Professional Documents
Culture Documents
Corrosion Control Using Anodic Protection
Uploaded by
WK SinnOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Corrosion Control Using Anodic Protection
Uploaded by
WK SinnCopyright:
Available Formats
Corrosion Control by Anodic
Protection
By c. Edeleanu, M.A., Ph.D.
Tube Investments Research Laboratories, Cambridge
It is well known that corrosion can somc-
times be controlled by cathodic currents and, The technique of cathodic protection is
even with an elementary knowledge of electro- well known and has been widely applied
chemistry, it is easy to appreciate why this to a number of corrosion problems. It
should be so. Corrosion involves the oxida- is not so well known that corrosion can
tion of the metal and it is reasonable to expect also be prevented in suitable cases by
that cathodic polarisation, which discourages anodic protection, using a platinum
oxidation and favours reductions at the metal electrode system. The author shows that,
surface, should tend to cause protection. In with adequate laboratory work before-
fact, the position is somewhat more compli- hand and proper instrumentation, the
cated and, in many cases, other factors use of anodic protection can make an
override this apparently simple one. efectiue contribution to the life of a
It is not so well known that corrosion can chemical plant.
also be prevented in suitable cases by anodic
polarisation, and it is certainly very much
more difficult to understand why this should
the driving force available for corrosion to a
be so from the somewhat oversimplified
minimum, and the other is to ensure that the
theory of corrosion which the non-specialist
corrosion product itself stifles the reaction
is bound to have. It is probably because of
by forming a suitably protective film.
this that this method, which is extremely
Using the terminology devised by Pourbaix
powerful and is often applicable just when
(I), we say that we make use of immunity in
cathodic protection is not possible, has not
the first case while in the second we depend
been easily accepted as a practical proposi-
on passivity.
tion and is still regarded as only a laboratory
In practice we can achieve immunity by
curiosity. There is, it seems, a feeling, per-
doing one or more of the following:
haps unconscious, that the method is basically
unsound, and the purpose of the present paper (I) Using a suitably noble metal
is to explain, in as simple a way as possible, (2)Removing unnecessary oxidising
why anodic protection is possible, and when agents (e.g. air)
it may be expected to be useful.
(3) Adding a cathodic inhibitor (lessening
the effectiveness of the oxidising agents)
General Principles
in Corrosion Control (4) Applying cathodic protection
If the “brute force” methods of corrosion In chemical plant it is often not economic
control such as plastic, glass or other coatings to use noble metals, and if the solutions are
are neglected, there are two basic methods of highly oxidising the other methods are in-
corrosion control available. One is to reduce applicable.
Platinum Metals Rev., 1960, 4, (3), 86-91 86
Passivity is achieved by: 1.6
(I) Using a metal having an oxide (or other
1.2
similar corrosion product) which is
virtually insoluble in the medium
0.8
(2) Ensuring that sufficient oxidising agent
is always present for the oxide to be 0.4
J
formed -4
+
(3) Applying anodic polarisation to main- 2
w
0
c
tain the oxide in constant repair
2- 0 . 4
In principle therefore anodic protection
has much in common with the practice of
adding oxidising substances such as chromates
- 0.8
or nitrites as inhibitors. Cathodic protection
i
IMMUNITY
on the other hand is, in some ways, related
to practices such as de-aeration. -I-2
The similarity can be taken further. In a 1
metal,/solution system in which corrosion is
low because of immunity, corrosion is gener-
Fig. 1 Pourbaix diagram .for iron in aqueous
ally enhanced by either the addition of solutions
oxidising agents or by anodic polarisation,
while in a case depending on passivity it is
dangerous either to de-aerate or to apply practical way of avoiding corrosion both
cathodic currents. because of the very heavy current require-
ment and because there is little point in
Protection of Ferrous Materials preventing corrosion if to do so we have to
in Acid Solutions decompose the solution.
Anodic protection will probably prove most Raising the potential of iron by anodic
useful with iron-based alloys in acid solutions polarisation or by the addition of a suitable
and for this reason this case has been selected oxidising agent to sufficiently high values for
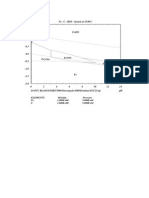
as an example. Fig. I shows the Pourbaix passivity does, on the other hand, seem to
diagram (I) for iron; the conditions for be a more promising way of avoiding corro-
passivity and immunity are indicated. From sion. This is particularly so since the area
this it will be seen that, in acid solutions, of passivity for iron, and especially for some
there is a considerable gap of potentials over of the iron-chromium alloys, is considerably
which neither of these conditions is estab- larger than indicated by Fig. I which was
lished and which should lead to heavy cor- obtained by calculation after making certain
rosion. assumptions.
Lines A and B in this diagram refer to the The actual relation between potential and
lower and upper limits of stability of water. corrosion rate at a given pH is shown dia-
Above A water is oxidised to oxygen and grammatically in a somewhat simplified
below A it is reduced to hydrogen. manner in Fig. 2 . This is an experimentally
If we place iron in a strong acid solution determinable curve for any given solution and
we can in theory protect it cathodically by alloy by using the potentiostatic techniques
lowering its potential to the region of im- which are becoming widely used in corrosion
munity. However, since water is not stable studies (2). From Fig. 2, which is typical
at such low potentials, continuous and rapid of many cases, it can be seen that once the
hydrogen evolution will occur. This is not a potential is raised sufficiently to establish
Platinum Metals Rev., 1960, 4, ( 3 ) 87
passivity the corrosion rate falls to really From the above it must have become
negligible values. For example with iron obvious that anodic protection is simply a
in normal sulphuric acid the rate falls to way of ensuring that the potential of the
approximately 0.I mgj cm2’day and the cur- metal is kept sufficiently high for passivity
rent density necessary to maintain passivity to be stable.
is 5 pA,lcm2. The rate of corrosion of passive
iron in this acid is therefore negligible and iron Instrumentation
could be a very satisfactory container material. If the potential of iron is raised appreciably
It is important to appreciate at this stage above line A in Fig. I , oxygen evolution takes
that the rate of corrosion of a metal in a given place (i.e. the solution starts being decom-
acid solution is an accurately determinable posed and current is wasted) so that this
property provided the potential is specified. imposes an upper limit to the desirable
The highly scattered and apparently meaning- potential. With the stainless steels oxygen is
less results often obtainable on conventional not generally evolved, but the corrosion rate
corrosion “test specimens” are entirely due to increases above a certain potential so that
the potential wandering in an uncontrolled again there is an upper limit for the potential,
manner, but once results such as those in With titanium (3), and some other metals
Fig. 2 have been obtained for a given metal’ which form non-conductive films, there is
solution system we can fully depend on them generally much greater latitude and it is
in practice, again provided we also ensure that often possible to raise the potential by some
the potential of the plant relative to the solu- tens of volts, but in these cases too the pro-
tion is kept at the correct value. Alternatively tection can break down if the potential is
we can monitor accurately the rate of corro- raised sufficiently.
sion by measuring the potential and referring The important fact is that there is an
to Fig. 2. upper, as well as a lower, limit to the range of
potentials which give satisfactory results.
This means that the instrument required for
anodic protection is a “potentiostat” but the
exact nature of the instrument depends
greatly on the system.
If the range of satisfactory potentials is
large, as with titanium, a very simple constant
voltage device such as an accumulator or
even a dry cell will meet the requirements.
I n such a case it can safely be assumed that
the potential of the inert cathode will not
wander by more than a few hundreds of
millivolts no matter what the current may be,
and if the potential between the cathode and
the plant is kept sufficiently great there will
be no danger that the potential of the plant
will fall to the breakdown point. Cotton has
in point of fact found this system completely
satisfactory for titanium in hydrochloric acid.
This simple method should also be applic-
CORROSION R A T E
able in certain cases for ferrous alloys, even
Fig.2 Relation between potential and corrosion though the useful potential range is only a
rate for iron i n sulphuric acid few hundreds of millivolts but, in general, it
Platinum Metals Rev., 1960, 4, ( 3 ) 88
would be safer to use a true potentiostat. itself, the rate of corrosion is very high. In
This instrument measures the potential of some cases this rate can be many orders of
the plant against a standard electrode, and magnitude greater than that of the passive
maintains it at the desired value by passing a metal. If a vessel were to go active, in order
polarising current through an inert auxiliary to re-establish passivity the protective device
electrode. would have to be able to supply a current
There are numerous potentiostat circuits equivalent to the highest possible rate of
available and the laboratory types are fully corrosion. This means that the potentiostat
electronic and can control potentials very must be able to provide a current many
accurately but have a rather low current out- orders of magnitude above that necessary for
put. For industrial use output is the main protection, and if it cannot it may lose control.
requirement, and a servo-operated instrument This is the reason why monitoring is thought
would be more satisfactory. to be advisable. This danger may be one
The cost of equipment for anodic protec- reason why the method has not found much
tion should not be high even if a true potentio- support up to now. Serious as it is, it has
static system is called for but, if the method is certainly been overstated possibly because, in
to be used to best advantage, it is worth an effort PO demonstrate the spectacular
installing, at the same time, a monitoring possibilities of the method, the solution used
system to provide a record of the performance in the first pilot plant experiments was one
of the plant from the corrosion point of of the most difficult to handle (6). In that
view (4). This could also provide a warning case the potentiostat available was highly in-
should anything unforeseen occur. adequate for the purpose (having been con-
The position is exactly analogous to the structed for laboratory studies on small
use of a temperature controller on, for in- specimens) and could supply a current great
stance, a furnace, which will protect the enough for protection, but there was little in
furnace from overheating, but, without a hand to allow for even small local accidents.
temperature recorder or at least an indicator, Nevertheless the plant ran successfully for
the system is incomplete. many hundreds of hours. More recent
American work (7, 8, 9) has shown that the
Dangers and Limitations in the risk is not unduly great, and with suitable
instrumentation it should be possible to
Application of Anodic Protection
overcome this difficulty entirely.
The method is particularly suitable for It is not possible to enumerate all the
application in the heavy chemical field, but limitations of the method but it is just worth
the solutions handled in chemical plant differ pointing out that not all metals show an
so greatly that each case has to be studied on adequate range of passivity, and that with
a laboratory scale before anodic protection any given metal passivity will not be stable in
can be safely applied. all solutions. The method depends on an
This preliminary work must include a electrolytic current arriving at the metal so
metallographic study, since there are various that it is inapplicable above the wash line
types of corrosion such as intercrystalline in a vessel or in similar places.
corrosion and selective attack that can limit
the use of alloys to a smaller range of poten- Applications of Anodic Protection
tial than might be appreciated (5). Although there have been some reports in
The greatest danger comes, however, from the technical press (9, 10)of the use of anodic
the shape of the curve sketched in Fig. 2. protection, and there have been a few other
In this it can be seen that at potentials just trials, the method has as yet hardly been
below those at which protection establishes tried in practice.
Platinum Metals Rev., 1960, 4, ( 3 ) 89
From a corrosion point of view all chemical the solution is a good conductor. Naturally,
plant tends to be grossly over-designed, since it is somewhat morc difficult to deal with an
it is like a furnace without a temperature con- accidental breakdown at the end of a tube
troller or recorder. The scope for the use of than inside a vessel, but it is relatively easy to
protection and/or monitoring is therefore assess the risks involved.
enormous. With stainless steel plant, for It is not possible to protect above the wash
instance, it is usual to maintain acid strength, line in a vessel where corrosion may be due to
temperatures, pressures or other such vari- spray. Some parts of valves and pumps are
ables below values which give trouble. Since also difficult, but there is no reason why
there is generally no means of telling how materials which are naturally resistant should
near the plant is to losing passivity the not be used at the danger points in conjunc-
materials are not used to their limit. Another tion with inferior materials elsewhere. Pro-
way of saying the above is that unnecessarily vided the materials are suitably selected there
expensive grades of material are usually should be no complications with stray
selected for chemical plant in order to currents.
provide some degree of safety. In so far as electrodes are concerned the
It seems that it is possible to make a dis- standard, if used, could be similar to that
tinction between two uses of anodic protec- which would be used for pH measurements
tion. In the first instance it should be possible in the same medium. Bearing in mind how-
to employ it in order to allow existing plant ever that the accuracy required of the standard
and materials to be used to their limit, with for this application is not great, very simple
anodic protection and/or monitoring only as and robust standards could be used instead.
a safety device. With courage however there For example, a platinum wire responding to
seems no reason why plant should not be the natural redox potential of the solution
specially designed from inferior materials would be adequate if this were reasonably
which would depend for survival entirely on stable.
anodic protection. In this case, of course, As far as the cathode is concerned there is
the anodic protection system may have to be again considerable latitude, but it is worth
expensive but the economics could turn out remembering one point. If a potentiostatic
to be attractive if there were a substantial system is used there may be short periods
saving on construction material, or if the plant when the polarity of the current is reversed
could be run under conditions much beyond so that the cathode becomes an anode. For
anything that could be visualised without this reason if this electrode is made from, say,
protection. copper or nickel, in the hope that it will be
protected cathodically, it may well vanish
Plant and Electrode Design during these reversals of polarity and, for
There seems to be only one plant design this reason, it is felt that noble metals are
feature to take into account. An electrolytic more convenient. Platinum is a natural choice
current must flow to the plant for protection. because of its good electrical conductivity,
The current necessary is generally lower than low hydrogen overvoltage, good sealing to
IopA/cm2, and it is relatively easy to calcu- glass and not least the ease of cleaning were a
late how far it will “throw” if the conductivity deposit to be formed as a result of the passage
of the solution is known and if the available of a current.
voltage range has been established. In
practice it is found that the throwing power Summary and Conclusions
is enormous, as has been demonstrated by From a corrosion point of view anodic
recent American work (9), and reasonably protection is, to a chemical plant, what a
long tubes can be protected easily provided temperature controller is to a furnace. With-
Platinum Metals Rev., 1960, 4, ( 3 ) 90
out anodic protection chemical plant has to point of fact there is nothing more strange in
be overdesigned and best use is not made of protection by an anodic current than there is
materials. in protection by oxidising agents such as
The method has hardly been used in prac- chromates, which are universally accepted.
tice although it is simple to apply. This is There are of course dangers and limitations
probably partly due to an inadequate under- but, with adequate laboratory work and suit-
standing of how the method works and a able instrumentations these do not amount to
feeling that it is a laboratory curiosity. In a serious objection to the technique.
References
I M. Pourbaix . Thermodynamics of Dilute Aqueous Solutions,
Arnold, London, 1949
2 V. Cihal and M. IPrazak .. .. J . Iron & Steel Znst., 1959, 193, 360
C. Edeleanu . . .. .. .. J. Iron & Steel Inst., 1958, 188, 122
3 J. B. Cotton .. .. .. .. Chem. and Znd., 1958, p. 68; 1958, p. 492
4 C. Edeleanu .. .. .. .. Corrosion Technology, 1955, 2, 204
5 C. Edeleanu .. .. .. .. J. Iron & Steel Inst., 1957, 185,482
6 C . Edeleanu .. ,. .. .. Metallurgia, 1954, 50, 113
7 J. D. Sudbury, 0. L. Riggs,and D. A. Corrosion, 1960, 16, 91
Shock
8 D. A. Shock, 0. L. Riggs, and J. D. Corrosion, 1960, 16,99
Sudbury
9 0. L. Riggs, M. 13utchison, and N. L. Corrosion, 1960, 16,102
Conger
I0 W. Mueller .. .. .. .. Canadian J. of Technology, 1956, 34, 162
Properties of Platinum Metals and Alloys
AN ANNOTATED BIBLIOGRAPHY
The literature dealing with the properties The review of this mass of literature ex-
of platinum and the platinum group metals is, tends to 105 pages and is reasonably compre-
on the whole, sparse and widely scattered. On hensive. The publication as a whole is likely
this account a recent publication, called a to prove an invaluable source book to anyone
“technical phasc report”, prepared by R. W. interested in the literature of the platinum
Douglass, F. C. Holden and R. I. JafTee, of metals, but it is rather less valuable as a
Battelle Memorial Institute for the U.S. critical survey. The brief introductory notes
Office of Naval Research, is particularly on extraction and benefication are, for
welcome. This was written with the special instance, misleading as far as modem condi-
intention that it should serve as a guide to tions are concerned, for today South Africa
planning experimental work on the platinum is undoubtedly the most significant world
group metals, “revealing”, as the authors put source of the platinum metals. A few of the
it, “areas where concentrated study is needed figures quoted for the physical and mechanical
and preventing duplication of previous work” properties are certainly in error-at least as
and was produced as the first part of a study far as the pure metals are concerned-and
at Battelle of the metallurgical properties of need to be treated with much more reserve
the refractory platinum group metals. than is accorded them by the authors. How-
As it is presented, this report provides a ever, if this is treated as a first-class annotated
very careful survey of the literature of the bibliography-which it primarily is-the
past fifty years on the properties of the report will be found a most useful work of
metals and on the constitution of their reference by all interested in the platinum
binary alloys, listing 281 references. metals. J. C. C.
Platinum Metals Rev., 1960, 4, ( 3 ) 91
You might also like
- Anodic Protection Lecture23 PDFDocument5 pagesAnodic Protection Lecture23 PDFKantilal MalwaniaNo ratings yet
- Anodic ProtectionDocument30 pagesAnodic ProtectionLuis Eduardo PereiraNo ratings yet
- The Electrochemistry and Characteristics of Embeddable Reference Electrodes for ConcreteFrom EverandThe Electrochemistry and Characteristics of Embeddable Reference Electrodes for ConcreteNo ratings yet
- Anodic ProtectionDocument50 pagesAnodic ProtectionEngr Arfan Ali DhamrahoNo ratings yet
- A Fully Differential PotentiostatDocument8 pagesA Fully Differential PotentiostatguiburNo ratings yet
- BCGA GN 41 Original 03 08 2020Document63 pagesBCGA GN 41 Original 03 08 2020natee8632No ratings yet
- IEEE 515 2011 Pre PDFDocument12 pagesIEEE 515 2011 Pre PDFSachin SehgalNo ratings yet
- 2008 MATCOR Technical Bulletin - Deep Well Anode System Design FINALDocument10 pages2008 MATCOR Technical Bulletin - Deep Well Anode System Design FINALNWALLL100% (1)
- CH 06 - Corrosion & ErosionDocument22 pagesCH 06 - Corrosion & ErosionvegaronNo ratings yet
- Hydrogen Permeation ExperimentsDocument2 pagesHydrogen Permeation ExperimentssgarrabNo ratings yet
- Telluric Currents-Paper No. 00741Document7 pagesTelluric Currents-Paper No. 00741Juan David Benitez MonroyNo ratings yet
- TR External Polymeric FailureDocument3 pagesTR External Polymeric FailureJuliano SampaioNo ratings yet
- NES 704 Part 5 Requirements For Cathodic Protection - Gneral Information On Bi-Metallic Couples Category 2Document26 pagesNES 704 Part 5 Requirements For Cathodic Protection - Gneral Information On Bi-Metallic Couples Category 2JEORJE0% (1)
- Breaking Down AC Corrosion of PipelinesDocument7 pagesBreaking Down AC Corrosion of PipelinesHenryNo ratings yet
- Brosur Soft Iron AnodeDocument2 pagesBrosur Soft Iron AnodekaryantoherlambangNo ratings yet
- Chloride SCC of 316 SSTDocument5 pagesChloride SCC of 316 SSTSH1961100% (1)
- Pipeline Current Mapping Overview v2.0Document2 pagesPipeline Current Mapping Overview v2.0AmanSharmaNo ratings yet
- Prediction of The Dew PointDocument9 pagesPrediction of The Dew PointBaptiste RéaudNo ratings yet
- ME1112 Engineers Guide To Corrosion Causes Protection and ControlDocument162 pagesME1112 Engineers Guide To Corrosion Causes Protection and ControlFarid TataNo ratings yet
- Evaluation and Validation of Aboveground Techniques For Coating Condition AssessmentDocument63 pagesEvaluation and Validation of Aboveground Techniques For Coating Condition AssessmentMajid SattarNo ratings yet
- Anodic and Cathodic ProtectionDocument42 pagesAnodic and Cathodic Protectionavinash rampureNo ratings yet
- Ansi - Nema MG 1-2016 Contents and ForewordDocument80 pagesAnsi - Nema MG 1-2016 Contents and Forewordary rizku100% (1)
- Cathodic Protection An Overview 2Document18 pagesCathodic Protection An Overview 2Johnson KurienNo ratings yet
- Soil Pollution EvsDocument10 pagesSoil Pollution EvsSayan MandalNo ratings yet
- Corrosion Swimming)Document6 pagesCorrosion Swimming)skenny1No ratings yet
- INDUSTRIAL GAS PIPELINE INTEGRITY MANAGEMENT, EIGA, Doc 23521Document35 pagesINDUSTRIAL GAS PIPELINE INTEGRITY MANAGEMENT, EIGA, Doc 23521A NacerNo ratings yet
- Duplex Stainless Steel 2304 Spec Sheet for Weight Savings & Corrosion ResistanceDocument2 pagesDuplex Stainless Steel 2304 Spec Sheet for Weight Savings & Corrosion ResistanceDeepak TdNo ratings yet
- A Model To Estimate The Failure Rates of Offshore PipelinesDocument11 pagesA Model To Estimate The Failure Rates of Offshore Pipelinesrovlad2000No ratings yet
- AL 6XN SourceBookDocument56 pagesAL 6XN SourceBookdrbeyerNo ratings yet
- Corrosion Resistance of High Nitrogen Steels PDFDocument27 pagesCorrosion Resistance of High Nitrogen Steels PDFAnil Kumar TNo ratings yet
- Shielding Vs Non-Shielding CoatingsDocument62 pagesShielding Vs Non-Shielding CoatingsAce AceNo ratings yet
- Acegirdle DNVGL RP F101CorrodedPipelinesDocument42 pagesAcegirdle DNVGL RP F101CorrodedPipelinesDimkNo ratings yet
- Dga Interpretation of Oil Filled Transformer: 1. Individual and Total Dissolved Combustible (TDCG) Gas ConcentrationsDocument3 pagesDga Interpretation of Oil Filled Transformer: 1. Individual and Total Dissolved Combustible (TDCG) Gas ConcentrationsjjrrsrNo ratings yet
- The Practical Salinity Scale 1978 and Its AntecedentsDocument6 pagesThe Practical Salinity Scale 1978 and Its AntecedentsVinícius MartinsNo ratings yet
- Hydrogen Embrittlement - Identifying AppearanceDocument2 pagesHydrogen Embrittlement - Identifying AppearanceeragornNo ratings yet
- DNV RP F103Document70 pagesDNV RP F103Bruno Nascimento100% (2)
- Advanced Internal Corrosion QuizDocument3 pagesAdvanced Internal Corrosion QuizHesham badawy100% (1)
- Antea Company BrochureDocument6 pagesAntea Company BrochureSabino LaraNo ratings yet
- IEEE 2014 Paper - Infrared Windows Applied in Switchgear Assemblies - Taking Another LookDocument6 pagesIEEE 2014 Paper - Infrared Windows Applied in Switchgear Assemblies - Taking Another Lookvenkat chakNo ratings yet
- Corrosion Rev02aDocument500 pagesCorrosion Rev02aDany Gonzalez HerreraNo ratings yet
- Longtermoxidationbehaviour PDFDocument10 pagesLongtermoxidationbehaviour PDFAnonymous lmCR3SkPrKNo ratings yet
- PREn - Pitting Resistance Equivalent NumberDocument2 pagesPREn - Pitting Resistance Equivalent NumberJacinto Gomez EmbolettiNo ratings yet
- 29784-The Impact of Corrosion On Oil and Gas IndustryDocument5 pages29784-The Impact of Corrosion On Oil and Gas IndustryhersystinNo ratings yet
- 4853Document8 pages4853mero2110No ratings yet
- Tubular Vent BinderDocument12 pagesTubular Vent BinderbayuNo ratings yet
- Sensor TechnologiesDocument10 pagesSensor TechnologiesMiguel LiceagaNo ratings yet
- CorossionDocument76 pagesCorossionviswamanojNo ratings yet
- DNV RP-G101 - 2010Document74 pagesDNV RP-G101 - 2010Mohammad TaherNo ratings yet
- High Temperature CorrosionDocument3 pagesHigh Temperature CorrosiontechzonesNo ratings yet
- Norwegian Oil and Gas Life Extension GuidelinesDocument20 pagesNorwegian Oil and Gas Life Extension GuidelinesPar MadNo ratings yet
- 9 CorrosDocument25 pages9 CorrosFrancisco Beltran100% (1)
- Ieee 1106Document26 pagesIeee 1106mahdi parsaNo ratings yet
- Cold End CorrosionDocument15 pagesCold End Corrosiondafteri11No ratings yet
- Hydrogen Permeation ThesisDocument16 pagesHydrogen Permeation ThesisAlberto SerranoNo ratings yet
- CorrISA Course Brochure JULY 2018Document23 pagesCorrISA Course Brochure JULY 2018Fredy MapsanganheNo ratings yet
- Cathodic ProtectionDocument22 pagesCathodic ProtectionFakhr-e-Alam100% (1)
- Anodic ProtectionDocument6 pagesAnodic ProtectionMazen AlhazeefNo ratings yet
- 2012 11 Full Paper BROESDER Coatings-And-cathodic-disbondmentDocument7 pages2012 11 Full Paper BROESDER Coatings-And-cathodic-disbondmentkakrasana100% (1)
- Cathodic Protection PDFDocument24 pagesCathodic Protection PDFJorge Luis Clavijo Iturri50% (2)
- 100 106 PMR Apr08Document7 pages100 106 PMR Apr08rrrogggerrrNo ratings yet
- P&G01Document3 pagesP&G01rrrogggerrrNo ratings yet
- Caracteristicas Normas ASME B31Document3 pagesCaracteristicas Normas ASME B31darthneoNo ratings yet
- CFD Analysis of Eductor Agitation in Electroplating TankDocument7 pagesCFD Analysis of Eductor Agitation in Electroplating TankrrrogggerrrNo ratings yet
- CFD Analysis of Eductor Agitation in Electroplating TankDocument7 pagesCFD Analysis of Eductor Agitation in Electroplating TankrrrogggerrrNo ratings yet
- AnodeDocument4 pagesAnoderrrogggerrrNo ratings yet
- Frequency of Maintenance TestingDocument5 pagesFrequency of Maintenance TestingIsmael AhmedNo ratings yet
- Equipment Costing: Chapter 22 (p558-597) CH EN 4253 Terry A. RingDocument20 pagesEquipment Costing: Chapter 22 (p558-597) CH EN 4253 Terry A. RingLívia AlmeidaNo ratings yet
- AnodeDocument4 pagesAnoderrrogggerrrNo ratings yet
- Composition High Temperarute AlloysDocument1 pageComposition High Temperarute AlloysrrrogggerrrNo ratings yet
- 56057Document11 pages56057rrrogggerrrNo ratings yet
- Knop - Iron DeterminationDocument7 pagesKnop - Iron DeterminationrrrogggerrrNo ratings yet
- Cathodic Pipeline PDFDocument6 pagesCathodic Pipeline PDFElias KapaNo ratings yet
- Anodic ProtectionDocument6 pagesAnodic ProtectionrrrogggerrrNo ratings yet
- 100Document40 pages100rrrogggerrrNo ratings yet
- Protección AnódicaDocument11 pagesProtección AnódicarrrogggerrrNo ratings yet
- Kjs 33 Mstankovic 63Document10 pagesKjs 33 Mstankovic 63rrrogggerrrNo ratings yet
- NORSOK STANDARD CATHODIC PROTECTIONDocument14 pagesNORSOK STANDARD CATHODIC PROTECTIONbreeeeezzzzzeNo ratings yet
- NORSOK Standard for Cathodic Protection M-CR-503Document16 pagesNORSOK Standard for Cathodic Protection M-CR-503Yetkin ErdoğanNo ratings yet
- Eh (Volts) Fe - C - H2O - System at 25.00 CDocument1 pageEh (Volts) Fe - C - H2O - System at 25.00 CrrrogggerrrNo ratings yet
- Kjs 33 Mstankovic 63Document10 pagesKjs 33 Mstankovic 63rrrogggerrrNo ratings yet
- Paginas de MontajeDocument1 pagePaginas de MontajerrrogggerrrNo ratings yet
- Syllabus - EU Institutions and Comparative Political System - 2023springDocument10 pagesSyllabus - EU Institutions and Comparative Political System - 2023springreif annieNo ratings yet
- Simha Lagna: First House Ruled by The Planet Sun (LEO) : The 1st House Known As The Ascendant orDocument3 pagesSimha Lagna: First House Ruled by The Planet Sun (LEO) : The 1st House Known As The Ascendant orRahulshah1984No ratings yet
- Details of Nodal Officer - HD Officers of Other DepttDocument46 pagesDetails of Nodal Officer - HD Officers of Other DepttManoj KashyapNo ratings yet
- Activity No.1 in GED 103Document2 pagesActivity No.1 in GED 103Kenneth HerreraNo ratings yet
- Manual Reductora IVECO TM 265 - TM 265ADocument31 pagesManual Reductora IVECO TM 265 - TM 265ARomà ComaNo ratings yet
- Structural Crack Identification in Railway Prestressed Concrete Sleepers Using Dynamic Mode ShapesDocument3 pagesStructural Crack Identification in Railway Prestressed Concrete Sleepers Using Dynamic Mode ShapesNii DmNo ratings yet
- 5.talisayon Chapter 3Document12 pages5.talisayon Chapter 3Jinky Marie TuliaoNo ratings yet
- AR Ageing FinalDocument13 pagesAR Ageing FinalHAbbunoNo ratings yet
- Baghouse Compressed AirDocument17 pagesBaghouse Compressed Airmanh hung leNo ratings yet
- GCSE Combined Science PDFDocument198 pagesGCSE Combined Science PDFMpumelelo Langalethu MoyoNo ratings yet
- Autonomy Necessity and Love by Harry FrankfurtDocument14 pagesAutonomy Necessity and Love by Harry FrankfurtjamesdigNo ratings yet
- Strategic ManagementDocument7 pagesStrategic ManagementSarah ShehataNo ratings yet
- Comprehensive Exam SchedDocument16 pagesComprehensive Exam SchedMark ErvinNo ratings yet
- Phy 111 - Eos Exam 2015Document7 pagesPhy 111 - Eos Exam 2015caphus mazengeraNo ratings yet
- Eugene A. Nida - Theories of TranslationDocument15 pagesEugene A. Nida - Theories of TranslationYohanes Eko Portable100% (2)
- Baikal Izh 18mh ManualDocument24 pagesBaikal Izh 18mh ManualMet AfuckNo ratings yet
- Occupational Health and Safety ProceduresDocument20 pagesOccupational Health and Safety ProceduresPRINCESS VILLANo ratings yet
- Text EditorDocument2 pagesText EditorVarunNo ratings yet
- Power Electronics Lab 1 (07DEM19F1005)Document15 pagesPower Electronics Lab 1 (07DEM19F1005)Mohd Faizul Idham AhmadNo ratings yet
- Net CallDocument2 pagesNet CallFerdinand Monte Jr.100% (2)
- Limit of Outside Usage Outside Egypt ENDocument1 pageLimit of Outside Usage Outside Egypt ENIbrahem EmamNo ratings yet
- Ici-Ultratech Build Beautiful Award For Homes: Indian Concrete InstituteDocument6 pagesIci-Ultratech Build Beautiful Award For Homes: Indian Concrete InstituteRavi NairNo ratings yet
- Optimizing Blended Learning with Synchronous and Asynchronous TechnologiesDocument24 pagesOptimizing Blended Learning with Synchronous and Asynchronous TechnologiesAnonymous GOUaH7FNo ratings yet
- Basic Vacuum Theory PDFDocument17 pagesBasic Vacuum Theory PDFada guevarraNo ratings yet
- Coolit SystemsDocument5 pagesCoolit SystemsUsama NaseemNo ratings yet
- 18.443 MIT Stats CourseDocument139 pages18.443 MIT Stats CourseAditya JainNo ratings yet
- Modern Tips For The Modern Witch (/)Document5 pagesModern Tips For The Modern Witch (/)Rori sNo ratings yet
- KireraDocument3 pagesKireramurithiian6588No ratings yet
- Sistem Informasi Geografis Pemetaan Lahan PertaniaDocument12 pagesSistem Informasi Geografis Pemetaan Lahan PertaniaRizal Nurrahman HabibNo ratings yet
- Module 5 Greek ArchDocument22 pagesModule 5 Greek ArchKyla A. EstoestaNo ratings yet