Professional Documents
Culture Documents
Sample Flow Chart of QMS
Uploaded by
Anonymous BcT42WLnOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sample Flow Chart of QMS
Uploaded by
Anonymous BcT42WLnCopyright:
Available Formats
4321
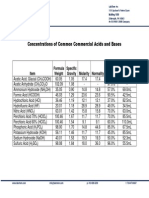
QUALITY MANAGEMENT SYSTEM (QMS) E00 GOOD ENGINEERING
Q00-00-00 Policy Statement – QMS Reference: PRACTICE (GEP)
Q00-00-01 QMS Flowchart FDA Quality System Approach to cGMP Sept.2006;
m
.co
FDA 21 CFR 210/211; ISO 9001-2000; ICH-Q10
te s
For details of GEP - see separate sheet
· Q04-0102
· Q04-0103
Document Control
p la
· Q04-0104
Good Documentation Practices
Change Control Q04 CORE CONCEPTS
te m E02
· Q04-0105 Document Change Control
M P PROJECT
w . G ENGINEERING
ww
H T© E03
Q05 Management Responsibilities RESOURCE
Q06 IG COMMON
YR
Q07 Manufacturing Operations Q08 Evaluation Activities
& Requirements REQUIREMENTS & MANAGEMENT PRACTICES &
P
ISO Section 7; FDA Section IV/C ISO section 8; FDA IV/D
CO SYSTEMS
ISO Section 5; FDA Section IV/A ISO Section 6; FDA Section IV/B
Policy Statement
Policy Statement Policy Statement Policy Statement
Q07-10
Q08-01 · Site Quality Council
Q06-01 Plan Product · Product Design Plan
Monitoring & · Customer Complaints E04
Personnel/Human Resources Realization · Quality Plan
measurement · Internal Audit
Requirements ISO 7.1, FDA C/1 OPERATIONS &
ISO Section 6.2, FDA IV/B/2
ISO 8.2, FDA D/2 · Annual Product Review
MAINTENANCE
m m
· Quality Policy, Quality Manual, · Training Policy s.co .co
Identify & Review Customer’s
· Site Master Plan/File, · Training Management System te
Q07-20 Requirements;
e s
· Organization Chart, · Competency p la Customer Related
lat
Customer Communications – Q08-02
mp
D D
tem
Control of Non-
om
· Quality Review Board, Process product information, contracts, · Deviation (CAPA)
C C
B · Validation Review Board,
P
ISO 7.2, FDA C/1
P te orders, complaints conforming
Product
· Product Recall
s.c B
GM
· Validation Master Plan,
M e
lat
A ISO 8.3, FDA D4/5 A
· Master Equipment List .
w Q06-30 .G · Design & Development Plan; p
w Q07-30
w m
wFacility & Equipment Requirements ww · Define Inputs, Generate Outputs;
e
Design, Develop &
© Document Product · Design Reviews; P t
©
T GM
ISO Section 6.3, FDA IV/B/3
& Processes · Design Verification/Validation;
GH H T ISO 7.3, FDA C/1
· Design Change Control
Q08-03
.
· Monitor trends
ft
I IG Analysis of Data w · Process Improvements so m
PY
R R ww
ISO 8.4, FDA D1
r o o
PY
· Facility Requirements c
O · Facility Design Guidelines © n Mi es.c
C · System Impact Assessment C O Q07-40 · Control Purchasing; H T i
ble mp
la t
· Component Criticality Analysis Purchasing &
Examine Inputs
· Document Purchasing;
R IG ila
va mpt
e
· Equipment & Drawing Control · Verify purchased products a
ISO 7.4, FDA C/2
P Y is . g
art www
· Equipment Pre-Qualification Process &
CO Q08-04 h
c at
Documentation
Make Quality flow at
Improvements is rm
· Q06-32 Equipment Qualification Q07-50
Perform & Monitor
· Validation (CV, PV, CSV) ISO 8.5, FDA D6 Th io fo
· Q06-36 Maintenance Program
· Q06-37 Laboratory Facility Mfg Operations
· Production & Process Control
· QC Criteria (In-process testing,
Vis
ISO 7.5, FDA C/3
Stability Program)
· QA & QC Checkpoints – batch
m
.co
release, sampling
Q06-40 · QC Lab Operations
Control Outsourced Operation,
te s · Storage & Warehousing
Work Environment
ISO 6.4, FDA IV/B/4
p la The drawing is the property & copyright of www.GMPtemplates.com.
Do not copy, distribute or reproduce without prior written permission from
te m GMPtemplates.
P
· Suppliers – Technical Agreement
.GM Q07-60
Control of · Calibration Program &
· Suppliers – External Audit, w
ww
monitoring & Schedules of Devices;
Monitoring measuring devices · Equipment Control www.GMPtemplates.com
· Q06-46 Environmental MonitoringT
© ISO 7.6, FDA C/3
Online Store for GMP Document Templates
· Q06-47 EH&S H
R IG Model for
O PY Q07-70
Address
· Document Deviations;
Address & control
Quality Management System
C Nonconformities
ISO 7.7, FDA C/4
CAPA
SIZE Filename DWG NO REV
info@GMPtemplates.com
A3 Q00-00-01 QMS Flowchart 2
SCALE 1:1 SHEET 1 OF 1
4321
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Analysis of Fats and OilsDocument19 pagesAnalysis of Fats and Oilsvishnoi1988% (8)
- PDG02 Documents and Records Process Description PDFDocument40 pagesPDG02 Documents and Records Process Description PDFAnonymous BcT42WLnNo ratings yet
- Where are we now and where should we go? GAP ANALYSIS OF FOOD SECTORDocument30 pagesWhere are we now and where should we go? GAP ANALYSIS OF FOOD SECTORSreekumarNo ratings yet
- EURAMET CG 11.01 Temperature IndicatorsDocument21 pagesEURAMET CG 11.01 Temperature IndicatorsSriniramu SriniramuNo ratings yet
- PDG02 Documents and Records Process Description PDFDocument40 pagesPDG02 Documents and Records Process Description PDFAnonymous BcT42WLnNo ratings yet
- White Paper 10 Golden RulesDocument19 pagesWhite Paper 10 Golden RulessweekarNo ratings yet
- Refining of Soya Bean Oil PDFDocument79 pagesRefining of Soya Bean Oil PDFjackully100% (8)
- Iodine ValueDocument3 pagesIodine ValuearshadgilaniNo ratings yet
- Anisfield - Investigating PDFDocument31 pagesAnisfield - Investigating PDFKuldeepNo ratings yet
- Peroxide Value DeterminationDocument5 pagesPeroxide Value DeterminationJohn Paul Pasicaran75% (20)
- Concentrations of Common Commercial Acids and BasesDocument1 pageConcentrations of Common Commercial Acids and BasesNazimah MaqboolNo ratings yet
- Persuasion Skills BasicsDocument114 pagesPersuasion Skills BasicsErmiza100% (1)
- Basicv 7Document29 pagesBasicv 7Anonymous BcT42WLnNo ratings yet
- Kjeldhal MethodDocument18 pagesKjeldhal MethodLaksilu Viduraga Peiris100% (4)
- AOCS-Method Free Fatty AcidDocument2 pagesAOCS-Method Free Fatty AcidAnonymous BcT42WLn100% (7)
- Kjeldhal MethodDocument18 pagesKjeldhal MethodLaksilu Viduraga Peiris100% (4)
- AOCS Acid ValueDocument1 pageAOCS Acid ValueAnonymous BcT42WLnNo ratings yet
- This Information Is Not Meant To Replace Company Policies or ProceduresDocument3 pagesThis Information Is Not Meant To Replace Company Policies or ProceduresAnonymous BcT42WLnNo ratings yet
- Self QualificationDocument30 pagesSelf QualificationAnonymous BcT42WLn100% (1)
- 2 4 1 Rev 9 Classification enDocument51 pages2 4 1 Rev 9 Classification enTowhidulIslamNo ratings yet
- BC34.1 E9 Determination of Acid Value of FatsDocument3 pagesBC34.1 E9 Determination of Acid Value of FatsGlenn Vincent Tumimbang96% (26)
- Good Documentation PracticesDocument34 pagesGood Documentation PracticesAnonymous BcT42WLn100% (1)
- White Paper 10 Golden RulesDocument19 pagesWhite Paper 10 Golden RulessweekarNo ratings yet
- HR Function Audit Checklist: Recruitment, Training & DevelopmentDocument4 pagesHR Function Audit Checklist: Recruitment, Training & DevelopmentAnonymous BcT42WLn100% (1)
- Keys To Good DocumentationDocument21 pagesKeys To Good DocumentationAnonymous BcT42WLnNo ratings yet
- Good Documentation PracticeDocument26 pagesGood Documentation PracticeAnonymous BcT42WLn100% (1)
- Total Acid Number (TAN) (ASTM D664) : Potentiometric Titration Application: Petrochemical OilsDocument5 pagesTotal Acid Number (TAN) (ASTM D664) : Potentiometric Titration Application: Petrochemical OilsI H AnsariNo ratings yet
- Good Documentation PracticeDocument37 pagesGood Documentation PracticeAnonymous BcT42WLnNo ratings yet
- Reactionsofalcohols PDFDocument9 pagesReactionsofalcohols PDFAnonymous BcT42WLnNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Synthesis Without Meta-Analysis (Swim) in Systematic Reviews: Reporting GuidelineDocument6 pagesSynthesis Without Meta-Analysis (Swim) in Systematic Reviews: Reporting GuidelineAngela TjungNo ratings yet
- ICE 3006A-Continuing Professional DevelopmentDocument2 pagesICE 3006A-Continuing Professional DevelopmentAmar MistryNo ratings yet
- Event Management A New ProfessionDocument7 pagesEvent Management A New ProfessionMustafa Tariq ButtNo ratings yet
- Assessment of School Security Practices Implemented at Visayas State University Tolosa in The New NormalDocument13 pagesAssessment of School Security Practices Implemented at Visayas State University Tolosa in The New NormalInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Software Quality ConceptsDocument38 pagesSoftware Quality Conceptskiran reddyNo ratings yet
- NLE Test Taking StrategiesDocument31 pagesNLE Test Taking StrategiesAr-jay JubaneNo ratings yet
- Cste 01Document4 pagesCste 01api-3733726No ratings yet
- Field Development PlanDocument23 pagesField Development PlanDjamel EddineNo ratings yet
- National Teachers College: Graduate Program, School of Teacher EducationDocument4 pagesNational Teachers College: Graduate Program, School of Teacher Educationariane galenoNo ratings yet
- Manajemen Proyek Teknologi InformasiDocument5 pagesManajemen Proyek Teknologi InformasiLiyaNo ratings yet
- Study - ISO 13485 PDFDocument15 pagesStudy - ISO 13485 PDFAnonymous 78Ezy46qvNo ratings yet
- Intern Report Format 8th Semester TUDocument10 pagesIntern Report Format 8th Semester TUPratibha YadavNo ratings yet
- Accounting Technologies and SustainabilityDocument13 pagesAccounting Technologies and Sustainabilitycharme4109No ratings yet
- Consumer Decision Making Process Model For Housing Demand: November 2015Document12 pagesConsumer Decision Making Process Model For Housing Demand: November 2015Ahmad HafizNo ratings yet
- (BS ISO GUIDE 80) - Guidance For In-House Production of Reference Materials For Quality Control (QCMS) PDFDocument60 pages(BS ISO GUIDE 80) - Guidance For In-House Production of Reference Materials For Quality Control (QCMS) PDFmrphongvnctNo ratings yet
- Helping Process in Social Work With GroupsDocument59 pagesHelping Process in Social Work With GroupsMohaimen EsmailNo ratings yet
- Corporate GovernanceDocument20 pagesCorporate GovernanceSyed Israr HussainNo ratings yet
- CBPRDocument60 pagesCBPREdgar MandengNo ratings yet
- DepEd Region 3 Research Policies MemoDocument36 pagesDepEd Region 3 Research Policies MemoAlvin Benavente100% (1)
- Quate For FSSC 22000Document2 pagesQuate For FSSC 22000AnkurNo ratings yet
- ICAO-Doc-9906-AN472-Volume 6-Quality Assurance Manual For Flight Procedure Design-1st EditionDocument64 pagesICAO-Doc-9906-AN472-Volume 6-Quality Assurance Manual For Flight Procedure Design-1st Editionjimi47gonzalezNo ratings yet
- IRIS Certification BDDocument2 pagesIRIS Certification BDMaciel Moreira SantosNo ratings yet
- Performance Budgeting: Muhammad Salim 07217003909Document6 pagesPerformance Budgeting: Muhammad Salim 07217003909salim1321No ratings yet
- Transport Canada TP 9685EDocument160 pagesTransport Canada TP 9685EbradnixonNo ratings yet
- RSPN PINS ER 3 ME Framework Final 15 Apr 19Document116 pagesRSPN PINS ER 3 ME Framework Final 15 Apr 19Sajid MallahNo ratings yet
- Log BookDocument131 pagesLog BookFahad Basheer100% (2)
- BPM tool guide for process automationDocument2 pagesBPM tool guide for process automationShiela TenidoNo ratings yet
- Contoh Table of ContentDocument3 pagesContoh Table of Contentmuhammad surooNo ratings yet
- Case Analysis:: PV Technologies, Inc.: Were They Asleep at The Switch?Document4 pagesCase Analysis:: PV Technologies, Inc.: Were They Asleep at The Switch?msbinuNo ratings yet
- National Council of Teachers of EnglishDocument14 pagesNational Council of Teachers of EnglishFitria Permata SariNo ratings yet