Professional Documents
Culture Documents
Isotopes: Chemschool As Chemistry
Uploaded by
ninaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Isotopes: Chemschool As Chemistry
Uploaded by
ninaCopyright:
Available Formats
ChemSchool AS CHEMISTRY
ISOTOPES
1. Fill in the blank spaces in the table below which refers to atoms of different
elements and the fundamental particles present in them.
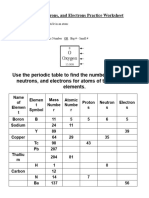
Element Atomic no. Mass no. Protons Neutrons Electrons
Aluminium 13 14
Carbon 6 7
Neon 10 12
Chlorine 37 17
Gallium 40 31
Potassium 19 22
Titanium 48 22
Cobalt 59 27
Chlorine 17 35
Copper 29 34
Cobalt 60 27
Gallium 31 69
2. Fill in the blank spaces in the table below which refers to ions of different elements
and the fundamental particles present in them.
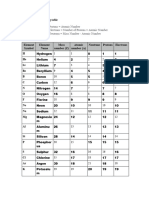
Ion Atomic no. Mass no. Protons Neutrons Electrons
Al3+ 13 14
+
K 19 22
71
Ga3+ 31
37 -
Cl 37 17
-
F 9 10
35 -
Cl 17 18
Ti4+ 48 18
69
Ga3+ 31
+
Li 3 7
Cu2+ 29 34
Copyright © GreenAPLEducation Ltd 2011
You might also like
- KCSE Form 2 NotesDocument139 pagesKCSE Form 2 NotesN KatanaNo ratings yet
- Atomic Structure and Periodic Table PDFDocument51 pagesAtomic Structure and Periodic Table PDFKevin NdanyiNo ratings yet
- FORM 2 CHEMISTRY NOTEzS (2023 - 11 - 13 08 - 17 - 14 UTC)Document254 pagesFORM 2 CHEMISTRY NOTEzS (2023 - 11 - 13 08 - 17 - 14 UTC)joshuamumo588No ratings yet
- Atomic MassDocument1 pageAtomic MassDeepti JainNo ratings yet
- Number of Protons WorksheetDocument4 pagesNumber of Protons WorksheetIrene SanchezNo ratings yet
- W.atoms and IonsDocument2 pagesW.atoms and IonsMahmoud AladdasiNo ratings yet
- 1 Grade 11 Review AnswersDocument9 pages1 Grade 11 Review Answersapi-363234558No ratings yet
- Protons Neutrons Electrons Review KEYDocument3 pagesProtons Neutrons Electrons Review KEYMiguel Jimenez OsorioNo ratings yet
- Ion PracticeDocument2 pagesIon Practicehart0% (1)
- 1 - Atomic Mass and Atomic Number WorksheetDocument1 page1 - Atomic Mass and Atomic Number WorksheetprevendidosamanthaNo ratings yet
- CH 5 Ion PracticeDocument2 pagesCH 5 Ion PracticeMahmoud AladdasiNo ratings yet
- Chemistry Assignment - 03Document2 pagesChemistry Assignment - 03K.TejasviNo ratings yet
- Part 2 Microscopic World (I) LQ AnswersDocument17 pagesPart 2 Microscopic World (I) LQ AnswersWing LamNo ratings yet
- Atomic Structure and Periodic Table - Chem - f2 - V1Document50 pagesAtomic Structure and Periodic Table - Chem - f2 - V1Lubanga N JamesNo ratings yet
- All About The Periodic Table - Home Laboratory WorksheetDocument4 pagesAll About The Periodic Table - Home Laboratory WorksheetFrank Ed SerranoNo ratings yet
- Basic Atomic Structure WorksheetDocument4 pagesBasic Atomic Structure WorksheetTrisha GolesNo ratings yet
- Activity 2 Gen. Chem 1Document2 pagesActivity 2 Gen. Chem 1Mark AtienzaNo ratings yet
- Element Atomic Number Proton S Electrons Group # Valence Electrons Cation or Anion?Document2 pagesElement Atomic Number Proton S Electrons Group # Valence Electrons Cation or Anion?Issa SherryNo ratings yet
- Lesson 10: The Symbol of Elements Name: Class: DateDocument2 pagesLesson 10: The Symbol of Elements Name: Class: Dateg-46005995No ratings yet
- Unit 2 Lesson 2Document1 pageUnit 2 Lesson 2seokitsukiNo ratings yet
- Week 4 Experiment Atomic Structure B1. Complete This TableDocument2 pagesWeek 4 Experiment Atomic Structure B1. Complete This TableLindsey StilleyNo ratings yet
- History and Subatomic Particle Review Take Two KEYDocument5 pagesHistory and Subatomic Particle Review Take Two KEYAlliya DaymonNo ratings yet
- CH 2Document14 pagesCH 2dwarriorsNo ratings yet
- Subatomic StructuresDocument18 pagesSubatomic StructuresArlyn RoblesNo ratings yet
- Atomic Mass and Atomic Number WorksheetDocument1 pageAtomic Mass and Atomic Number WorksheetGuayNo ratings yet
- 1.chem Review & Aquesous Solutions Key.Document34 pages1.chem Review & Aquesous Solutions Key.Calo Is TrashNo ratings yet
- Atoms and Ions Worksheet AnswersDocument1 pageAtoms and Ions Worksheet AnswersFrancis Olila0% (1)
- CH 5 Ion PracticeDocument2 pagesCH 5 Ion PracticeMAHJABEEN NASEEMNo ratings yet
- GENCHEM 1 - Week 5-1 - Activity 10Document1 pageGENCHEM 1 - Week 5-1 - Activity 10Contreras JerwhinNo ratings yet
- Module 1 Core CompetenciesDocument25 pagesModule 1 Core Competenciesdũng nguyễnNo ratings yet
- Summative Atomic StructureDocument3 pagesSummative Atomic StructureNovie Mae ReambonanzaNo ratings yet
- G 8 TestDocument1 pageG 8 TestlouNo ratings yet
- Subatomic Particles - FILLDocument2 pagesSubatomic Particles - FILLALMERA SHELLA CABOGONo ratings yet
- Table of IonsDocument2 pagesTable of IonsLucia Jimenez AlvarezNo ratings yet
- Atomic STRCDocument2 pagesAtomic STRCZoya AdnanNo ratings yet
- Periodic TableDocument1 pagePeriodic TablelingarajugowdaNo ratings yet
- 02 Atomic Number and MassDocument2 pages02 Atomic Number and Masskshitijshandilya2010No ratings yet
- Activities Unit 2 Physics and Chemistry. "The Composition of Matter" KEYSDocument4 pagesActivities Unit 2 Physics and Chemistry. "The Composition of Matter" KEYSgenusxyzNo ratings yet
- Full Download Chemistry 12th Edition Chang Solutions Manual PDF Full ChapterDocument36 pagesFull Download Chemistry 12th Edition Chang Solutions Manual PDF Full Chaptermohur.auszug.zai8x100% (12)
- Lilavatibai Podar High School (Isc) : Holding Capacity of Shells: 2 N Formula (N Position of The Shell From The NucleusDocument3 pagesLilavatibai Podar High School (Isc) : Holding Capacity of Shells: 2 N Formula (N Position of The Shell From The NucleusMahesh hamneNo ratings yet
- 1.2 Gen ChemDocument1 page1.2 Gen ChemClariza GarmaNo ratings yet
- Atoms & Ions Worksheet 1 /63: Atomic Number and Mass NumberDocument4 pagesAtoms & Ions Worksheet 1 /63: Atomic Number and Mass Numbercate christineNo ratings yet
- ValencyDocument7 pagesValencyrajindia698No ratings yet
- Annotated-Atomic Structure Bohr Models-1Document2 pagesAnnotated-Atomic Structure Bohr Models-1Ivania Joselina Lobo MontoyaNo ratings yet
- Atomic Weights 2013Document8 pagesAtomic Weights 2013LuisCastilloNo ratings yet
- Atomic Structure Form 3Document21 pagesAtomic Structure Form 3Kupakwashe KampiniNo ratings yet
- Periodic Table WorksheetDocument1 pagePeriodic Table WorksheetJahbuki ReynoldsNo ratings yet
- Ion Worksheet KEYDocument1 pageIon Worksheet KEYAna Marie Corales TabunarNo ratings yet
- The Periodic Table of Elements: Daniel LundbergDocument2 pagesThe Periodic Table of Elements: Daniel LundbergEZLYEN AZLINNo ratings yet
- The Main Postulates of Dalton's Atomic Theory AreDocument6 pagesThe Main Postulates of Dalton's Atomic Theory ArePriyanshu PalNo ratings yet
- Group Activity1Document1 pageGroup Activity1ansalerochNo ratings yet
- 9PS Ion Notation WorksheetDocument4 pages9PS Ion Notation WorksheetKaden AguiarNo ratings yet
- Class: M3 Subject: Chemistry Chapter 1: Basic Concepts of ChemistryDocument6 pagesClass: M3 Subject: Chemistry Chapter 1: Basic Concepts of Chemistrysamarth chawlaNo ratings yet
- Chemistry Unit 2: ST ND RD THDocument24 pagesChemistry Unit 2: ST ND RD THjontstufNo ratings yet
- Week Six Lesson NoteDocument9 pagesWeek Six Lesson Notepalmer okiemuteNo ratings yet
- Stretch SheetDocument2 pagesStretch Sheetjoe bloggNo ratings yet
- Elements ActivityDocument59 pagesElements ActivitypazangelicamaevalladolidNo ratings yet
- Atomic Structure: Pre-Lab Study QuestionsDocument4 pagesAtomic Structure: Pre-Lab Study QuestionsKailaNo ratings yet
- Atomic Number, Mass Number, and IsotopesDocument48 pagesAtomic Number, Mass Number, and IsotopesDave Cercado BugadorNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- SAMPLE Topic 5 On The Wild SideDocument8 pagesSAMPLE Topic 5 On The Wild SideRishita SinghNo ratings yet
- A2 Biology Core Practical SummaryDocument3 pagesA2 Biology Core Practical SummarySQ100% (2)
- Sea Prayer Khaled Hoesseini PDFDocument193 pagesSea Prayer Khaled Hoesseini PDFninaNo ratings yet
- Reported StatementsDocument3 pagesReported StatementsIlir NikaNo ratings yet
- C1, Topic 1.7 Student Activity: Ideas About IonsDocument3 pagesC1, Topic 1.7 Student Activity: Ideas About IonsninaNo ratings yet
- Edexcel Biology AS Revision NotesDocument67 pagesEdexcel Biology AS Revision NotesTim Filtness95% (184)
- Edexcel Biology AS Revision NotesDocument67 pagesEdexcel Biology AS Revision NotesTim Filtness95% (184)
- English Language: Pearson Edexcel Level 3 GCEDocument28 pagesEnglish Language: Pearson Edexcel Level 3 GCEninaNo ratings yet
- Ions Atoms and Isotope Questions From A Text BookDocument3 pagesIons Atoms and Isotope Questions From A Text BookninaNo ratings yet
- Mapping GuideDocument28 pagesMapping GuideBekhiet AhmedNo ratings yet
- C1.7 Ions, Atoms and Isotopes: Task 2Document2 pagesC1.7 Ions, Atoms and Isotopes: Task 2ninaNo ratings yet