Professional Documents
Culture Documents
Please Attach A Copy of The Excel Calibration Graph
Please Attach A Copy of The Excel Calibration Graph
Uploaded by
Luyanda MnisiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Please Attach A Copy of The Excel Calibration Graph
Please Attach A Copy of The Excel Calibration Graph
Uploaded by
Luyanda MnisiCopyright:
Available Formats
Name Date ____________
Experiment 5: Spectrophotometric Analysis
Spectrophotometric Determination of Phosphate in Cola
Aim:
Procedure: the procedure was as given in the laboratory manual. (Make a note
here of any deviations from the procedure, and of any observations you made.)
Concentration of PO42- Measured absorbance
mL phosphate standard
solution @ 400 nm
added
0
8.0
16
24
32
40
- Sample R1
Sample R2
Sample R3
Calculation of PO42-in cola sample:
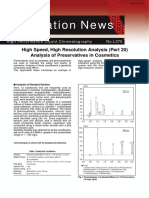
(Please attach a copy of the Excel calibration graph)
The graph must include the equation of the line, the trend-line and the R2 value.
Remove the gridlines and greyscale background.
Equation of the line
Calculation of Concentration of PO42- in
aliquot (ppm)
Concentration of phosphate in cola sample
% Relative Error
Comprehension questions:
1. Show, with calculations, if your results are significantly different from the stated
value.
2. Why does the solution need to stand for 10 minutes?
3. Discuss the function of the ammonium molybdate and vanadate solutions in this
experiment.
4. Does the colour of the cola sample matter? Why?
5. Calculate the mass of phosphate in the sample.
You might also like
- Feed Analysis Standard Operation (Analysis) Procedure SOPDocument27 pagesFeed Analysis Standard Operation (Analysis) Procedure SOPZiauddeen Noor100% (1)
- 31 New Interesting Applications For CCP - SW TES SeminarDocument48 pages31 New Interesting Applications For CCP - SW TES SeminarAlfonso José García LagunaNo ratings yet
- Science Group 9424713991Document48 pagesScience Group 9424713991Shahzad50% (2)
- Experiment 3 Redox Titration Percent Purity AnalysisDocument5 pagesExperiment 3 Redox Titration Percent Purity AnalysisnanaNo ratings yet
- Phosphate Test: SpectroquantDocument1 pagePhosphate Test: SpectroquantMariana RochaNo ratings yet
- Phosphorus Analysis by UV-VISDocument4 pagesPhosphorus Analysis by UV-VISPrasanth AddagtalaNo ratings yet
- Co2 LDocument3 pagesCo2 LARIF AHAMMED P100% (1)
- Treatability Study: Eco Chem Sales & ServiceDocument11 pagesTreatability Study: Eco Chem Sales & ServicenarendraNo ratings yet
- Deter Polarografica - AB-190 - 2 - EN PDFDocument3 pagesDeter Polarografica - AB-190 - 2 - EN PDFCorina StanculescuNo ratings yet
- Ferric Reducing Antioxidant Power (FRAP) Assay Kit (Colorimetric)Document2 pagesFerric Reducing Antioxidant Power (FRAP) Assay Kit (Colorimetric)jayven minguillanNo ratings yet
- Experiment 3 Anion Analysis by Ion ChromatographyDocument6 pagesExperiment 3 Anion Analysis by Ion ChromatographyYuying FengNo ratings yet
- Alkaline Phosphatase P-Nitrophenol ConcDocument1 pageAlkaline Phosphatase P-Nitrophenol ConcAlbertochoNo ratings yet
- Alkaline Phosphatase P-Nitrophenol ConcDocument1 pageAlkaline Phosphatase P-Nitrophenol ConcAlbertochoNo ratings yet
- 2nd Sem Chemistry ManualDocument19 pages2nd Sem Chemistry ManualOliver Ryan FernandesNo ratings yet
- USP-NF Acetaminophen and Codeine Phosphate Oral SolutionDocument3 pagesUSP-NF Acetaminophen and Codeine Phosphate Oral SolutionStalin VacaNo ratings yet
- Cytec Solutions 2013 14Document1 pageCytec Solutions 2013 14aktivrudarpNo ratings yet
- H P L C: Igh Erformance Iquid HromatographyDocument45 pagesH P L C: Igh Erformance Iquid HromatographyPranalosaNo ratings yet
- FDGFDGFDDocument5 pagesFDGFDGFDHugo JoãoNo ratings yet
- A Steady State Model For Describing The PhosphateDocument9 pagesA Steady State Model For Describing The PhosphateNguyen Khanh DuyNo ratings yet
- Lab 4 - Soda Titrations 1Document9 pagesLab 4 - Soda Titrations 1api-385516219No ratings yet
- 10 5923 J Ijme 20140406 01 PDFDocument7 pages10 5923 J Ijme 20140406 01 PDFVizzy VishalNo ratings yet
- Methods BeerDocument62 pagesMethods BeerTruong Anh TuanNo ratings yet
- Phenoxyethanol-Cosmetics en PDFDocument2 pagesPhenoxyethanol-Cosmetics en PDFHiềnNo ratings yet
- Normality N MolarityDocument10 pagesNormality N Molaritynavigcp100% (9)
- Wet Practical Frcpath2014Document7 pagesWet Practical Frcpath2014monday125No ratings yet
- For METTLER TOLEDO Titration Excellence Line: Selected ApplicationsDocument31 pagesFor METTLER TOLEDO Titration Excellence Line: Selected ApplicationsKeila ChavesNo ratings yet
- Thiobarbituric Acid Reactive Substances (TBARS PDFDocument5 pagesThiobarbituric Acid Reactive Substances (TBARS PDFderboNo ratings yet
- HPLCDocument46 pagesHPLCJunaidi HidayatNo ratings yet
- 3 22EM02P3 Handling and Analysis InstrucionsDocument4 pages3 22EM02P3 Handling and Analysis InstrucionsknbiolabsNo ratings yet
- Application Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerDocument4 pagesApplication Note: A Cleaning Validation Swab Recovery Study Using A UV/Persulfate AnalyzerPrianurraufikachmadNo ratings yet
- Fast 8Document44 pagesFast 8regtorrezNo ratings yet
- USP-NF Acepromazine Maleate InjectionDocument2 pagesUSP-NF Acepromazine Maleate InjectionStalin VacaNo ratings yet
- APP Water Analysis Using LAMBDA Ortho Phosphate Determination 012575 01Document3 pagesAPP Water Analysis Using LAMBDA Ortho Phosphate Determination 012575 01shai dunayaNo ratings yet
- Ab 190Document4 pagesAb 190swapon kumar shillNo ratings yet
- Wastewater Characterization Lab 020409 FinalDocument9 pagesWastewater Characterization Lab 020409 FinalgiabrunNo ratings yet
- Vinita Katlamudi 20Cs01024 Section 4 Group 1: Ka (H+) (MR) / (HMR) Pka Ph-Log (MR) / (HMR)Document10 pagesVinita Katlamudi 20Cs01024 Section 4 Group 1: Ka (H+) (MR) / (HMR) Pka Ph-Log (MR) / (HMR)VINITA KATLAMUDINo ratings yet
- Determination of Phosphate in Clinical SamplesDocument3 pagesDetermination of Phosphate in Clinical SamplesDaria VîrticNo ratings yet
- Aluminum AnodizationDocument19 pagesAluminum AnodizationDally Esperanza GafaroNo ratings yet
- HTBC003Document10 pagesHTBC003Amal ..No ratings yet
- PI e BICARB 16Document2 pagesPI e BICARB 16Jaydev DegloorkarNo ratings yet
- USP-NF Acesulfame PotassiumDocument3 pagesUSP-NF Acesulfame PotassiumIVAN BERNALNo ratings yet
- AU150 V27 releasedJC121305Document6 pagesAU150 V27 releasedJC121305juakfuenmayorNo ratings yet
- USP-NF Acepromazine MaleateDocument2 pagesUSP-NF Acepromazine MaleateStalin VacaNo ratings yet
- Iso 4325 1990Document4 pagesIso 4325 1990Edu CorrêaNo ratings yet
- Sulfate Analysis Spectrophotometry Method - English Report TextDocument11 pagesSulfate Analysis Spectrophotometry Method - English Report Textnadazalfa.fitriaNo ratings yet
- Standardization of Naoh 1Document3 pagesStandardization of Naoh 1api-309208977No ratings yet
- 08 Percentage of H2O2Document3 pages08 Percentage of H2O2cpetrillo773No ratings yet
- Iodometric Determination of Formalin in Stabilizer: ECR-1803G ReagentsDocument2 pagesIodometric Determination of Formalin in Stabilizer: ECR-1803G ReagentsJamilah Ghozy (Mila)No ratings yet
- Nalco 77262: Boiler Treatment MultifunctionalDocument7 pagesNalco 77262: Boiler Treatment MultifunctionalMunir AbdullahNo ratings yet
- PDS Chemfos Liquid AdditiveDocument3 pagesPDS Chemfos Liquid AdditiveKenieta100% (1)
- Datos IsopiésticoDocument4 pagesDatos IsopiésticoJuanMeMooMillaNo ratings yet
- Technial Informations CA80TPDocument36 pagesTechnial Informations CA80TPchudungk57No ratings yet
- 5 2 Limewater As Indicator of Carbon Dioxide GasDocument5 pages5 2 Limewater As Indicator of Carbon Dioxide Gasshanea bucknorNo ratings yet
- Iron LabDocument13 pagesIron Labsenthilkumar100No ratings yet
- Chemistry II Lab 7Document2 pagesChemistry II Lab 7Jake OxleyNo ratings yet
- Lkbsis53 Potassium 30343Document2 pagesLkbsis53 Potassium 30343nmakrygNo ratings yet
- Experiment 3 Redox Titration Percent Purity Analysis PDFDocument5 pagesExperiment 3 Redox Titration Percent Purity Analysis PDFnanaNo ratings yet
- SPECTROPHOTOMETRIC DETERMINATION OF AN ACID DISSOCIATION CONSTANT - EditedDocument5 pagesSPECTROPHOTOMETRIC DETERMINATION OF AN ACID DISSOCIATION CONSTANT - Editedsindiswamngadi076No ratings yet
- HTBC007Document7 pagesHTBC007Anirban MullickNo ratings yet