatbatNo ratings yet
- Precision 32 CT Product Recommendation, Campo ImagingDocument15 pagesPrecision 32 CT Product Recommendation, Campo ImagingNur HusenNo ratings yet
- BioVisc Ortho SINGLE Treatment Joint Pain ReliefDocument8 pagesBioVisc Ortho SINGLE Treatment Joint Pain ReliefAnaNo ratings yet
- NCM 117: Psychiatric Nursing Mental Status ExamDocument8 pagesNCM 117: Psychiatric Nursing Mental Status ExamGabrielle Acabo100% (1)
- Wondfo Fia Finecare Plus Se Fs 114Document2 pagesWondfo Fia Finecare Plus Se Fs 114Toto FollyNo ratings yet
- Encyclopedia of Diseases and Disorders (Gnv64)Document482 pagesEncyclopedia of Diseases and Disorders (Gnv64)Florin Ciobanu100% (12)
- List of Problems and PrioritizationDocument3 pagesList of Problems and PrioritizationAsthra R.No ratings yet
- Nocardia: Gram-Positive Bacteria Found in SoilDocument3 pagesNocardia: Gram-Positive Bacteria Found in Soilsujithas0% (1)
- Oms Global Report On InfectionDocument182 pagesOms Global Report On InfectionMonica FerreiraNo ratings yet
- %pediatric TracheostomyDocument25 pages%pediatric TracheostomyFabian Camelo OtorrinoNo ratings yet
- 1 UsmleDocument6 pages1 UsmleKeaira KcNo ratings yet
- Diagnostic Accuracy in The Detection of Depth of Myometrial Invasion With MRI in Early-Stage Endometrial CancerDocument5 pagesDiagnostic Accuracy in The Detection of Depth of Myometrial Invasion With MRI in Early-Stage Endometrial CancerBOHR International Journal on GynaecologyNo ratings yet
- Amaryl 1mg Tablets - (eMC) Print Friendly PDFDocument9 pagesAmaryl 1mg Tablets - (eMC) Print Friendly PDFrakeshNo ratings yet
- EchinococcosisDocument52 pagesEchinococcosisZezo TarekNo ratings yet
- Picornaviridae, Orthomyxoviridae-1Document45 pagesPicornaviridae, Orthomyxoviridae-1Farrah BenoitNo ratings yet
- Young2022 Article OxygenTargetsDocument4 pagesYoung2022 Article OxygenTargetsBrenda Serrano LaraNo ratings yet
- Pain Assessment ScalesDocument4 pagesPain Assessment ScalesREMILYN ROSE ASUNCIONNo ratings yet
- Nursing Diagnoses For PT With Altered Level of ConsciousnessDocument5 pagesNursing Diagnoses For PT With Altered Level of Consciousnessmikaela_pascua95% (40)
- Treatment of Angular Cheilitis A Narrative Review and AuthorsDocument9 pagesTreatment of Angular Cheilitis A Narrative Review and AuthorsElizabeth SihiteNo ratings yet
- June 2021 MCQ Psychiatry at DAMSDocument61 pagesJune 2021 MCQ Psychiatry at DAMSAli HusseinNo ratings yet
- Nurse-Midwife - Trend or Treason by Nancy Jo ReedyDocument7 pagesNurse-Midwife - Trend or Treason by Nancy Jo ReedyDivine OublietteNo ratings yet