Professional Documents
Culture Documents
FDA Approves Smart Continuous Glucose Monitoring System
FDA Approves Smart Continuous Glucose Monitoring System
Uploaded by
Ankit VermaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
FDA Approves Smart Continuous Glucose Monitoring System
FDA Approves Smart Continuous Glucose Monitoring System
Uploaded by
Ankit VermaCopyright:
Available Formats
FDA Approves Smart Continuous Glucose
Monitoring System
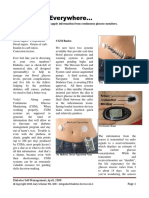
Officials with the FDA have approved Medtronic’s continuous glucose monitoring (CGM)
system (Guardian Connect) for use by individuals living with diabetes, according to a company
press release.
The Guardian Connect System is indicated for patients aged 14 to 75 years old with diabetes.
According to the press release, the product is the first standalone CGM system that can alert
patients of potential high or low glucose events up to 60 minutes in advance.
The approval is based on a clinical study that demonstrated the system’s ability to accurately
alert patients of 98.5% of hypoglycemic events while using Medtronic’s advanced glucose
sensor, Guardian Sensor 3. The Guardian Connect system gives caregivers the opportunity to
track glucose in real-time or receive text alerts.
Additionally, the system offers access to the Sugar.IQ smart diabetes assistant, an artificial
technology product from IBM Watson Health, which continually analyzes how an individual’s
glucose levels respond to their food intake, insulin dosages, daily routines, and other factors.
“Newer sensors paired with intelligent algorithms that help to both predict and understand
glucose excursions, particularly hypoglycemia, will make diabetes safer and more
comprehensible for people who inject insulin,” Timothy Bailey, director of the AMCR Institute
and clinical associate professor at University of California, San Diego, said in the press release.
“Greater utilization of smarter CGM systems promises to allow our patients to achieve more
glycemic time-in-range and to further reduce the risk of hypoglycemia.”
The Guardian Connect System will be available in the first quarter of Medtronic’s fiscal year
2019, according to the press release.
Article By-
MR. TARUN KUMAR PANCHAL
AP62046
You might also like
- Capillary Blood Glucose Monitoring or CBG MonitoringDocument5 pagesCapillary Blood Glucose Monitoring or CBG MonitoringRonilyn Mae Alvarez100% (1)
- Extended Assignment 2020 (Technical English)Document11 pagesExtended Assignment 2020 (Technical English)Fakhriah AzmiNo ratings yet
- Electronics: A Smart Glucose Monitoring System For Diabetic PatientDocument18 pagesElectronics: A Smart Glucose Monitoring System For Diabetic PatientKriesha MariceNo ratings yet
- Continuous Glucose Monitoring 508Document4 pagesContinuous Glucose Monitoring 508Hadi jameel HadiNo ratings yet
- Glucose Level Monitoring Arduino GlucometerDocument15 pagesGlucose Level Monitoring Arduino GlucometerNebitno UopsteNo ratings yet
- LibreView Guide - Italian PaperDocument12 pagesLibreView Guide - Italian PaperJesus MuñozNo ratings yet
- Monitoring Your Glucose LevelDocument4 pagesMonitoring Your Glucose LevelSarah OmarNo ratings yet
- Sensors: Advanced Diabetes Management Using Artificial Intelligence and Continuous Glucose Monitoring SensorsDocument18 pagesSensors: Advanced Diabetes Management Using Artificial Intelligence and Continuous Glucose Monitoring SensorsShujian ZhaoNo ratings yet
- Pick Two Common Chronic Illness and Do A Research On The New Technological Care For Each IllnessDocument3 pagesPick Two Common Chronic Illness and Do A Research On The New Technological Care For Each IllnessLore Anne Mhae SantosNo ratings yet
- Chemical SensorsDocument3 pagesChemical Sensorsarcelio emiyaNo ratings yet
- Data, Data Everywhere - How To Analyze, Interpret and Apply Information Fro M Continuous Glucose MonitorsDocument6 pagesData, Data Everywhere - How To Analyze, Interpret and Apply Information Fro M Continuous Glucose MonitorsSeunNo ratings yet
- 2019 09 23 Participation-Information-Sheet ConfidentialDocument2 pages2019 09 23 Participation-Information-Sheet ConfidentialMirzania Mahya FathiaNo ratings yet
- Flash Glucose MonitoringDocument9 pagesFlash Glucose MonitoringAneed AnandNo ratings yet
- Automated Blood Glucose Testing GadgetDocument6 pagesAutomated Blood Glucose Testing GadgetAtharva AnchalwarNo ratings yet
- Saint Paul University Philippines: Master of Science in NursingDocument15 pagesSaint Paul University Philippines: Master of Science in NursingGorgieNo ratings yet
- Saint Paul University Philippines: Master of Science in NursingDocument17 pagesSaint Paul University Philippines: Master of Science in NursingGorgieNo ratings yet
- Sxcribnd Reaction Paper DAY2 PDFDocument2 pagesSxcribnd Reaction Paper DAY2 PDFYlenol HolmesNo ratings yet
- Accenture Still Waiting For MhealthDocument2 pagesAccenture Still Waiting For MhealthSteveEpsteinNo ratings yet
- Adult PICF 2GO CGMDocument12 pagesAdult PICF 2GO CGMadil shabbirNo ratings yet
- Topic: Health: Blood Glucose Levels For Patient XDocument2 pagesTopic: Health: Blood Glucose Levels For Patient XChristian RiveraNo ratings yet
- How To Access A Free Blood Glucose Meter - Diabetes AustraliaDocument5 pagesHow To Access A Free Blood Glucose Meter - Diabetes AustraliaWilliam AbdinegaraNo ratings yet
- Remote DiabetesDocument14 pagesRemote DiabetesAtikah AbayNo ratings yet
- Practical CGM: Improving Patient Outcomes through Continuous Glucose MonitoringFrom EverandPractical CGM: Improving Patient Outcomes through Continuous Glucose MonitoringNo ratings yet
- Technology Report Freestyle Libre Nursing InformaticsDocument25 pagesTechnology Report Freestyle Libre Nursing InformaticsBea Dela CenaNo ratings yet
- Doctor Bot ProjectDocument8 pagesDoctor Bot Projectnitishksingh2018No ratings yet
- Gluco For PhonesDocument10 pagesGluco For PhonesFaradilla KarnesiaNo ratings yet
- Sugar DefenderDocument3 pagesSugar DefenderleachpoopNo ratings yet
- Pocket ManualDocument142 pagesPocket ManualPubudu PereraNo ratings yet
- TAKUNDA ZOU PROJECT PRESENTATION ProposalDocument28 pagesTAKUNDA ZOU PROJECT PRESENTATION ProposaltawandaNo ratings yet
- Diabetes Technology Stack - 231222 - 081814Document23 pagesDiabetes Technology Stack - 231222 - 081814Srinivas PingaliNo ratings yet
- Finaldocx 2Document14 pagesFinaldocx 2Vegesana SumythriNo ratings yet
- Glucose Sensors CWDDocument83 pagesGlucose Sensors CWDJelenaNo ratings yet
- Artificial Pancreas Technology Offers Hope For Childhood DiabetesDocument11 pagesArtificial Pancreas Technology Offers Hope For Childhood DiabetesDiana PeñalozaNo ratings yet
- Classification of Diabetes Using Machine Learning Algorithm: 1.1 Methods To Measure GlucoseDocument23 pagesClassification of Diabetes Using Machine Learning Algorithm: 1.1 Methods To Measure GlucoseVegesana SumythriNo ratings yet
- Guidance Documents (Medical Devices and Radiation-Emitting Products) _ Review Criteria Assessment of Portable Blood Glucose Monitoring in Vitro Diagnostic Devices Using Glucose Oxidase, Dehydrogenase or Hexokinase MethodologyDocument11 pagesGuidance Documents (Medical Devices and Radiation-Emitting Products) _ Review Criteria Assessment of Portable Blood Glucose Monitoring in Vitro Diagnostic Devices Using Glucose Oxidase, Dehydrogenase or Hexokinase MethodologyVidyasagar DeshpandeNo ratings yet
- 2021 - ES - CGM Pocket GuideDocument32 pages2021 - ES - CGM Pocket GuideLuiz Fernando Fonseca VieiraNo ratings yet
- Tellspec - QA Tellspec - 09 18 2015 3Document4 pagesTellspec - QA Tellspec - 09 18 2015 3Pando DailyNo ratings yet
- Glucometer Details 1Document3 pagesGlucometer Details 1jitendersingh23No ratings yet
- Project Seminar ReportDocument18 pagesProject Seminar Reportchava varshithaNo ratings yet
- Dexcom G - Continous Glucose Monitoring (CGM) : VJ'S COLLEGE OF PHARMACY, 7731051115Document1 pageDexcom G - Continous Glucose Monitoring (CGM) : VJ'S COLLEGE OF PHARMACY, 7731051115A SIVA RAMA PRASADNo ratings yet
- Finaldocx 2Document17 pagesFinaldocx 2Kola SuprajaNo ratings yet
- Hcin 557 Business PlanDocument18 pagesHcin 557 Business Planapi-575725863No ratings yet
- Picot: Student Proffessor Course Affiliation DateDocument7 pagesPicot: Student Proffessor Course Affiliation DateStephen WangaiNo ratings yet
- CGM Comparison For People With Insulin Treated Research July 2023Document11 pagesCGM Comparison For People With Insulin Treated Research July 2023shahirahNo ratings yet
- Artificial Pancreas Device Systems For The Closed-Loop Control of Type 1 Diabetes: What Systems Are in Development?Document10 pagesArtificial Pancreas Device Systems For The Closed-Loop Control of Type 1 Diabetes: What Systems Are in Development?efNo ratings yet
- IQ4I Research & Consultancy Published A New Report On "Glucose Monitoring Global Market - Forecast To 2027"Document6 pagesIQ4I Research & Consultancy Published A New Report On "Glucose Monitoring Global Market - Forecast To 2027"VinayNo ratings yet
- Continuous Glucose Monitoring: Evidence and Consensus Statement For Clinical UseDocument20 pagesContinuous Glucose Monitoring: Evidence and Consensus Statement For Clinical UseBianca CosovanuNo ratings yet
- Code FreeDocument87 pagesCode FreeJabbari Paydar Gholam RezaNo ratings yet
- The Use of Cgms in Redefining Care Delivery For Type 2 DiabetesDocument8 pagesThe Use of Cgms in Redefining Care Delivery For Type 2 DiabetesChrisSandersNo ratings yet
- Metrik Kualitas Pada Tingkat Skrining Diabetes Pasca Persalinan Setelah Kehamilan Dengan Diabetes Melitus GestasionalDocument23 pagesMetrik Kualitas Pada Tingkat Skrining Diabetes Pasca Persalinan Setelah Kehamilan Dengan Diabetes Melitus GestasionalAulia Dwi JuanitaNo ratings yet
- REFERENCESDocument5 pagesREFERENCESvillorente.jerichozNo ratings yet
- Benefits of TelehealthDocument14 pagesBenefits of TelehealthDeanna LoraNo ratings yet
- The Reminder Current Problem: Surname 1Document4 pagesThe Reminder Current Problem: Surname 1Wislay 'sivivatu' ObwogeNo ratings yet
- Fasting Blood Glucose TestDocument10 pagesFasting Blood Glucose TestBrylle ArbasNo ratings yet
- GDH-PQQ (Glucose Dehydrogenase Pyrroloquinoline Quinone) Glucose Monitoring TechnologyDocument5 pagesGDH-PQQ (Glucose Dehydrogenase Pyrroloquinoline Quinone) Glucose Monitoring Technologyahkhan57No ratings yet
- Smart Health Monitoring System Using GSM Technology For Pregnant Lady in Rural Areas With Audio Interaction Project Reference No.: 38S0245Document4 pagesSmart Health Monitoring System Using GSM Technology For Pregnant Lady in Rural Areas With Audio Interaction Project Reference No.: 38S0245OSAMANo ratings yet
- Name Netid Group Number: Website Link: Tutorial Details Time Spent On AssignmentDocument12 pagesName Netid Group Number: Website Link: Tutorial Details Time Spent On AssignmentAmandeepTanNo ratings yet
- Patient Monitoring System With Automatic SMS AlertDocument14 pagesPatient Monitoring System With Automatic SMS Alert227r1a0519No ratings yet
- Cutting-Edge AI and ML Technological Solutions: Healthcare IndustryFrom EverandCutting-Edge AI and ML Technological Solutions: Healthcare IndustryNo ratings yet