Professional Documents
Culture Documents
JPediatrNeurosci132176-312375 084037
Uploaded by
afriska chandraOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
JPediatrNeurosci132176-312375 084037
Uploaded by
afriska chandraCopyright:
Available Formats
[Downloaded free from http://www.pediatricneurosciences.com on Thursday, April 25, 2019, IP: 180.247.194.
95]
Original Article
Outcome Analysis of Ventriculoperitoneal Shunt Surgery in Pediatric
Hydrocephalus
Pradyumna Pan
Pediatric Surgery Unit, Aim: To study the clinical outcome of shunt surgeries in children with
Abstract
Ashish Hospital and
hydrocephalus and evaluate the risk factors for ventriculoperitoneal (VP) shunt
Research Centre, Jabalpur,
Madhya Pradesh, India
failure. Materials and Methods: Patients who underwent VP shunt surgery for
hydrocephalus were included. Medical charts, operative reports, imaging studies,
and clinical follow-up evaluations were reviewed and analyzed retrospectively.
Results: A total of 137 patients with the average age of 20.7 months, range
from 1.5 months to 8.5 years at the time of VP shunt surgery were included.
The incidence of overall shunt complications was 35.76%; incidence of shunt
revision was 27%, shunt blockade 45.94%, shunt infection 16.21%, shunt
migration 10.81%, and shunt malfunction due to abdominal pseudocyst 10.81%.
The mortality rate was 5.10%. The shunt revisions in the first 6 months after
shunt placement was observed in n = 9 (24%). Hydrocephalus was associated
with post-tubercular meningitis and intraventricular hemorrhage (IVH) in shunt
placement was associated with multiple shunt revisions (n = 13, 35.13%) (n = 5,
45.4%), respectively. Conclusion: The findings of this study indicate that etiology
of hydrocephalus, were associated with the shunt survival. Further prospective
controlled studies are required to address the observed associations
Keywords: Complications, hydrocephalus, ventriculoperitoneal shunt
Introduction the subdural collection, inguinal hernia, hydrocele,
ascites, pseudocyst formation, perforation of a viscus,
H ydrocephalus is one of the most common clinical
conditions affecting the central nervous system
with an incidence of 3–4 per 1000 births for congenital
or extrusion of the shunt.[2] We studied 137 patients
with hydrocephalus and analyzed the predisposing risk
factors and range of complications.
hydrocephalus.[1] Ventriculoperitoneal (VP) shunt is the
most frequently utilized diversion procedure because of
the capability of the peritoneum to absorb fluid. Initial
Materials and Methods
and subsequent peritoneal catheter placements can We performed a retrospective review of children with
be performed with relative ease. They are associated hydrocephalus that required VP shunt placement
with complications and may require multiple surgical between 2004 and 2014. Patient diagnoses were
procedures during a patient’s life span. divided into the following etiologies: congenital
anomaly, post-tubercular meningitis (TBM),
Complications of VP shunt surgery may be broadly hydrocephalus associated with spinal dysraphism, and
divided into (1) mechanical and (2) infective post-intraventricular hemorrhage (IVH) of newborn.
complications. Mechanical complications include A detailed record was kept with regard to name, age,
obstruction, disconnection or migration of any
components of a shunt system either at the ventricular Address for correspondence: Dr. Pradyumna Pan,
or peritoneal end. Infective complications include Pediatric Surgery Unit, Ashish Hospital and
Research Centre, Jabalpur,
ventriculitis, shunt tract abscess, and skin necrosis Madhya Pradesh 482001, India.
overlying the shunt device. Other complications are E-mail: dr_pan@rediffmail.com
This is an open access journal, and articles are distributed under the terms of the
Access this article online
Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows
Quick Response Code: others to remix, tweak, and build upon the work non-commercially, as long as
Website: appropriate credit is given and the new creations are licensed under the identical terms.
www.pediatricneurosciences.com
For reprints contact: reprints@medknow.com
How to cite this article: Pan P. Outcome analysis of ventriculoperitoneal
DOI: 10.4103/jpn.JPN_29_18
shunt surgery in pediatric hydrocephalus. J Pediatr Neurosci
2018;13:176-81.
176 © 2018 Journal of Pediatric Neurosciences | Published by Wolters Kluwer - Medknow
[Downloaded free from http://www.pediatricneurosciences.com on Thursday, April 25, 2019, IP: 180.247.194.95]
Pan: Outcome of VP shunt in pediatric hydrocephalus
sex, etiology, and clinical features. Investigations Increase in head circumference was the most common
included ultrasonography and computed tomography sign (74%) followed by tense anterior fontanel and splayed
(CT) scan of head to assess ventricular dilatation and cranial sutures (67%). A headache was present in 12%
ventricular–parenchyma thickness ratio. patients, vomiting in 11% and a refusal of feed in 9%,
behavioral changes in 3%, and lethargy in 7%. Papilledema
Causes of revision were divided into obstruction,
and sunset sign were seen in 6.5% and 5%, respectively.
infection, malposition, catheter fracture, and abdominal
problems. The causes of shunt revision were analyzed The incidence of shunt failure is highest in the first
with respect to the age of the patients and according to 6 months following the VP shunt,[3] however, may
the time interval of original shunt surgery and revision.
All patients underwent VP shunt in a standard protocol. Table 1: Demographic distribution of patients
Chhabra medium pressure slit and spring value shunt Etiology Number Age
system (Gurgiwear Ltd, Shahjahanpur, India) was Congenital 62 1.5–9 months
used. Early and late shunt complication was defined Post-TBM 35 4–8.5 years
according to the duration between the initial shunt Postspinal dysraphism 29 2–8 months
placement and appearance of the first shunt problem. Post-IVH 11 3–7 months
Those occurring within 6 months were early and later
than 6 months were late complications. Table 2: Relationship between the cause of revision surgery
and the time interval after initial shunt placement (months)
Results Causes of revision <6 6–12 12–24 >24
The study included 137 patients who underwent primary Obstruction 2 1 1 4
Infection 2 1 0 1
VP shunt. There were 78 male and 59 female patients.
Pseudocyst 0 0 1 3
Age distribution: 103 patients were below 1 year and
Extrusion 0 0 1 2
34 above 2 years. The median age of the patients at the Over drainage 0 1 0 0
time of VP shunt placement was 20.7 months (range Disconnection 0 0 0 1
1.5 months to 8.5 years). The etiologies of hydrocephalus Ventricular end 0 1 0 0
in the patient sample are shown in Table 1. Nine patients displacement
(6.5%) were operated on within 6 months after shunt
placement. Twenty-three patients required 1 shunt Table 3: Relationship between the cause of revision surgery
revision, 2 revisions in 11 patients, and 3 revisions in 3 and the time interval after second revision (months)
patients. Distribution of revision causes and time intervals Causes of revision <6 6–12 12–24 >24
between shunt placement and revision according to each Obstruction 3 0 1 2
complication are shown in Tables 2-4. Obstruction and Infection 2 1 0 0
infection were the most common causes of revision, Disconnection 0 0 0 1
occurring in 17 (45.9%) and 6 (16.2%) cases, respectively. Under drainage 0 1 0 0
Surgical procedures performed during shunt revisions in Extrusion 0 0 0 1
the order of frequency were the revision of peritoneal
part of the shunt (n = 12, 32.43%), revision of ventricular Table 4: Relationship between the cause of revision surgery
part of the shunt (n = 11, 29.72%), revision of the entire and the time interval after third revision (months)
shunt system (n = 7, 18.91%), cysts excision and revision Causes of revision <24 >24
of peritoneal catheter (n = 4, 10.81%), extra ventricular Pseudocyst 0 1
drainage and delayed re-shunt (n = 4, 10.21%), shunt Extrusion 0 1
removal and delayed re-shunt (n = 2, 5.4%), and opposite Obstruction 0 1
side shunting (n = 2, 5.40%). The mortality was 7 (5.10%).
Other complications that did not require shunt revisions Table 5: Complications not requiring shunt revision
are shown in Table 5. Complications n
Seizures 13
Abdominal pain 11
Discussion Hernia/hydrocele 7
Hydrocephalus is a common neurosurgical disease Superficial wound infection 7
that develops through a variety of etiologies, including Ascites 5
congenital anomaly, intracranial hemorrhage, infection, Shunt tract collection 3
and tumor. Hydrocephalus has been customarily Subdural collection 2
classified into obstructive and communicating variety. Craniosynostosis 1
Journal of Pediatric Neurosciences ¦ Volume 13 ¦ Issue 2 ¦ April-June 2018 177
[Downloaded free from http://www.pediatricneurosciences.com on Thursday, April 25, 2019, IP: 180.247.194.95]
Pan: Outcome of VP shunt in pediatric hydrocephalus
occur at all times. The incidence of complications
following VP shunt placement is reported to be around
20%–40%.[3] However, Stone et al.[4] reported 84.5%.
In this study, the most common complication was
blockage of the shunt that causes it to function
intermittently or not at all. We encountered shunt
blockage in 15 patients (10.94%). Among the nine
patients who had primary VP shunt, five were
post-TBM, three were post-IVH and one was
congenital hydrocephalus. The reasons for this were Figure 1: Blockage of the ventricular end
the presence of higher protein and cellular content in
the cerebrospinal fluid (CSF), leading to early shunt
obstruction. Ventricular end blockage was seen in nine
[Figure 1] and peritoneal end blockage was in six. Distal
obstruction was caused by collection of the tissue in
the slit valve and wrapping of omentum. To improve
the proper placement of the shunt, intraoperative
ultrasound[5] and neuro-navigation guidance are being
used particularly in patients with a small ventricle.[6]
Laparoscopic insertion of a peritoneal catheter was
also suggested for distal catheter insertion or revision.[7]
The incidence of shunt blockage complications has
been reported from 5% to 47%.[8] In this study, 24.32%
patients had proximal catheter blockage and 16.21%
patients had distal catheter blockage.
In this study, infection was the second most common
complication following shunt surgery responsible for
significant morbidity and mortality. An infection rate
of 4.37% was found in this study. Reported series has
shown the incidence ranging from 5% to 15%. Although
many factors seem to contribute to shunt infection, it is
likely that the contamination of the shunt system at the
time of surgery is the primary cause but also exposure
during cleaning of the surgical wound is the risk factor.
Approximately 70% of shunt infections present within
3 months and the remaining by 6 months of the surgical
Figure 2: X-ray showing disconnection of chamber and
procedure. In our study, n = 4 (66.6%) developed
ventricular end
infection within 6 months. The most common
organism was coagulase negative staphylococci (n = 4). suggested the simultaneous use of doubles gloves during
Staphylococcus epidermidis (n = 1) and Staphylococcus surgical procedures; we followed the same to reduce the
aureus (n = 1) were also seen.[9] infection. Shunt infection was treated by intermittent
Infection rates are much higher in those patients in whom CSF tapping and intravenous antibiotics based on
a longer period of hospitalization is required. Presence culture and sensitivity of CSF and exteriorization of
of sepsis markedly increases the probability of VP shunt. Revision shunt was performed only when CSF
shunt infections.[10] This emphasizes the need to reduce samples confirmed the absence of infection. Recently,
the duration of hospitalization and to maintain special antibiotic-impregnated shunt catheters have been shown
surgical wound care. Some authors[11] have reported an to reduce the infection rate significantly.[13] Others have
increased frequency of infection in children less than not found any reduction in the rates of complication.[14]
6 months of ages as compared to older than 1 year. Disconnections occur at any section of the shunt, but
Other studies did not find differences in the infection they are especially frequent at the sites of mobility and
rate between different children’s ages.[12] In this study, we with patient growth. Shunt disconnection was seen in
did not find any correlation with age. Kulkarni et al.[11] 2 patients aged 13 and 14.6 years [Figure 2]. Both had
178 Journal of Pediatric Neurosciences ¦ Volume 13 ¦ Issue 2 ¦ April-June 2018
[Downloaded free from http://www.pediatricneurosciences.com on Thursday, April 25, 2019, IP: 180.247.194.95]
Pan: Outcome of VP shunt in pediatric hydrocephalus
disconnection between chamber and upper end. It is
related to fibrosis and tight adhesion of the catheter to
the surrounding soft tissue, which prevented the sliding
of the catheter necessary to adjust to the growth of
children.[15]
Overdrainage was observed in one leading to slit-like
ventricles [Figure 3]. To restore a balanced flow of
CSF, it was necessary to change with a more accurate
pressure valve.
Underdrainage was seen in one not being able to relieve
the symptoms of hydrocephalus.
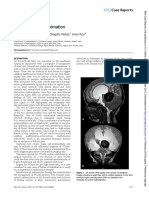
Subdural hematoma was observed in two [Figure 4]
and craniosynostosis in one [Figure 5]. Both subdural
hematoma formation and the occurrence of postshunt
craniosynostosis are due to craniocephalic disproportion.
The skull is not in a position to decrease in size to the
same extent that the brain does after placement of the
shunt, and this creates a space between the skull and
the brain. Postshunt craniosynostosis is the result of
apposition and overlapping of the cranial sutures in an
infant following treatment of hydrocephalus.
Seizures occurred in 13 patients. Epilepsy has been
involved in shunt-treated hydrocephalus. However, it Figure 3: CT scan of head showing over drainage with ventricular
is not believed to be directly related to the placement collapse
of the shunt. The reported percentage of children with
VP shunts and seizures amounts to 48%, but there is a
high association of seizures with underlying neurologic
disorders.[16] There is no relationship between the
number of shunt revisions or other complications. There
is insufficient direct confirmation of an association
between seizures and shunt surgery to necessitate
routine prophylaxis with antiepileptic drugs. With the
onset of further seizures in a child with a shunt, an
infection and/or obstruction should be excluded, and
then antiepileptic drugs should be considered.
Formation of CSF abdominal pseudocyst is a
well-recognized but not very frequent complication
(1%–4%).[17] We encountered pseudocyst in five patients
presented with increased abdominal girth and shunt
malfunction. All required excision of the pseudocyst
and revision of the peritoneal end of the shunt
[Figure 6].
Migration of shunt was observed in 3.64% (n = 5)
of cases. The distal catheter had migrated and was
extruded perrectally in two [Figure 7] and through the
abdominal wound in two cases. Displacement of the Figure 4: MRI of head showing subdural collection
ventricular end was observed in one.
Developmental milestones were delayed in 11 patients The role of, age at the time of shunt insertion has been
(20%); of them, 7 were of post-TBM, 3 were of IVH, analyzed previously in several studies.[18] Increased
and 1 was of spinal dysraphism. shunt failure was seen in patients who had undergone
Journal of Pediatric Neurosciences ¦ Volume 13 ¦ Issue 2 ¦ April-June 2018 179
[Downloaded free from http://www.pediatricneurosciences.com on Thursday, April 25, 2019, IP: 180.247.194.95]
Pan: Outcome of VP shunt in pediatric hydrocephalus
Figure 7: Rectal extrusion of the peritoneal end
of shunt placement. On literature review, event-free
survival at 1 year ranged from 62% to 80%[22] and at
10 years from 35% to 48%.[22] In this study, we found
56.12% and 37.7% event free at 1 year and 5 years,
Figure 5: Craniosynostosis respectively. The shunt-related mortality reported
being 8.6%–13.7%.[23] In our series, it was n = 7 (5.10%).
Conclusion
Hydrocephalus is a common neurological disorder.
VP shunt placement has been the main treatment
modality for hydrocephalus. It has amazingly changed
the neurological outcome in these patients with
enhanced chances of leading a normal life. VP shunts
procedures are still associated with complications and
morbidity. The development of shunt complications
was found to be significantly associated with the
etiology of the hydrocephalus. Other characteristics,
such as age and gender, did not affect the overall shunt
function. Prospective controlled studies are needed
to address the observed associations between the
Figure 6: Operative photo showing shunt tube in the abdominal risk factors and incidence of shunt revisions in these
pseudocyst patients.
shunt placement at age less than 1 year.[19] On the other Declaration of patient consent
hand, there are studies showing no effect of age on The authors certify that they have obtained all
the incidence of shunt infection.[20] This study did not appropriate patient consent forms. In the form the
support the age at the time of shunt placement to be patient(s) has/have given his/her/their consent for his/
an important predictor of shunt function. In our study, her/their images and other clinical information to be
only six patients had shunt failure who were shunted reported in the journal. The patients understand that
during initial 1 year of age. their names and initials will not be published and
due efforts will be made to conceal their identity, but
In our series, 67.56% were mechanical, and 16.2% were
anonymity cannot be guaranteed.
infective complications. Similar findings were seen in
other study.[21] In our study, 32.43% of patients with Financial support and sponsorship
shunt-related complications presented within 6 months Nil.
180 Journal of Pediatric Neurosciences ¦ Volume 13 ¦ Issue 2 ¦ April-June 2018
[Downloaded free from http://www.pediatricneurosciences.com on Thursday, April 25, 2019, IP: 180.247.194.95]
Pan: Outcome of VP shunt in pediatric hydrocephalus
Conflicts of interest 11. Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal
fluid shunt infection: a prospective study of risk factors. J
There are no conflicts of interest.
Neurosurg 2001;94:195-201.
12. Faillace WJ. Shunt infection. J Neurosurg 2001;94:1019-20.
References 13. Kandasamy J, Dwan K, Hartley JC, Jenkinson MD, Hayhurst C,
1. Laurence KM, Coates S. The natural history of hydrocephalus. Gatscher S, et al. Antibiotic-impregnated ventriculoperitoneal
Detailed analysis of 182 unoperated cases. Arch Dis Child shunts–a multi-centre British paediatric neurosurgery group
1962;37:345-62. (BPNG) study using historical controls. Childs Nerv Syst
2. Sgouros S, Malluci C, Walsh AR, Hockley AD. Long-term 2011;27:575-81.
complications of hydrocephalus. Pediatr Neurosurg 14. Costa BS, Maitrot TD. Prise en charge de l’hidrocéphalie
1995;23:127-32. de l’enfant au Brésil. Congres Table Ronde de La SNCLF,
3. Reddy GK, Bollam P, Caldito G. Long-term outcomes Paris, 2001.
of ventriculoperitoneal shunt surgery in patients with 15. Park MK, Kim M, Park KS, Park SH, Hwang JH, Hwang SK. A
hydrocephalus. World Neurosurg 2014;81:404-10. retrospective analysis of ventriculoperitoneal shunt revision cases
4. Stone JJ, Walker CT, Jacobson M, Phillips V, Silberstein HJ. of a single institute. J Korean Neurosurg Soc 2015;57:359-63.
Revision rate of pediatric ventriculoperitoneal shunts after 16. Klepper J, Büsse M, Strassburg HM, Sörensen N. Epilepsy
15 years. J Neurosurg Pediatr 2013;11:15-9. in shunt-treated hydrocephalus. Dev Med Child Neurol
5. Crowley RW, Dumont AS, Asthagiri AR, Torner JC, Medel 1998;40:731-6.
R, Jane JA Jr, et al. Intraoperative ultrasound guidance for 17. Sharma AK, Pandey AK, Diyora BD, Mamidanna R, Sayal
the placement of permanent ventricular cerebrospinal fluid PP. Abdominal CSF pseudocyst in a patient with ventriculo-
shunt catheters: a single-center historical cohort study. World peritoneal shunt. Indian J Surg 2004;66:360-3.
Neurosurg 2014;81:397-403. 18. Agarwal N, Shukla RM, Agarwal D, Gupta K, Luthra R,
6. Levitt MR, O’Neill BR, Ishak GE, Khanna PC, Temkin Gupta J, et al. Pediatric ventriculoperitoneal shunts and
NR, Ellenbogen RG, et al. Image-guided cerebrospinal fluid their complications: an analysis. J Indian Assoc Pediatr Surg
shunting in children: catheter accuracy and shunt survival. J 2017;22:155-7.
Neurosurg Pediatr 2012;10:112-7. 19. Liptak GS, McDonald JV. Ventriculoperitoneal shunts in
7. Martin K, Baird R, Farmer JP, Emil S, Laberge JM, Shaw K, children: factors affecting shunt survival. Pediatr Neurosci
et al. The use of laparoscopy in ventriculoperitoneal shunt 1985;12:289-93.
revisions. J Pediatr Surg 2011;46:2146-50. 20. Kinasha AD, Kahamba JF, Semali IT. Complications of
8. Tyagi DK, Balasubramaniam S, Jayaswal SA, Savant HV, ventriculoperitoneal shunts in children in Dar es Salaam. East
Gandhi AS. Outcome analysis of ventriculoperitoneal Cent Afr J Surg 2005;10:55-9.
shunt procedures in hydrocephalus due to tubercular 21. Lee JY, Wang KC, Cho BK. Functioning periods and
meningitis and non-infective cases. Int J Contemp Pediatr complications of 246 cerebrospinal fluid shunting procedures
2016;3:1210-5. in 208 children. J Korean Med Sci 1995;10:275-80.
9. Braga MH, Carvalho GTC, Brando RACS, Lima FBF, 22. Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop
Costa BS. Early shunt complications in 46 children with F, et al. Long-term follow-up data from the shunt design trial.
hydrocephalus. Arq Neuro-Psiquiatr 2009;67:145-8. Pediatr Neurosurg 2000;33:230-6.
10. Pople IK, Bayston R, Hayward RD. Infection of cerebrospinal 23. Tuli S, Tuli J, Drake J, Spears J. Predictors of death in pediatric
fluid shunts in infants: a study of etiological factors. J Neurosurg patients requiring cerebrospinal fluid shunts. J Neurosurg
1992;77:29-36. 2004;100:442-6.
Journal of Pediatric Neurosciences ¦ Volume 13 ¦ Issue 2 ¦ April-June 2018 181
You might also like
- Herbal Medicine Guide: Benefits, Uses and Safety TipsDocument12 pagesHerbal Medicine Guide: Benefits, Uses and Safety TipsVer BautistaNo ratings yet
- Learning Iron PalmDocument5 pagesLearning Iron Palmtenbears m.100% (2)
- Food PoisoningDocument11 pagesFood PoisoningLeanne Teh100% (1)
- Laser Tattoo RemovalDocument13 pagesLaser Tattoo RemovalAnonymous GUXjVMXUNo ratings yet
- SUCCEESSFULLY Navigating The First Year of Surgical ResidencyDocument56 pagesSUCCEESSFULLY Navigating The First Year of Surgical ResidencyYoya LawiNo ratings yet
- Schizoid Personality Disorder: Social Withdrawal and Lack of EmotionDocument3 pagesSchizoid Personality Disorder: Social Withdrawal and Lack of EmotionEzraManzanoNo ratings yet
- Dr. Dr. Tri Maharani, M.si., Sp. em - Snake Bite Medical Management CrebonDocument33 pagesDr. Dr. Tri Maharani, M.si., Sp. em - Snake Bite Medical Management Crebonrio100% (1)
- Dr. Dr. Tri Maharani, M.si., Sp. em - Snake Bite Medical Management CrebonDocument33 pagesDr. Dr. Tri Maharani, M.si., Sp. em - Snake Bite Medical Management Crebonrio100% (1)
- Dr. Dr. Tri Maharani, M.si., Sp. em - Snake Bite Medical Management CrebonDocument33 pagesDr. Dr. Tri Maharani, M.si., Sp. em - Snake Bite Medical Management Crebonrio100% (1)
- Normal and Pathological Bronchial Semiology: A Visual ApproachFrom EverandNormal and Pathological Bronchial Semiology: A Visual ApproachPierre Philippe BaldeyrouNo ratings yet
- Nursing Documentation Using the DAR Method for Patient with FeverDocument15 pagesNursing Documentation Using the DAR Method for Patient with FeverSergio Rivas100% (3)
- Quality Assurance in ICCUDocument61 pagesQuality Assurance in ICCUnayan4uNo ratings yet
- Pedoman Pengelolaan DM Tipe 2 Dewasa Di Indonesia Ebook PDFDocument132 pagesPedoman Pengelolaan DM Tipe 2 Dewasa Di Indonesia Ebook PDFcyntiaNo ratings yet
- NICCA Implementing Rules and RegulationsDocument34 pagesNICCA Implementing Rules and RegulationsFaith MorillaNo ratings yet
- VP Shunt ComplicationDocument6 pagesVP Shunt ComplicationMary Christina ElsaNo ratings yet
- VP shunt and complicationDocument5 pagesVP shunt and complicationFabiola VaniaNo ratings yet
- Management of Adult Hydrocephalus With Ventriculoperitoneal Shunts: Long-Term Single-Institution ExperienceDocument8 pagesManagement of Adult Hydrocephalus With Ventriculoperitoneal Shunts: Long-Term Single-Institution ExperienceFelipe Anduquia GarayNo ratings yet
- VP Shunt For Meningitis TBDocument9 pagesVP Shunt For Meningitis TBNindi LizenNo ratings yet
- Shunt Exposure As A Ventriculoperitoneal Shunt Complication A CaseDocument8 pagesShunt Exposure As A Ventriculoperitoneal Shunt Complication A CasePutri Tamara DasantosNo ratings yet
- HydrocephalusDocument9 pagesHydrocephalusDeby AnditaNo ratings yet
- LSM RepairDocument4 pagesLSM RepairDanily Faith VillarNo ratings yet
- The Incidence of Peripheral IntravenousDocument5 pagesThe Incidence of Peripheral IntravenousAlvin Josh CalingayanNo ratings yet
- A Study On Complications of Ventriculoperitoneal Shunt Surgery in Bir Hospital, Kathmandu, NepalDocument4 pagesA Study On Complications of Ventriculoperitoneal Shunt Surgery in Bir Hospital, Kathmandu, NepalHaziq AnuarNo ratings yet
- Scrotal Migration of The Distal End of The Ventriculoperitoneal Shunt Is There A Role For Early Closure of Patent Processus Vaginalis in The Pediatric PopulationDocument4 pagesScrotal Migration of The Distal End of The Ventriculoperitoneal Shunt Is There A Role For Early Closure of Patent Processus Vaginalis in The Pediatric PopulationAthenaeum Scientific Publishers100% (1)
- 1 s2.0 S2214751921001584 MainDocument7 pages1 s2.0 S2214751921001584 MainfitriNo ratings yet
- Pediatric PIV Study CharacteristicsDocument9 pagesPediatric PIV Study CharacteristicspareshNo ratings yet
- Frozen Shoulder New FalonDocument10 pagesFrozen Shoulder New FalonFalon PapalangiNo ratings yet
- Factors Related To Delayed Extubation in Cervical SpineDocument6 pagesFactors Related To Delayed Extubation in Cervical SpineAhmad Rizal MNo ratings yet
- 236409871Document13 pages236409871AlemsegedNo ratings yet
- Tuberculous Meningitis and HydrocephalusDocument10 pagesTuberculous Meningitis and HydrocephalusDwi Ayu KusumawardaniNo ratings yet
- Surgical Management of Hydrocephalus Secondary To Intraventricular Hemorrhage in The Preterm InfantDocument7 pagesSurgical Management of Hydrocephalus Secondary To Intraventricular Hemorrhage in The Preterm InfantcogajoNo ratings yet
- Suprasellar and Recurrent Pediatric Craniopharyngiomas: Expanding Indications For The Extended Endoscopic Transsphenoidal ApproachDocument9 pagesSuprasellar and Recurrent Pediatric Craniopharyngiomas: Expanding Indications For The Extended Endoscopic Transsphenoidal Approachbenjamin6macindewar6No ratings yet
- Ivh and PHVDDocument12 pagesIvh and PHVDSebastian Mora LópezNo ratings yet
- Fixation of The Short-Term Central Venous Catheter. A Comparison of Two TechniquesDocument11 pagesFixation of The Short-Term Central Venous Catheter. A Comparison of Two TechniquesJaime Augusto Quimbayo GuarnizoNo ratings yet
- New 2 PDFDocument9 pagesNew 2 PDFImanuel SoniNo ratings yet
- AJA2019 Cspine ExtubationDocument7 pagesAJA2019 Cspine ExtubationSeveNNo ratings yet
- 433-Article Text-896-1-10-20170620Document7 pages433-Article Text-896-1-10-20170620Tri AdityaNo ratings yet
- Sattar M. Hashim JPMCDocument5 pagesSattar M. Hashim JPMCprastiaNo ratings yet
- 1 s2.0 S0165587617302975 Main PDFDocument4 pages1 s2.0 S0165587617302975 Main PDFAchmad YunusNo ratings yet
- WOS - Neurodevelopmental outcomes of premature infants born at ≤ 32 weeks of gestational age with post-hemorrhagic hydrocephalus treated with ventriculoperitoneal shuntDocument11 pagesWOS - Neurodevelopmental outcomes of premature infants born at ≤ 32 weeks of gestational age with post-hemorrhagic hydrocephalus treated with ventriculoperitoneal shuntanto.fernandez0107No ratings yet
- 1 PBDocument9 pages1 PBcorinaNo ratings yet
- Tip 2Document5 pagesTip 2Anis Eka SukmadadariNo ratings yet
- Zero Tolerance To Shunt Infections: Can It Be Achieved?: PaperDocument5 pagesZero Tolerance To Shunt Infections: Can It Be Achieved?: PaperChristian Sanchez AlvaNo ratings yet
- Peritonitis in Children On Peritoneal Dialysis: 12 Years of Tertiary Center ExperienceDocument7 pagesPeritonitis in Children On Peritoneal Dialysis: 12 Years of Tertiary Center ExperienceMarselya GaniNo ratings yet
- Peripherally Inserted Central Venous Acces - 2021 - Seminars in Pediatric SurgerDocument8 pagesPeripherally Inserted Central Venous Acces - 2021 - Seminars in Pediatric Surgeralergo.ramirezNo ratings yet
- Presentation and Management of Congenital Dacryocystocele: PediatricsDocument7 pagesPresentation and Management of Congenital Dacryocystocele: PediatricsTalix MadrigalNo ratings yet
- Outcome Analysis of Shunt Surgery in Hydrocephalus: Riginal RticleDocument4 pagesOutcome Analysis of Shunt Surgery in Hydrocephalus: Riginal RticleiqbalNo ratings yet
- AygunDocument5 pagesAygunotheasNo ratings yet
- KOHORTDocument8 pagesKOHORTSharonLorisaSimamoraNo ratings yet
- Endoscopic Choroid Plexus Coagulation in InfantsDocument7 pagesEndoscopic Choroid Plexus Coagulation in InfantsCarlos Daniel Giménez MéndezNo ratings yet
- English Jurnal HidrosefalusDocument6 pagesEnglish Jurnal Hidrosefalusthe OGs. 96No ratings yet
- Opth 14 2087Document4 pagesOpth 14 2087Savitri IndrasariNo ratings yet
- A Proposed Protective Protocol Predicting Reduction of Shunt InfectionDocument9 pagesA Proposed Protective Protocol Predicting Reduction of Shunt InfectionRamon VenturaNo ratings yet
- 05 N293 33108Document23 pages05 N293 33108Arjay G ParaniNo ratings yet
- International Journal of Health Sciences and ResearchDocument4 pagesInternational Journal of Health Sciences and Researchwound and ostomy careNo ratings yet
- El Beheiry2019Document4 pagesEl Beheiry2019achmadaNo ratings yet
- VivianecatheterinfectionDocument8 pagesVivianecatheterinfectionRobithoh AlamsyahNo ratings yet
- 2018, J, (Prevalensi) 1-S2.0-S2405857217300931-MainDocument8 pages2018, J, (Prevalensi) 1-S2.0-S2405857217300931-MainAyashopia AyaNo ratings yet
- ava_poster_cincinnati_childrens_hospital_medical_centerDocument1 pageava_poster_cincinnati_childrens_hospital_medical_centeringrid.naruto.fbNo ratings yet
- 1 IiomainDocument6 pages1 IiomainyandaputriNo ratings yet
- Management Outcomes of Hydrocephalus Among Under Five Children in A Tertiary Hospital in Gombe North Eastern NigeriaDocument4 pagesManagement Outcomes of Hydrocephalus Among Under Five Children in A Tertiary Hospital in Gombe North Eastern NigeriaInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Ecommons@Aku Ecommons@Aku: Section of Paediatric Surgery Department of SurgeryDocument5 pagesEcommons@Aku Ecommons@Aku: Section of Paediatric Surgery Department of SurgeryJane PengNo ratings yet
- The Open Access Journal of Science and TechnologyDocument6 pagesThe Open Access Journal of Science and TechnologyNatalia SilahooyNo ratings yet
- 001 Hipoglucemia NeonatalDocument6 pages001 Hipoglucemia NeonatalErick Lozada OlivaNo ratings yet
- Hydro ManagementDocument7 pagesHydro ManagementHadaAtiyehNo ratings yet
- Nursing Management Protocol For Mothers of Children Having Ventricular Peritoneal ShuntDocument9 pagesNursing Management Protocol For Mothers of Children Having Ventricular Peritoneal ShuntZikrina RamadhaniNo ratings yet
- Long-Term Outcomes of Pediatric Penetrating Keratoplasty For Herpes Simplex Virus KeratitisDocument6 pagesLong-Term Outcomes of Pediatric Penetrating Keratoplasty For Herpes Simplex Virus KeratitisgallaNo ratings yet
- Risk Factors for Phlebitis Among IV Cannulated PatientsDocument9 pagesRisk Factors for Phlebitis Among IV Cannulated PatientswindysandyNo ratings yet
- JurnalDocument7 pagesJurnalskocabzzNo ratings yet
- Hestin 2022 Time To Medical Fitness For DischarDocument8 pagesHestin 2022 Time To Medical Fitness For DischarAmro MahmoudNo ratings yet
- Use of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes WorldwideDocument7 pagesUse of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes WorldwidemochkurniawanNo ratings yet
- International Journal of Pediatric OtorhinolaryngologyDocument5 pagesInternational Journal of Pediatric OtorhinolaryngologyTuis PreviyantiNo ratings yet
- Sci J Al Azhar Med Fac Girls 2020 4 1 34 41 EngDocument8 pagesSci J Al Azhar Med Fac Girls 2020 4 1 34 41 EngDr vishnu rathwaNo ratings yet
- Mini Test 1Document6 pagesMini Test 1afriska chandraNo ratings yet
- Post Test Practice Test CDocument8 pagesPost Test Practice Test Cafriska chandra0% (1)
- Questions Native Americans Arrival in Successive Waves Across Land BridgeDocument2 pagesQuestions Native Americans Arrival in Successive Waves Across Land BridgeSuprapto ToNo ratings yet
- Pre Test Practice Test ADocument6 pagesPre Test Practice Test Aafriska chandraNo ratings yet
- Chemical Injury of The EyeDocument28 pagesChemical Injury of The EyeAyesya KamilahNo ratings yet
- Abstract Icran 2019Document2 pagesAbstract Icran 2019afriska chandraNo ratings yet
- 38 Acne PDFDocument7 pages38 Acne PDFRamadhan Harya Puja KusumaNo ratings yet
- The Influence of Upper Limb Elevation On Balance in Elderly Women 2165 7025 1000265Document5 pagesThe Influence of Upper Limb Elevation On Balance in Elderly Women 2165 7025 1000265afriska chandraNo ratings yet
- Vein of Galen Malformation: Images inDocument2 pagesVein of Galen Malformation: Images inafriska chandraNo ratings yet
- Pathophysiology of Gestational Diabetes Mellitus: The Past, The Present and The FutureDocument25 pagesPathophysiology of Gestational Diabetes Mellitus: The Past, The Present and The Futureafriska chandraNo ratings yet
- Journal Pone 0165122Document26 pagesJournal Pone 0165122afriska chandraNo ratings yet
- Cuci Tangan, Sarung Tangan, Gaun Dan Mask 05.06Document18 pagesCuci Tangan, Sarung Tangan, Gaun Dan Mask 05.06Dewi Ayu Puspitasari TwinNo ratings yet
- Cross-Cultural Conference Presentation 02072018 StandardDocument33 pagesCross-Cultural Conference Presentation 02072018 Standardapi-298976119No ratings yet
- ABCDE of Melanoma DetectionDocument51 pagesABCDE of Melanoma DetectionclikgoNo ratings yet
- K.Saikrishna: Under The Guidance ofDocument28 pagesK.Saikrishna: Under The Guidance ofRaj SekharNo ratings yet
- FOAT Readings and ResourcesDocument1 pageFOAT Readings and Resourcesagnes windramNo ratings yet
- Restless Leg SyndromeDocument29 pagesRestless Leg SyndromeMarisa FatkiyaNo ratings yet
- A Systematic Review of The Soteria Paradigm For The Treatment of People Diagnosed With SchizophreniaDocument12 pagesA Systematic Review of The Soteria Paradigm For The Treatment of People Diagnosed With SchizophreniaAmit VasudevNo ratings yet
- Nasogastric Tube InsertionDocument17 pagesNasogastric Tube InsertionMichael Jude MedallaNo ratings yet
- Manual For Preventive Dentistry I-Ii & Clinical Periodontics I - IiDocument77 pagesManual For Preventive Dentistry I-Ii & Clinical Periodontics I - IiSalma MentariNo ratings yet
- Draft Flyer Sandplay TherapyDocument5 pagesDraft Flyer Sandplay TherapyNasri ZulhaidiNo ratings yet
- Bach Flower Therapy for Animal Emotional IssuesDocument4 pagesBach Flower Therapy for Animal Emotional Issuesmajik100% (1)
- Pacemaker Failure - WikipediaDocument17 pagesPacemaker Failure - WikipediaهادىNo ratings yet
- Tetanus Immunity: Book DDocument13 pagesTetanus Immunity: Book Djm_ftaa100% (1)
- UzemaDocument7 pagesUzemaShawn DavisNo ratings yet
- Foot phalanges anatomy and growthDocument9 pagesFoot phalanges anatomy and growthAfif MansorNo ratings yet
- Barcode WardahDocument1 pageBarcode WardahKontol LodonNo ratings yet
- Protein-Calories, Malnutrition & Nutritional DeficienciesDocument14 pagesProtein-Calories, Malnutrition & Nutritional DeficienciesChino Paolo SamsonNo ratings yet
- Survanta 25mg - ML Suspension - Summary of Product Characteristics (SMPC) - (Emc)Document7 pagesSurvanta 25mg - ML Suspension - Summary of Product Characteristics (SMPC) - (Emc)Mohadese hosseini zabetNo ratings yet
- Ecl501 Final Exam Paper (Dec 16)Document12 pagesEcl501 Final Exam Paper (Dec 16)ali mubarak25% (4)
- Scabies Management: Community Control of ScabiesDocument3 pagesScabies Management: Community Control of Scabiespet_trinNo ratings yet
- Learning Contract SamplesDocument25 pagesLearning Contract SamplesClayton BaxterNo ratings yet
- Tramadol: (Trade Names: Ultram®, Ultracet®)Document1 pageTramadol: (Trade Names: Ultram®, Ultracet®)Marthaluzy CarinaNo ratings yet