Professional Documents
Culture Documents
Rta PDF
Uploaded by
traceyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Rta PDF
Uploaded by
traceyCopyright:
Available Formats
CME Renal medicine Clinical Medicine 2012, Vol 12, No 5: 476–9
• Still further along is the proximal and the collecting duct (together sometimes
Renal tubular disorders straight tubule (S3) where many drugs called the ‘distal nephron’). This limits
and their metabolites are secreted further bicarbonate loss and the urine pH
Stephen B Walsh, consultant nephrologist (eg loop and thiazide diuretics). becomes more acid unlike distal RTA – see
and honorary senior lecturer; Robert J later. This form of RTA can occur in an
All this active transport depends on the
Unwin, professor of nephrology and isolated monogenic form1 and is also
physiology
‘sodium pump’ (Na⫹-K⫹-ATPase) on the
caused by carbonic anhydrase inhibitors
basolateral side of the proximal tubular cell
(eg acetazolamide) or derivative drugs such
UCL Centre for Nephrology, Royal Free (PTC). This requires energy, so PTCs are
as the anticonvulsant topiramate.
Campus and Hospital, University College full of mitochondria and are highly
London Medical School dependent on aerobic respiration, and are Renal Fanconi syndrome. However, pRTA is
therefore vulnerable to hypoxia – one usually associated with uricosuria,
The physiology of the renal tubule and the reason why PTCs are particularly suscep- glycosuria, phosphaturia, aminoaciduria
diseases that can affect its function are tible to injury or necrosis from renal and low molecular weight proteinuria,
often thought of as complicated and con- ischaemia and drug nephrotoxicity. which comprises the renal Fanconi
fusing. This article will attempt to make syndrome. This syndrome can occur in a
Disturbance of active transport number of acquired diseases such as
tubular disorders slightly easier to under-
processes myeloma2 and Wilson disease,3 but also as
stand by linking the physiology of the four
main nephron segments with the clinical a side effect of some drugs (notably
Failure of these active transport processes
features of the more commonly encoun- ifosfamide, tenofovir4 and aminoglycoside
in the PTCs results in reduced reabsorption
tered renal tubular disorders (Fig 1). antibiotics) and in mitochondrial disease,
of the solutes already mentioned, which
which can be drug-related or inherited.
can then appear in the final urine.
The proximal tubule A genetic form of the renal Fanconi syn-
Glucose. Various genetic defects affect drome associated with nephrocalcinosis
The proximal convoluted tubule (PCT) is glucose (isolated renal glycosuria) and and nephrolithiasis occurs in Dent disease,
the main site of active transport and reab- amino acid (aminoacidurias) transport, a recessive X-linked condition due to a
sorption of the majority of solutes present such as cystine (dimeric cysteine) in mutation in a PCT intracellular chloride
in the glomerular filtrate, as well as the cystinuria, one of the commonest clinically transporter CLC-5,5 or in the intracellular
location of the production of the key uri- significant inherited defects of amino acid phosphatase enzyme OCRL1, which causes
nary buffer ammonium (NH4⫹): transport causing stones in humans. a Dent-like syndrome known as Dent-2.6
• In the early part of the PCT (S1), reab- Cystinuria must be distinguished from OCRL1 mutations are also the cause of
sorbed solutes include glucose, cystinosis, a lysosomal storage disease Lowe syndrome, an inherited renal Fanconi
amino acids, phosphate, bicarbonate affecting the PCT and due to the syndrome associated with mental retarda-
and various filtered low molecular intracellular accumulation of cystine. tion and congenital cataracts (oculocere-
weight proteins. brorenal syndrome).
Bicarbonate. Disturbance of bicarbonate
• In the later part of the PCT (S2) urate reabsorption presents as proximal renal
is reabsorbed and secreted, and citrate tubular acidosis (pRTA or type 2 RTA). The loop of Henle
is also reabsorbed. Initially, urine pH will be alkaline and The loop of Henle is the site of the counter-
systemic bicarbonate current multiplier that serves to generate
concentration will the corticomedullary osmotic gradient, and
fall, causing an hence the kidney’s ability to concentrate
acidosis. When the and dilute the final urine. This ability
Distal Convoluted Tubule threshold for depends on the reabsorption of sodium
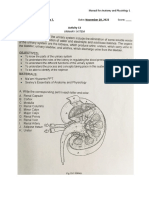
Proximal Convoluted Tubule Gitelman, Gordon (NCC)
pRTA bicarbonate (Na⫹) and chloride (Cl⫺) along the water
Fanconi reabsorption is impermeable thick ascending limb (TAL)
Dent/Lowe
exceeded (usually at of the loop of Henle (also known as the
a plasma or serum ‘diluting segment’).
concentration of
Loop of Henle
Bartter (NKCC2) around 16 mmol/l) Bartter syndrome
Collecting Duct
Familial hypomagnesaemia Liddle, PHA1 (ENaC) any bicarbonate not
with hypercalciuria NDI (AVPR1, Aquaporin 2) reabsorbed by the Bartter syndrome results from a failure of
(Claudin 16 or 19) Hereditary SIADH (AVP-NP2)
dRTA (vH+ATPase, AE1) PCT is reabsorbed Na+ and Cl- reabsorption in the TAL, and
by the thick thus a failure to concentrate the urine. This
Fig 1. Renal tubular disorders. ascending limb of results in salt wasting, polyuria and volume
the loop of Henle depletion (often with hypotension).
476 © Royal College of Physicians, 2012. All rights reserved.
CMJ-1205-CME-Unwin.indd 476 9/21/12 10:02:35 PM
CME Renal Medicine
Consequent secondary hyperaldosteronism • Type 5 is an autosomal dominant The distal convoluted tubule
and increased delivery of Na⫹ to the down- form caused by activating mutations
stream collecting duct lead to increased of the calcium sensing receptor The distal convoluted tubule (DCT) is
urinary excretion of potassium (K⫹) and (CaSR) on the basolateral membrane involved mainly in Na⫹ and Cl- transport,
hydrogen (H⫹) ions, producing hypoka- of TAL cells. CaSR activation inhibits as well as some Ca2⫹ and Mg2⫹ reabsorption,
laemia and metabolic alkalosis. The TAL is NaCl reabsorption, explaining the although in this case across (transcellular)
also the major site of calcium (Ca2⫹) and renal effects of hypercalcaemia. rather than between (paracellular) the DCT
magnesium (Mg2⫹) reabsorption, which Affected patients also have hypocal- cells. Na⫹ and Cl⫺ transport occurs via a
depends on normal NaCl reabsorption (see caemia from parathyroid hormone thiazide-sensitive apical NaCl co-
below). Thus, urinary losses of Ca2⫹ and suppression (due to parathyroid gland transporter (NCC).
Mg2⫹ are increased in Bartter syndrome expression of the CaSR), nephrocalci-
and hypomagnesaemia is not uncommon nosis and stones (for review, see Gitelman syndrome
in types 1 and 2 (see below). Ref. 7).
Loss-of-function mutations of NCC cause
NaCl reabsorption in the TAL relies on a
Gitelman syndrome,8 characterised by:
number of transporters working in con-
cert. The furosemide-sensitive apical trans- Familial hypomagnesaemia with • milder renal salt losses and volume
porter NKCC2 transports Na⫹, K⫹ and hypercalciuria contraction than in Bartter syndrome
2Cl- together into the cell, driven by the low • hypokalaemia and metabolic alkalosis,
Another inherited tubular disease affecting
intracellular Na⫹ concentration, main- but with
this nephron segment is familial hypomag-
tained by the basolateral Na+-K+-ATPase. • hypocalciuria, similar to the effect of thi-
nesaemia with hypercalciuria. This is due
However, the K⫹ concentration in tubular azide diuretic administration rather than
to a defect in the paracellular pathway
fluid is much less than the concentrations the hypercalciuria of Bartter syndrome.
(mentioned above) for Ca2⫹ and Mg2⫹
of Na⫹ and Cl-. This would be limiting if it
reabsorption, which depends on the selec- Gitelman syndrome is usually asympto-
was not for an apical K⫹ channel, ROMK,
tive permeability of cell junction proteins matic, often diagnosed late in childhood or
which recycles K⫹ back across the apical
known as claudins. Mutations in claudin incidentally in adulthood.
membrane into the lumen. This K+ recy-
16 or 19 cause this autosomal recessive
cling generates a lumen positive potential
syndrome with nephrocalcinosis and ill-
difference that drives the reabsorption of Gordon syndrome
understood recurrent urinary tract infec-
Ca2⫹ and Mg2⫹ (and some Na⫹) paracel-
tions. It invariably progresses to renal The mirror image of Gitelman syndrome is
lularly between the TAL cells. Meanwhile,
failure. the autosomal dominant (usually) Gordon
Cl⫺ is transported out of the TAL cell baso-
laterally via the Cl⫺ channels, ClC-Kb and
ClC-Ka, which are both regulated by an
accessory protein known as barttin. Genetic
Key points
mutations of any of these transport and Disorders of the proximal tubule can cause renal Fanconi syndrome with glycosuria,
regulatory proteins can cause Bartter syn- amino aciduria, bicarbonaturia, phosphaturia (often, though not always, with
drome by reducing NaCl transport along hypercalciuria), uricosuria, and low molecular weight tubular proteinuria (not usually
detectable by routine urine dipstick)
the TAL, with its local and downstream
effects on Ca2⫹, Mg2⫹, K⫹ and H⫹ Bartter syndrome is caused by mutations that inactivate the loop diuretic-sensitive
excretion. NKCC2 cotransporter in the thick ascending limb of Henle’s loop. The electrolyte
abnormalities found in Bartter syndrome are similar to those occurring on loop
Types of Bartter syndrome. Types 1–4 of diuretics
Bartter syndrome are autosomal recessive.
Gitelman syndrome is caused by mutations that inactivate the thiazide diuretic-
• Types 1 and 2 arise from NKCC2 and sensitive NCC cotransporter. The electrolyte abnormalities of Gitelman syndrome are
ROMK mutations, respectively, and can similar to those occurring on thiazide diuretics
be associated with nephrocalcinosis. Genetic causes of hypertension can result from activating mutations of NCC (Gordon
• Type 3 is caused by ClC-Kb mutations syndrome) or of ENaC (Liddle syndrome). They are a ‘mirror image’ of Gitelman
and has a milder phenotype, probably syndrome and pseudohypoaldosteronism type 1a, respectively
due to some redundancy in Cl- channel
Proximal (tubular) RTA is caused by failure to reabsorb bicarbonate and is usually part
function.
of the renal Fanconi syndrome. Whereas distal (tubular) RTA is caused by failure to
• A barttin mutation results in a more secrete H+, proximal (tubular) RTA is often associated with autoimmune disease in
severe form known as type 4 which, adults and causes a more severe form of acidosis with hypokalaemia, complicated by
because this protein is also present in stones and nephrocalcinosis
the inner ear, is associated with sen-
KEYWORDS: renal tubule, proximal tubule loop of Henle, collecting duct, renal
sorineural deafness. Fanconi syndrome, renal tubular acidosis
© Royal College of Physicians, 2012. All rights reserved. 477
CMJ-1205-CME-Unwin.indd 477 9/21/12 10:02:35 PM
CME Renal Medicine
syndrome (or pseudohypoaldosteronism to loss-of-function mutations in the enzyme of its chronic use) because it enters the prin-
type 2, or familial hyperkalaemic hyperten- 11-hydroxysteroid dehydrogenase-2 cipal cells through ENaC. This effect can be
sion). This is caused by NCC overactivity (11HSD2). The mineralocorticoid receptor blocked or ameliorated by amiloride.13
due to mutations in the upstream (MR) in the collecting duct can bind cortisol
regulators of NCC, WNK (With No lysine (present in much higher concentrations Hereditary central diabetes insipidus
(K)) kinases 1 and 4.9 The result is hyper- than aldosterone), as well as aldosterone,
tension, hyperkalaemia, metabolic acidosis and the intracellular enzyme 11HSD2 nor- Hereditary central diabetes insipidus is due
and hypercalciuria. Patients with Gordon mally metabolises cortisol, preventing it to autosomal dominant mutations in the
syndrome are particularly sensitive to thi- from activating the MR in place of aldos- AVP-neurophysin II gene (AVP-NPII),
azide diuretics which can correct most of terone. Licorice inhibits this enzyme, which leading to loss of vasopressin secretion
its clinical features. can cause hypertension. (unlike NDI, this is not usually evident at
birth, but progressive) and circulating
Pseudohypoaldosteronism type 1 vasopressin levels are low.
The collecting duct
(PHA1)
The collecting duct comprises two main Hereditary syndrome of antidiuretic
cell types: A mirror image of Liddle syndrome is hormone secretion (SIADH) and
pseudohypoaldosteronism type 1, which is hyponatraemia
• Na⫹ and water reabsorbing, and K⫹ like Addison's disease. Typical features are
secreting, principal cells, and salt wasting, hypotension, acidosis and In contrast to X-linked NDI (see above)
• acid or bicarbonate secreting interca- hyperkalaemia. The syndrome has two SIADH can be due to a gain-of-function
lated cells. forms due to: mutation in the AVRP2 gene and V2 vaso-
pressin receptor.14 A loss-of-function poly-
1 Autosomal recessive loss-of-function
Principal cells morphism of the TRPV4 cation channel
ENaC mutations unresponsive to
has also been linked to SIADH and hyponat-
Principal cells are the majority cell type and aldosterone (type 1a). There is wide
raemia, and it seems that this ion channel is
have the apical Na⫹ channel, ENaC and the distribution of ENaC in the lung,
involved in osmosensing by the hypotha-
K⫹ channel ROMK, as well as in the basola- kidney, skin and gastrointestinal tract,
lamus.15 Those affected have a blunted
teral Na⫹-K⫹-ATPase, which is present in all so the phenotype is often more severe.
response to hypotonicity and behave as if
polarised renal epithelial cells. The amilo- 2 Autosomal dominant mutations of
they have a reset osmostat, and can regulate
ride-sensitive ENaC is regulated by aldos- the MR that cannot bind aldosterone
their plasma osmolality normally, although
terone, which increases both the number of (type 1b). In this form the pheno-
at a lower than normal value. This poly-
open ENaCs and the activity of the Na⫹-K⫹- type is milder and see is confined to
morphism has a dominant-negative effect
ATPase, and the number of ROMK chan- the kidney.10
on the normal wild type allele.
nels. The net effect is to increase Na⫹ reab-
Hereditary nephrogenic diabetes
sorption and K⫹ secretion/excretion.
Alpha intercalated cells
Hereditary nephrogenic diabetes insipidus
Liddle syndrome (NDI) is a condition of resistance to the Alpha intercalated cells excrete acid into
action of vasopressin caused either by: the urine by generating H⫹ from the intra-
Water reabsorption also occurs across the cellular conversion of CO2 and water to
principal cells via an apical water channel, • recessive loss-of-function mutations in
carbonic acid, followed by its breakdown to
aquaporin 2, controlled by vasopressin the V2 vasopressin receptor gene
bicarbonate and H⫹ catalysed by carbonic
(antidiuretic hormone). An autosomal (AVRP2) on the X chromosome
anhydrase 2 (CA2). The H⫹ is secreted into
dominant form of hypertension, Liddle (>90%),11 or
the tubular lumen by the electrogenic H⫹
syndrome, is caused by mutations in ENaC • recessive and autosomal dominant
vH-ATPase and the bicarbonate transferred
that prevent its removal from the apical mutations in the water channel
to blood via the Cl⫺/bicarbonate anion
membrane, thus maintaining increased aquaporin 2 gene (AQP2).12
exchanger AE1 (SLC4A1).
ENaC activity.10 In addition to hyperten- Vasopressin levels are elevated and the
sion, there is hypokalaemia and metabolic main features are polyuria, nocturia and
alkalosis. Not surprisingly, patients with Hereditary distal renal tubular acidosis
polydipsia, usually associated with mild
Liddle syndrome respond well to amiloride, hypernatraemia. The urine concentrating Hereditary distal renal tubular acidosis (type
whereas spironolactone is ineffective. ability is lost, so urine osmolality is low and 1 RTA or dRTA) can be caused by loss-of-
plasma or serum osmolality raised. Thirst is function mutations of the subunits B1 or a4
Apparent mineralocorticoid excess
normal, so severe hypernatraemia is of the vH-ATPase, or of AE1. An impaired
syndrome
uncommon. Lithium interferes with vaso- ability to excrete acid in the urine can lead to
A similar syndrome, apparent mineralocor- pressin signalling via cyclic AMP and can metabolic acidosis, complicated by rickets,
ticoid excess, is autosomal recessive and due cause an acquired form of NDI (a side effect osteomalacia or reduced bone mineral
478 © Royal College of Physicians, 2012. All rights reserved.
CMJ-1205-CME-Unwin.indd 478 9/21/12 10:02:35 PM
CME Renal Medicine
density, nephrocalcinosis and stones. There References insipidus are impaired in their cellular
is also increased urinary potassium excre- routing. J Clin Invest 1995;95:2291–6.
1 Igarashi T, Inatomi J, Sekine T et al. Novel 13 Kortenoeven ML, Li Y, Shaw S et al.
tion, leading to hypokalaemia, although this
nonsense mutation in the Na+/HCO3- Amiloride blocks lithium entry through
is more difficult to explain. The tendency to cotransporter gene (SLC4A4) in a patient the sodium channel thereby attenuating the
form calcium phosphate stones is because of with permanent isolated proximal renal resultant nephrogenic diabetes insipidus.
the alkaline urine and hypercalciuria in aci- tubular acidosis and bilateral glaucoma. J Kidney Int 2009;76:44–53.
dotic patients (so-called ‘complete’ dRTA); Am Soc Nephrol 2001;12:713–8. 14 Feldman BJ, Rosenthal SM, Vargas GA et al.
2 Maldonado JE, Velosa JA, Kyle RA et al. Nephrogenic syndrome of inappropriate
urine pH is always above 5.3 in the presence
Fanconi syndrome in adults. A manifesta- antidiuresis. N Engl J Med 2005;352:1884–90.
of a systemic acidosis. Patients can still have tion of a latent form of myeloma. Am J 15 Tian W, Fu Y, Garcia-Elias A et al. A loss-
an acidification defect but without acidosis Med 1975;58:354–64. of-function nonsynonymous polymor-
(‘incomplete’ dRTA). If this is suspected (in 3 Morgan HG, Stewart WK, Lowe KG, phism in the osmoregulatory TRPV4 gene
the presence of nephrocalcinosis or with a Stowers JM, Johnstone JH. Wilson’s disease is associated with human hyponatremia.
and the Fanconi syndrome. QJM Proc Natl Acad Sci U S A 2009;106:14034–9.
family history of stones), a urinary acidifica-
1962;31:361–84. 16 Walsh SB, Shirley DG, Wrong OM, Unwin
tion test is necessary.16,17 4 Verhelst D, Monge M, Maynard JL et al. RJ. Urinary acidification assessed by simul-
Autosomal recessive mutations of the B1 Fanconi syndrome and renal failure taneous furosemide and fludrocortisone
subunit of the vH-ATPase (also present in induced by tenofovir: a first case report. treatment: an alternative to ammonium
the inner ear) cause dRTA with congenital Am J Kidney Dis 2002;40:1331–3. chloride. Kidney Int 2007;71:1310–6.
5 Lloyd SE, Pearce SH, Fisher SE et al. A 17 Wrong O, Davies HE. The excretion of acid
sensorineural deafness.18 With autosomal
common molecular basis for three inher- in renal disease. QJMed 1959;28:259–313.
recessive a4 mutations, the onset of deafness ited kidney stone diseases. Nature 18 Karet FE, Finberg KE, Nelson RD et al.
is often later in early adulthood. AE1 muta- 1996;379:445–9. Mutations in the gene encoding B1 subunit
tions cause autosomal dominant dRTA 6 Hoopes R Jr, Shrimpton AE, Knohl SJ et al. of H+-ATPase cause renal tubular acidosis
without deafness, which can present in Dent Disease with mutations in OCRL1. with sensorineural deafness. Nat Genet
Am J Hum Genet 2005;76:260–7. 1999;21:84–90.
childhood with rickets or in later life with
7 Seyberth HW. An improved terminology 19 Bruce LJ, Cope DL, Jones GK et al. Familial
recurrent renal stones and nephrocalcinosis. and classification of Bartter-like syn- distal renal tubular acidosis is associated
This form of dRTA is often recessive in the dromes. Nat Clin Pract Nephrol with mutations in the red cell anion
tropics due to associated inherited red cell 2008;4:560–7. exchanger (Band 3, AE1) gene. J Clin Invest
defects, such as South-East Asian ovalocy- 8 Simon D, Nelson-Williams C, Bia MJ et al. 1997;100:1693–707.
Gitelman’s variant of Bartter’s syndrome, 20 Sly WS, Hewett-Emmett D, Whyte MP, Yu
tosis.19 CA2 mutations can cause a rare
inherited hypokalaemic alkalosis, is caused YS, Tashian RE. Carbonic anhydrase II
mixed type of RTA with both pRTA and by mutations in the thiazide-sensitive deficiency identified as the primary defect
dRTA features, associated with osteopetrosis Na-Cl cotransporter. Nat Genet in the autosomal recessive syndrome of
and cerebral calcification.20 Acquired dRTA 1996;12:24–30. osteopetrosis with renal tubular acidosis
is more common in adult clinical practice 9 Wilson FH, Disse-Nicod me S, Choate KA and cerebral calcification. Proc Natl Acad
et al. Human hypertension caused by Sci U S A 1983;80:2752–6.
and typically is associated with autoimmune
mutations in WNK kinases. Science 21 Walsh S, Turner CM, Toye A et al.
diseases, especially Sjögren syndrome.21 2001;293:1107–12. Immunohistochemical comparison of a
10 Snyder PM, Price MP, McDonald FJ et al. case of inherited distal renal tubular aci-
Mechanism by which Liddle’s syndrome dosis (with a unique AE1 mutation) with
Conclusions mutations increase activity of a human an acquired case secondary to autoimmune
epithelial Na+ channel. Cell 1995;83: disease. Nephrol Dial Transplant
This article is not an exhaustive account of 969–78. 2007;22:807–12.
renal tubular disorders but has covered 11 Bichet DG, Arthus MF, Lonergan M et al.
many of those likely to be encountered X-linked nephrogenic diabetes insipidus Address for correspondence: Prof
clinically, especially in adult patients. The mutations in North America and the Robert J Unwin, UCL Centre for
Hopewell hypothesis. J Clin Invest
intention has been to link structure with Nephrology, UCL Medical School,
1993;92:1262–8.
function, and to make it easier to remember 12 Deen PM, Croes H, van Aubel RA et al. Royal Free Campus and Hospital,
and understand the pathophysiology of the Water channels encoded by mutant Rowland Hill Street, London NW3 2PF.
tubulopathies described. aquaporin-2 genes in nephrogenic diabetes Email: robert.unwin@ucl.ac.uk
© Royal College of Physicians, 2012. All rights reserved. 479
CMJ-1205-CME-Unwin.indd 479 9/21/12 10:02:36 PM
You might also like
- Electrolytes and Acid Base Common Fluid and Enlectrolyte DisordersDocument9 pagesElectrolytes and Acid Base Common Fluid and Enlectrolyte Disordersninta karinaNo ratings yet
- Molecular Developments in Renal Tubulopathies: Leading ArticlesDocument3 pagesMolecular Developments in Renal Tubulopathies: Leading ArticlesJulio César Valdivieso AguirreNo ratings yet
- Broodbank & Christian. Renal Tubular Disorders. 2018.Document10 pagesBroodbank & Christian. Renal Tubular Disorders. 2018.Jéssica Hilário BonomoNo ratings yet
- Pathophysiology of Chronic Renal Failure: Quentin Milner MB CHB FrcaDocument4 pagesPathophysiology of Chronic Renal Failure: Quentin Milner MB CHB FrcaFajar JarrNo ratings yet
- Management Surgery of The Oral and Maxillofacial Patient With End-Stage Renal DiseaseDocument6 pagesManagement Surgery of The Oral and Maxillofacial Patient With End-Stage Renal Diseasemehak malhotraNo ratings yet
- Drugs and The Kidney: Continuing Medical EducationDocument6 pagesDrugs and The Kidney: Continuing Medical EducationJANINE PASIONNo ratings yet
- Acute Tubular NecrosisDocument46 pagesAcute Tubular NecrosispadmaNo ratings yet
- Are All Fluids Bad For The Kidney?Document10 pagesAre All Fluids Bad For The Kidney?Xavier AbrilNo ratings yet
- Rational Use of Diuretics and Pathophysiology of Edema: OP Kalra, Amitesh AggarwalDocument10 pagesRational Use of Diuretics and Pathophysiology of Edema: OP Kalra, Amitesh AggarwalVina AnasthasiaNo ratings yet
- Review Article: Ijprbs Chirag Modi, IJPRBS, 2012: Volume1 (3) : 120-132Document13 pagesReview Article: Ijprbs Chirag Modi, IJPRBS, 2012: Volume1 (3) : 120-132Uci Ramadhanty D3 2018No ratings yet
- Cyclosporine HyperkalemiaDocument5 pagesCyclosporine HyperkalemiaAlba RosesNo ratings yet
- A Guide For The Assessment and Management of Post-Obstructive DiuresisDocument3 pagesA Guide For The Assessment and Management of Post-Obstructive DiuresisRara Aulia IINo ratings yet
- Pi Is 0085253815538106Document8 pagesPi Is 0085253815538106Romel BarrientosNo ratings yet
- GinjalDocument11 pagesGinjalOneng IfayaniNo ratings yet
- By B. Shalini Under The Guidance of Neelakant Reddy Patil M.PharmDocument25 pagesBy B. Shalini Under The Guidance of Neelakant Reddy Patil M.PharmShalini Reddy100% (1)
- Local Anesthetic Systemic Toxicity: Continuing Professional DevelopmentDocument20 pagesLocal Anesthetic Systemic Toxicity: Continuing Professional Developmentyounes kabNo ratings yet
- Mechanisms and Management of Diuretic Resistance I PDFDocument5 pagesMechanisms and Management of Diuretic Resistance I PDFErensina M MansnandifuNo ratings yet
- The Pathophysiology of Distal Renal Tubular AcidosisDocument17 pagesThe Pathophysiology of Distal Renal Tubular AcidosisPASMOXNo ratings yet
- Novedad en Terapia Injuria Renal AgudaDocument15 pagesNovedad en Terapia Injuria Renal AgudaAlicia Carrera TorresNo ratings yet
- Curt Hoys 2013Document12 pagesCurt Hoys 2013broken22No ratings yet
- Diuretic Treatment in Heart FailureDocument12 pagesDiuretic Treatment in Heart FailureRoberto López Mata100% (1)
- Gough 2019Document6 pagesGough 2019torres.maria240995No ratings yet
- Pi Is 0272638623009320Document18 pagesPi Is 0272638623009320Sa7arNo ratings yet
- SX FalconiDocument15 pagesSX FalconiJesús QuirarteNo ratings yet
- Paraprotein-Related Kidney Disease: Kidney Injury From Paraproteins-What Determines The Site of Injury?Document7 pagesParaprotein-Related Kidney Disease: Kidney Injury From Paraproteins-What Determines The Site of Injury?Miguel Odar SampeNo ratings yet
- Dysnatremia in The ICU: Milap Pokaharel and Clay A. BlockDocument13 pagesDysnatremia in The ICU: Milap Pokaharel and Clay A. BlockJonathan Gustavo MenaNo ratings yet
- Uremic Encephalopathy and Other Brain Disorders Associated With Renal FailureDocument5 pagesUremic Encephalopathy and Other Brain Disorders Associated With Renal FailuresrikandhihasanNo ratings yet
- Hypokalemic Distal Renal Tubular Acidos 2018 Advances in Chronic Kidney DiseDocument18 pagesHypokalemic Distal Renal Tubular Acidos 2018 Advances in Chronic Kidney DiseSiva RamanNo ratings yet
- Jansen Et Al-2003-Liver InternationalDocument8 pagesJansen Et Al-2003-Liver InternationalPablo Escalante ValdaNo ratings yet
- The Pathophysiology of Acute Renal Failure: D P P P, G P. K K K, A G. B B B, S V. SDocument2 pagesThe Pathophysiology of Acute Renal Failure: D P P P, G P. K K K, A G. B B B, S V. SLOLONo ratings yet
- Renal Blood Flow and OxygenationDocument12 pagesRenal Blood Flow and OxygenationnanreNo ratings yet
- Endocannabinoid System and The Kidneys: From Renal Physiology To Injury and DiseaseDocument11 pagesEndocannabinoid System and The Kidneys: From Renal Physiology To Injury and DiseaseAnimal-House TunjaNo ratings yet
- Drug Name MagsnDocument3 pagesDrug Name MagsnBryant Von Andrew EstradaNo ratings yet
- Molecular Pathways of Chronic Kidney Disease Progression: SciencedirectDocument4 pagesMolecular Pathways of Chronic Kidney Disease Progression: SciencedirectJosué VelázquezNo ratings yet
- Hyperkalemic Forms of Renal Tubular Acidosis Clin - 2018 - Advances in ChronicDocument13 pagesHyperkalemic Forms of Renal Tubular Acidosis Clin - 2018 - Advances in ChronicSiva RamanNo ratings yet
- Background: Acute Tubular NecrosisDocument23 pagesBackground: Acute Tubular NecrosisLuther ThengNo ratings yet
- Yaffe Chapter Renal MaturationDocument17 pagesYaffe Chapter Renal Maturationwalaa alsharanyNo ratings yet
- Role of Reactive Oxygen Species in Pathogenesis of Radiocontrast Induced NephropathyDocument7 pagesRole of Reactive Oxygen Species in Pathogenesis of Radiocontrast Induced NephropathyAlumno UstNo ratings yet
- Potassium and AnaesthesiaDocument16 pagesPotassium and AnaesthesiaAnonymous S0MyRHNo ratings yet
- Dr. Vineet ChaturvediDocument69 pagesDr. Vineet ChaturvediVinay PatilNo ratings yet
- Part 1 - US-Grade 4-BiochemistryDocument34 pagesPart 1 - US-Grade 4-BiochemistryFarah Bashar Al-RawachyNo ratings yet
- Hypernatremia: Kidney Case Conference: How I TreatDocument3 pagesHypernatremia: Kidney Case Conference: How I TreatJulian ViggianoNo ratings yet
- Acute Tubular NecrosisDocument38 pagesAcute Tubular Necrosisganesa ekaNo ratings yet
- 1 Patho5 - Kidney I 2015bDocument10 pages1 Patho5 - Kidney I 2015bmiguel cuevasNo ratings yet
- The Kidney - An Organ of Critical Importance in Physiology: Louise RobsonDocument2 pagesThe Kidney - An Organ of Critical Importance in Physiology: Louise Robsoncahyadi adityaNo ratings yet
- Fanconi Syndrome and Other Proximal Tubule Disorders: John W. ForemanDocument12 pagesFanconi Syndrome and Other Proximal Tubule Disorders: John W. ForemanphillymouNo ratings yet
- Acute Renal Failure Introduction - : PrognosisDocument8 pagesAcute Renal Failure Introduction - : PrognosisHepsibaNo ratings yet
- Clinical Approach To Renal Tubular Acidosis in AdultDocument11 pagesClinical Approach To Renal Tubular Acidosis in Adultnanang hidayatullohNo ratings yet
- Understanding Treatments For Gout: ReportsDocument8 pagesUnderstanding Treatments For Gout: ReportsTim LaneNo ratings yet
- Ac 1Document12 pagesAc 1Alamsyah TegarNo ratings yet
- Jurnal CardioDocument11 pagesJurnal Cardiomohamad safiiNo ratings yet
- Fluid, Electrolyte, and Acid-Base Disorders Robert B Schonberger 2018Document18 pagesFluid, Electrolyte, and Acid-Base Disorders Robert B Schonberger 2018Akhmad Fadhiel Noor100% (1)
- Renal Metabolism and Hypertension Review ArticleDocument12 pagesRenal Metabolism and Hypertension Review ArticlePATRICIA JACQUELINE SARMIENTO GOMEZNo ratings yet
- Food & Function: PaperDocument5 pagesFood & Function: PaperFrancisco RamirezNo ratings yet
- Drug-Induced Kidney Disease: ArticleDocument11 pagesDrug-Induced Kidney Disease: Articledwi harisNo ratings yet
- Chapter-1 INTRODUCTION 1.1 Nephrotoxicity:: Figure 1 Location of KidneysDocument29 pagesChapter-1 INTRODUCTION 1.1 Nephrotoxicity:: Figure 1 Location of Kidneysalam25No ratings yet
- Ellison Felker NEJM2017 Heart FailureDocument13 pagesEllison Felker NEJM2017 Heart FailureMihai CebotariNo ratings yet
- tmp8482 TMPDocument8 pagestmp8482 TMPFrontiersNo ratings yet
- CH 15 Excretory-SystemDocument94 pagesCH 15 Excretory-SystemAntonio Calleja II100% (1)
- Obstructive UropathyDocument47 pagesObstructive UropathyChristine Karen Ang Suarez100% (3)
- Poultry BrochureDocument12 pagesPoultry BrochureWayanad AyurvedaNo ratings yet
- Homeopathy in Intensive Care and Emergency Medicine Michael Frass Martin Buendner.14188 1Document10 pagesHomeopathy in Intensive Care and Emergency Medicine Michael Frass Martin Buendner.14188 1Mansoor Khanali100% (2)
- Tetada Kalimasad1Document16 pagesTetada Kalimasad1Rasaraj Ramon Castaneda100% (2)
- Kidney - WikipediaDocument122 pagesKidney - WikipediaMuhammad HuzaifaNo ratings yet
- Aki App ContentDocument6 pagesAki App ContentmimoNo ratings yet
- Common Med Surg Lab ValuesDocument5 pagesCommon Med Surg Lab ValuesToMorrowNo ratings yet
- Renovascular Hypertension (RVH) SeminarDocument58 pagesRenovascular Hypertension (RVH) SeminarfaizalmasoodiNo ratings yet
- Blood Chemistry TestsDocument3 pagesBlood Chemistry TestsMarcelina ElizabethNo ratings yet
- GUS1 - K5 - Renal Blood Flow and Glomerular FiltrationDocument23 pagesGUS1 - K5 - Renal Blood Flow and Glomerular FiltrationRudinNo ratings yet
- Urology DNB Old QuestionsDocument8 pagesUrology DNB Old QuestionssjulurisNo ratings yet
- Renal ExamDocument12 pagesRenal ExamCamille Espinosa100% (6)
- Chronic Kidney DiseaseDocument2 pagesChronic Kidney DiseasechyNo ratings yet
- KDIGO AKI Conference Public Comments FinalDocument66 pagesKDIGO AKI Conference Public Comments Finalcafl2309No ratings yet
- Somali National Imam Protocol 0Document209 pagesSomali National Imam Protocol 0Ahmed MohammedNo ratings yet
- Complications of Percutaneous Nephrostomy Tube Placement To Treat NephrolithiasisDocument4 pagesComplications of Percutaneous Nephrostomy Tube Placement To Treat NephrolithiasisPande Made FitawijamariNo ratings yet
- The Human Excretory System Functions To Remove Waste From The Human BodyDocument2 pagesThe Human Excretory System Functions To Remove Waste From The Human BodyrawatanandNo ratings yet
- BMJ 2022 074216.fullDocument20 pagesBMJ 2022 074216.fullArmando OrtegaNo ratings yet
- MEDICAL VI - Lesson 4 Renal UnitDocument34 pagesMEDICAL VI - Lesson 4 Renal Unitnathalie c. rodriguezNo ratings yet
- CreatinineDocument8 pagesCreatinineGul RockzzNo ratings yet
- Pex 09 03Document4 pagesPex 09 03Marcela Anco Sotomayor50% (4)
- Fisiologia Asa de HenleDocument24 pagesFisiologia Asa de HenleOscar SotoNo ratings yet
- Life Processes Notes (Yashvi Modi)Document15 pagesLife Processes Notes (Yashvi Modi)YASHVI MODI60% (5)
- Urinary SystemDocument5 pagesUrinary SystemJushelle Anne Tigoy Pilare100% (1)
- 05 N111 39956Document28 pages05 N111 39956Mahruri SaputraNo ratings yet
- Hypertension PDFDocument50 pagesHypertension PDFMochamad Ali Rosadi100% (1)
- Evaluation and Preoperative Management of Kidney Transplant Recipient and DonorDocument26 pagesEvaluation and Preoperative Management of Kidney Transplant Recipient and DonorqadiraftabNo ratings yet
- Acute Glomerulonephritis (AGN) : Universidad de ManilaDocument10 pagesAcute Glomerulonephritis (AGN) : Universidad de ManilaKristine_Bacan_2085No ratings yet
- Lesson Plan Science VDocument7 pagesLesson Plan Science VOlan Magbuhos0% (2)