100% found this document useful (2 votes)
1K views75 pages1 Primary-And-Secondary-Hemostasis PDF
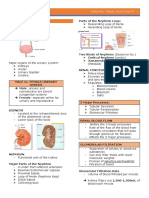
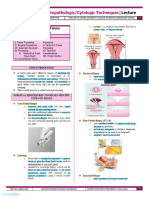
Primary hemostasis involves platelet plug formation and vasoconstriction to stop bleeding from damaged blood vessels. It can be assessed through tests like bleeding time, which measures how long bleeding continues after a small wound. Secondary hemostasis involves the coagulation cascade of clotting factor proteins culminating in fibrin clot formation. The clotting time test measures how long it takes a blood sample to form a solid clot, indicating the function of coagulation factors. There are several methods for measuring both bleeding time and clotting time to evaluate primary and secondary hemostasis.
Uploaded by
Sareene Joyce PepitoCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
100% found this document useful (2 votes)
1K views75 pages1 Primary-And-Secondary-Hemostasis PDF
Primary hemostasis involves platelet plug formation and vasoconstriction to stop bleeding from damaged blood vessels. It can be assessed through tests like bleeding time, which measures how long bleeding continues after a small wound. Secondary hemostasis involves the coagulation cascade of clotting factor proteins culminating in fibrin clot formation. The clotting time test measures how long it takes a blood sample to form a solid clot, indicating the function of coagulation factors. There are several methods for measuring both bleeding time and clotting time to evaluate primary and secondary hemostasis.
Uploaded by
Sareene Joyce PepitoCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Primary and Secondary Hemostasis Overview
- Aspects and Steps of Hemostasis
- Coagulation Factors
- Primary Hemostasis: Stages and Tests
- Bleeding Time
- Clotting Time
- Laboratory Tests for Secondary Hemostasis