Curare
Uploaded by
sai calderCurare
Uploaded by
sai calderNot logged in Talk Contributions Create account Log in
Article Talk Read Edit View history Search Wikipedia
Curare
From Wikipedia, the free encyclopedia
Main page This article is about the plant toxins. For the DC Comics character, see Curaré (Batman Beyond).
Contents Not to be confused with Curara.
Featured content
Current events This article may be expanded with text translated from the corresponding article in French. (March 2014) Click [show] for [show]
Random article important translation instructions.
Donate to Wikipedia
Wikipedia store The lead section of this article may need to be rewritten. Use the lead layout guide to ensure the section follows
Wikipedia's norms and is inclusive of all essential details. (May 2019) (Learn how and when to remove this template message)
Interaction
Help Curare (/kʊˈrɑːri/ or /kjʊˈrɑːri/; koo-rah-ree or kyoo-rah-ree)[1][2] is a common name for various plant extract alkaloid arrow poisons
About Wikipedia originating from Central and South America. These poisons function by competitively and reversibly inhibiting the nicotinic acetylcholine
Community portal
receptor (nAChR), which is a subtype of acetylcholine receptor found at the neuromuscular junction. This causes weakness of the
Recent changes
skeletal muscles and, when administered in a sufficient dose, eventual death by asphyxiation due to paralysis of the diaphragm.
Contact page
According to pharmacologist Rudolf Boehm's 1895 classification scheme, the three main types of curare are:[3]
Tools
What links here tube or bamboo curare: so named because of its packing into hollow bamboo tubes—of which the main toxin is D-tubocurarine—
Related changes derived from Chondrodendron and other genera in the Menispermaceae.
Upload file pot curare: originally packed in terra cotta pots—of which the main alkaloid components are protocurarine, protocurine, and
Special pages protocuridine (but see below re: inaccuracy/ambiguity of early analyses)(Protocurarine being the active ingredient; protocurine only
Permanent link
weakly toxic, and protocuridine non-toxic) comprising extracts from both Menispermaceae and Loganiaceae/Strychnaceae.
Page information
calabash or gourd curare: originally packed into hollow gourds, of which the main toxin is C toxiferine I, comprising extracts from
Wikidata item
Cite this page Loganiaceae/Strychnaceae alone.
Of these three types, some formulae referable to tube curare are the most toxic, relative to their LD50 values.[3]
In other projects
Wikimedia Commons Although this tripartite classification of curares into 'tube', 'pot' and 'calabash' was initially useful, it rapidly became outmoded:
Chondrodendron tomentosum,
main source plant of 'Tube Curare' and
Print/export Gill found that Boehm's classification became invalid shortly after his investigations, because the Indians began to use principal source of D-tubocurarine
Download as PDF various types of containers for their preparations [i.e. were not consistent in their use of the three types of container for (DTC), the alkaloid constituting
medicinal curare.
Printable version three distinct types of poison].[4]
Languages
Manske also observed in his 1955 The Alkaloids:
ا
Deutsch
Español The results of the early [pre-1900] work were very inaccurate because of the complexity and variation of the composition of
Français the mixtures of alkaloids involved ... these were impure, non-crystalline alkaloids ... Almost all curare preparations were and
Italiano are complex mixtures, and many of the physiological actions attributed to the early curarizing preparations were
⽇本語 undoubtedly due to impurities, particularly to other alkaloids present. The curare preparations are now considered to be of
Português
two main types, those from Chondrodendron or other members of the Menispermaceae family and those from Strychnos, a
Русский
中⽂ genus of the Loganiaceae [ now Strychnaceae ] family. Some preparations may contain alkaloids from both ... and the
majority have other secondary ingredients.[4]
22 more
Edit links
Contents [hide]
1 History
2 Classification and chemical structure
3 Pharmacological properties
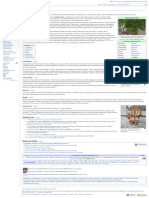
3.1 Anesthesia Strychnos toxifera, the Strychnos
species which is the principal source of
4 Plant sources
'Calabash Curare' and its main active
5 Toxicity constituent - the alkaloid toxiferine
6 Preparation
6.1 Adjuvants
7 Diagnosis and management of curare poisoning
7.1 Antidote
8 Gallery
9 See also
10 Notes
10.1 References
10.2 Further reading
History [ edit ]
This section is missing information about use of curare by Central American people. Please expand the section to include
this information. Further details may exist on the talk page. (March 2014)
Curare was used as a paralyzing poison by South American indigenous people. The prey was shot by arrows or blowgun darts dipped in
curare, leading to asphyxiation owing to the inability of the victim's respiratory muscles to contract. The word 'curare' is derived from
wurari, from the Carib language of the Macusi Indians of Guyana.[5] Curare is also known among indigenous peoples as Ampi, Woorari,
Woorara, Woorali, Wourali, Wouralia, Ourare, Ourari, Urare, Urari, and Uirary.
Curare was used by the Island Caribs, indigenous people of the Lesser Antilles in the Caribbean, on the tips of their arrows.[6]
In 1596, Sir Walter Raleigh mentioned the arrow poison in his book Discovery of the Large, Rich, and Beautiful Empire of Guiana (which
relates to his travels in Trinidad and Guayana), though the poison he described possibly was not curare.[7] In 1780, Abbe Felix Fontana Curare darts and quiver from the
discovered that it acted on the voluntary muscles rather than the nerves and the heart.[8] In 1832, Alexander von Humboldt gave the first Amazon rainforest.
western account of how the toxin was prepared from plants by Orinoco River natives.[9]
During 1811–1812, Sir Benjamin Collins Brody experimented with curare (woorara).[10][11] He was the first to show that curare does not
kill the animal and the recovery is complete if the animal's respiration is maintained artificially. In 1825, Charles Waterton described a
classical experiment in which he kept a curarized female donkey alive by artificial respiration with a bellows through a tracheostomy.[12]
Waterton is also credited with bringing curare to Europe.[13] Robert Hermann Schomburgk, who was a trained botanist, identified the vine
as one of the genus Strychnos and gave it the now accepted name Strychnos toxifera.[14]
George Harley (1829–1896) showed in 1850 that curare (wourali) was effective for the treatment of tetanus and strychnine
poisoning.[15][16] In 1857, Claude Bernard (1813–1878) published the results of his experiments in which he demonstrated that the
19th century depiction of hunting
mechanism of action of curare was a result of interference in the conduction of nerve impulses from the motor nerve to the skeletal
with blowguns in the Amazon
muscle, and that this interference occurred at the neuromuscular junction.[3][17] From 1887, the Burroughs Wellcome catalogue listed rainforest.
under its 'Tabloids' brand name, tablets of curare at 1⁄12 grain (price 8 shillings) for use in preparing a solution for hypodermic injection. In
1914, Henry Hallett Dale (1875–1968) described the physiological actions of acetylcholine.[18] After 25 years, he showed that
acetylcholine is responsible for neuromuscular transmission, which can be blocked by curare.[19]
The best known and historically most important (because of its medical applications) toxin is d-tubocurarine. It was isolated from the crude drug – from a museum sample of
curare – in 1935 by Harold King of London, working in Sir Henry Dale's laboratory. King also established its chemical structure.[20][21] Pascual Scannone, a Venezuelan
anesthesiologist[22] who trained and specialized in New York City, USA, did extensive research on curare as a possible paralyzing agent for patients during surgical procedures.
In 1942, he became the first person in all of Latin America to use curare during a medical procedure when he successfully performed a tracheal intubation in a patient to whom
he administered curare for muscle paralysis at the El Algodonal Hospital in Caracas, Venezuela.[22]
After its introduction in 1942, curare/curare-derivatives became a widely used paralyzing agent during medical and surgical procedures.[citation needed] In medicine, curare has
been superseded by a number of curare-like agents, such as pancuronium, which have a similar pharmacodynamic profile, but fewer side effects.[citation needed]
Curare is active – toxic or muscle-relaxing depending on the intended use – only by an injection or a direct wound contamination by poisoned dart or arrow. It is harmless if taken
orally[12][23] because curare compounds are too large and highly charged to pass through the lining of the digestive tract to be absorbed into the blood. For this reason, people
can safely eat curare-poisoned prey.[24]
Classification and chemical structure [ edit ]
This section needs expansion.
You can help by adding to it. (March
2014)
The various components of curare are organic compounds classified as either isoquinoline or indole alkaloids. Tubocurarine is the major active component in the South American
dart poison.[25] As an alkaloid, tubocurarine is a naturally occurring compound that consists of nitrogenous bases, although the chemical structure of alkaloids is highly variable.
Like most alkaloids, tubocurarine consists of a cyclic system with a nitrogen atom in an amine group.[26] Because of this structure, tubocurarine can bind readily to the receptors
for acetylcholine (ACh) at the neuromuscular junction, which blocks nerve impulses from being sent to the skeletal muscles, effectively paralyzing the muscles of the body. Since
tubocurarine binds reversibly to the ACh receptors, treatment for curare poisoning involves adding an acetylcholinesterase (AChE) inhibitor, which will stop the destruction of
acetylcholine so that it can compete with curare.[27]
Pharmacological properties [ edit ]
Curare is an example of a non-depolarizing muscle relaxant that blocks the nicotinic acetylcholine receptor (nAChR),[28] one of the two
types of acetylcholine (ACh) receptors, at the neuromuscular junction. The main toxin of curare, d-tubocurarine, occupies the same
position on the receptor as ACh with an equal or greater affinity, and elicits no response, making it a competitive antagonist. The antidote
for curare poisoning is an acetylcholinesterase (AChE) inhibitor (anti-cholinesterase), such as physostigmine or neostigmine. By blocking
ACh degradation, AChE inhibitors raise the amount of ACh in the neuromuscular junction; the accumulated ACh will then correct for the
effect of the curare by activating the receptors not blocked by toxin at a higher rate.
The time of onset varies from within one minute (for tubocurarine in intravenous administration, penetrating a larger vein), to between 15
and 25 minutes (for intramuscular administration, where the substance is applied in muscle tissue).[28]
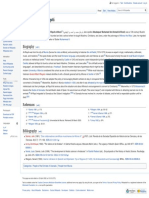
A neuromuscular junction. Curare
Curare has no effect if ingested so the meat of an animal killed by curare does not become poisonous, and it has no effect on its blocks Ach receptors (bottom left).
flavor.[24]
Anesthesia [ edit ]
Isolated attempts to use curare during anesthesia date back to 1912 by Arthur Lawen of Leipzig,[29] but curare came to anesthesia via psychiatry (electroplexy). In 1939 Abram
Elting Bennett used it to modify metrazol induced convulsive therapy.[30] Muscle relaxants are used in modern anesthesia for many reasons, such as providing optimal operating
conditions and facilitating intubation of the trachea. Before muscle relaxants, anesthesiologists needed to use larger doses of the anesthetic agent, such as ether, chloroform or
cyclopropane to achieve these aims. Such deep anesthesia risked killing patients who were elderly or had heart conditions.
The source of curare in the Amazon was first researched by Richard Evans Schultes in 1941. Since the 1930s, it was being used in hospitals as a muscle relaxant. He
discovered that different types of curare called for as many as 15 ingredients, and in time helped to identify more than 70 species that produced the drug.
In the 1940s, it was used on a few occasions during surgery as it was mistakenly thought to be an analgesic or anesthetic. The patients reported feeling the full intensity of the
pain though they were not able to do anything about it since they were essentially paralyzed.[31]
On January 23, 1942, Harold Griffith and Enid Johnson gave a synthetic preparation of curare (Intercostrin/Intocostrin) to a patient undergoing an appendectomy (to supplement
conventional anesthesia). Safer curare derivatives, such as rocuronium and pancuronium, have superseded d-tubocurarine for anesthesia during surgery. When used with
halothane d-tubocurarine can cause a profound fall in blood pressure in some patients as both the drugs are ganglion blockers.[32] However, it is safer to use d-tubocurarine with
ether.
In 1954, an article was published by Beecher and Todd suggesting that the use of muscle relaxants (drugs similar to curare) increased death due to anesthesia nearly sixfold.[33]
This was refuted in 1956.[34]
Modern anesthetists have at their disposal a variety of muscle relaxants for use in anesthesia. The ability to produce muscle relaxation irrespective of sedation has permitted
anesthetists to adjust the two effects independently and on the fly to ensure that their patients are safely unconscious and sufficiently relaxed to permit surgery. The use of
neuromuscular blocking drugs carries with it the risk of anesthesia awareness.
Plant sources [ edit ]
There are dozens of plants from which isoquinoline and indole alkaloids with curarizing effects can be isolated, and which were utilized by indigenous tribes of Central and South
America for the production of arrow poisons. Among them are:
In family Menispermaceae:
Genus Chondrodendron notably C. tomentosum
Genus Curarea, species toxicofera and tecunarum
Genus Sciadotenia toxifera
Genus Telitoxicum
Genus Abuta
Genus Caryomene
Genus Anomospermum
Genus Orthomene
Genus Cissampelos, section L. (Cocculeae) of genus
Other families:
several species of the genus Strychnos of family Loganiaceae including toxifera, guianensis, castelnaei, usambarensis
a plant in the subfamily Aroideae of family Araceae called taja
at least three members of the genus Artanthe of family Piperaceae
Paullinia cururu in the family Sapindaceae[35]
Some plants in the family Aristolochiaceae have also been reported as sources.
Alkaloids with curare-like activity are present in plants belonging to the fabaceous genus Erythrina.[4]
Toxicity [ edit ]
This section does not cite any sources. Please help improve this section by adding citations to reliable sources. Unsourced
material may be challenged and removed.
Find sources: "Curare" – news · newspapers · books · scholar · JSTOR (February 2016) (Learn how and when to remove this template message)
The toxicity of curare alkaloids in humans has not been established. Administration must be parenterally, as gastro-intestinal absorption is ineffective.
LD50 (mg/kg)
human: 0.735 est. (form and method of administration not indicated)
mouse: pot: 0.8–25; tubo: 5-10; calabash: 2–15.
Preparation [ edit ]
This section needs expansion.
You can help by adding to it. (March
2014)
Traditionally prepared curare is a dark, heavy, viscid paste with a very bitter taste.[36] In 1938, Richard Gill and his expedition collected samples of processed curare and
described its method of traditional preparation; one of the plant species used at that time was Chondrodendron tomentosum.[37]
Adjuvants [ edit ]
This section contains weasel words: vague phrasing that often accompanies biased or unverifiable information. Such
statements should be clarified or removed. (December 2014)
It is known[citation needed] that the final preparation is often more potent than the concentrated principal active ingredient. Various irritating herbs, stinging insects, poisonous
worms, and various parts of amphibians and reptiles are added to the preparation. Some of these accelerate the onset of action or increase the toxicity; others prevent the
wound from healing or blood from coagulating.
Diagnosis and management of curare poisoning [ edit ]
Curare poisoning can be indicated by typical signs of neuromuscular-blocking drugs such as paralysis including respiration but not directly affecting the heart.
Curare poisoning can be managed by artificial respiration such as mouth-to-mouth resuscitation. In a study of 29 army volunteers that were paralyzed with curare, artificial
respiration managed to keep an oxygen saturation of always above 85%,[38] a level at which there is no evidence of altered state of consciousness.[39] Yet, curare poisoning
mimics the total locked-in syndrome in that there is paralysis of every voluntarily controlled muscle in the body (including the eyes), making it practically impossible for the victim
to confirm consciousness while paralyzed.[40]
Spontaneous breathing is resumed after the end of the duration of action of curare, which is generally between 30 minutes[41] and 8 hours,[42] depending on the variant of the
toxin and dosage. Cardiac muscle is not directly affected by curare, but if more than four to six minutes[43] has passed since respiratory cessation the cardiac muscle may stop
functioning by oxygen-deprivation, making cardiopulmonary resuscitation including chest compressions necessary.
Antidote [ edit ]
Muscle paralysis can be reversed by administration of a cholinesterase inhibitor such as pyridostigmine,[44] neostigmine and edrophonium. These can be termed "anticurare"
drugs.
Gallery [ edit ]
Abuta selloana. Certain Anomospermum Cissampelos pareira.
species in the schomburgkii. Certain Certain species in the
menispermaceous species in the genus genus Cissampelos
genus Abuta— Anomospermum have have been employed in
particularly the been used in the the preparation of
Colombian species A. preparation of some curare.
imene—have sometimes forms of curare.
been used in the
preparation of curare.
See also [ edit ]
Arrow poison, what curare was originally used for
Poison dart frog, another source of arrow poison
Strychnine, a related alkaloid poison that occurs in some of the same plants as curare
Notes [ edit ]
References [ edit ]
1. ^ "curare" . Lexico. Oxford Online Dictionaries. 23. ^ Schaffner, Brynn (2000). "Curare" . Blue Planet Biomes. Retrieved 14 April 2020.
2. ^ "cu·ra·re also cu·ra·ri" . The American Heritage Dictionary of the English Language. 24. ^ a b Milner, Daniel (Summer 2009). "From the Rainforests of South America to The
Houghton Mifflin Harcourt. Retrieved 14 April 2020. Operating Room: A History of Curare" . Faculty of Medicine, Department of Innovation in
3. ^ a b c Gray, TC (1947). "The Use of D-Tubocurarine Chloride in Anæsthesia" . Ann R Medical Education. University of Ottawa. Archived from the original on 30 July 2011.
Coll Surg Engl. The Royal College of Surgeons of England. 1 (4): 191–203. 25. ^ Editors of Encyclopaedia Britannica (7 March 2016). "Curare, chemical compound" .
PMC 1940167 . PMID 19309828 . Encyclopædia Britannica. Retrieved 17 April 2020.
4. ^ a b c Manske, R. H. F., ed. (1955). The Alkaloids: Chemistry and Physiology – Volume 5, 26. ^ Verpoorte, R.; Choi, Y.H.; Kim, H.K. (August 2005). "Ethnopharmacology and systems
Pharmacology . New York, New York: Academic Press Inc. p. 269. LCCN 50-5522 . biology: A perfect holistic match". Journal of Ethnopharmacology. 100 (1–2): 53–56.
Retrieved 12 May 2014. doi:10.1016/j.jep.2005.05.033 . PMID 16026949 .
5. ^ "curare (n.)" . Online Etymology Dictionary. Douglas Harper. 27. ^ Saladin, Kenneth S. (2015). Anatomy and Physiology The Unity of Form and Function
6. ^ La Oficina del Indice Histórico de Puerto Rico [The Office of the Historical Index of (7th ed.). New York: McGraw Hill Education. ISBN 978-1259385513.
Puerto Rico] (1949). Tesauro de datos historicos: Indice compendioso de la literatura 28. ^ a b "Curare" . Drugs.com. Drugs.com. 8 November 2001.
histórica de Puerto Rico, incluyendo algunos datos inéditos, periodísticos y cartográficos, 29. ^ Lawen, A. (1912). "Über die Verbindung der Lokalanästhesie mit der Narkose, über
Tomo II [Thesaurus of historical data: Comprehensive index of Puerto Rico's historical hohe Extraduralanaesthesie und epidurale injektionen anasthesierender Losungen bei
literature, including some unpublished, journalistic and cartographic data, Volume II] (in tabischen Makenkrisen" [Over the connection of local anesthesia with anesthesia, through
Spanish). San Juan, Puerto Rico: El Gobierno de Puerto Rico. p. 306. Retrieved high extradural anesthesia and epidural injections of anesthetic solutions in tabetic
4 January 2020. macaques]. Beitrage zur klinischen Chirurgie [Contributions to Clinical Surgery] (in
7. ^ Carman, J. A. (October 1968). "History of curare". Anaesthesia. Association of German). 80: 168–189.
Anaesthesists. 23 (4): 706–707. doi:10.1111/j.1365-2044.1968.tb00142.x . 30. ^ Bennett, A. E. (1940). "Preventing traumatic complications in convulsive shock therapy
PMID 4877723 . by curare". Journal of the American Medical Association. American Medical Association
8. ^ The Gale Encyclopedia of Science (Third ed.). Gale Group. (114): 322–324. doi:10.1001/jama.1940.02810040032009 .
9. ^ Humboldt, Alexander von; Bonpland, Aimé (1907). Personal Narrative of Travels to the 31. ^ Dennett, Daniel C. (1978). Brainstorms: Philosophical Essays on Mind and Psychology.
Equinoctial Regions of America, During the Year 1799–1804 – Volume 2 . Translated by Cambridge, Massachusetts: The MIT Press. p. 209.
Ross, Thomasina. London: George Bell & Sons. 32. ^ Mashraqui, S. (October 1994). "Hypotension induced with d-tubocurarine and halothane
10. ^ Brodie, Benjamin Collins (1811). "X. Experiments and Observations on the different for surgery of patent ductus arteriosus". Indian Journal of Anesthesia. 42 (5): 346–50.
Modes in which Death is produced by certain vegetable Poisons. By B. C. Brodie, Esq. F. 33. ^ Beecher, H. K.; Todd, D. P. (1954). "A Study of the Deaths Associated with Anesthesia
R. S. Communicated by the Society for promoting the Knowledge of Animal Chemistry". and Surgery: Based on a Study of 599,548 Anesthesias in Ten Institutions 1948–1952,
Philosophical Transactions. The Royal Society. 101: 178–208. Inclusive" . Annals of Surgery. 140 (2): 2–35. doi:10.1097/00000658-195407000-
doi:10.1098/rstl.1811.0011 . 00001 . PMC 1609600 . PMID 13159140 ., reprinted in "Classical File" . Survey of
11. ^ Brodie, Benjamin Collins (1812). "XI. Further Experiments and Observations on the Anesthesiology. 15 (5): 496 ff. October 1971.
Action of Poisons on the Animal System. By B. C. Brodie, Esq. F. R. S. Communicated to 34. ^ Albertson, HA; Trout, HH; Morfin, E (June 1956). "The Safety of Curare in
the Society for the Improvement of Animal Chemistry, and by them to the Royal Society". Anesthesia" . Annals of Surgery. 143 (6): 833–837. doi:10.1097/00000658-195606000-
Philosophical Transactions. The Royal Society. 102: 205–227. 00012 . PMC 1465152 . PMID 13327828 .
doi:10.1098/rstl.1812.0013 . 35. ^ Lewis, Walter H.; Elvin-Lewis, Memory P.F. (1977). Medical Botany: Plants Affecting
12. ^ a b "CURARE (Chondrodendron tomentosum - Menispermaceae): From Arrow Poison to Man's Health. Wiley-Interscience. ISBN 0-471-53320-3.
Surgical Muscle Relaxant" . Ye Olde Log. n.d. Archived from the original on 9 May 36. ^ Gibson, Arthur C. "Curare, a South American Arrow Poison" . Plants and Civilization.
2008. Retrieved 23 August 2017. UCLA Mildred E. Mathias Botanical Garden, University of California, Los Angeles.
13. ^ Waterton, Charles (1891). "Chapter II". Wanderings in South America. London, Paris & Archived from the original on 28 July 2012.
Melbourne: Cassell & Company, Limited., reprinted in "Classical File" . Survey of 37. ^ Kemp, Christopher (17 January 2018). "The Amazonian arrow poison that made
Anesthesiology. 22 (1): 98 ff. February 1978. modern anaesthesia: Adventurer Richard Gill sought relief from symptoms of multiple
14. ^ Birmingham, A T (1999). "Waterton and Wouralia" . British Journal of Pharmacology. sclerosis in an Ecuadorian tribal weapon – with wider results that live on in medicine
The British Pharmacological Society. 126: 1685–1689. doi:10.1038/sj.bjp.0702409 . today" . New Scientist (3161).
PMC 1565951 . 38. ^ Idress, A.H.; Gabrielli, A. (2007). "Techniques of ventilation during CPR" . In Paradis,
15. ^ Paton, A. (December 1979). "George Harley (1829-1896)". Practitioner. 223 (1338): Norman A.; Halperin, Henry R.; Kern, Karl B.; Wenzel, Volker; Chamberlain, Douglas A.
849–51. PMID 396529 . (eds.). Cardiac Arrest: The Science and Practice of Resuscitation Medicine (2nd ed.).
16. ^ "George Harley" . Whonamedit? – A dictionary of medical eponyms. Retrieved Cambridge, UK: Cambridge University Press. p. 520. ISBN 978-0-521-84700-1.
14 April 2020. 39. ^ McEvoy, Mike (October 12, 2010), Oxymoron: Our Love-Hate Relationship with
17. ^ Bernard, Claude (1857). "Vingt-cinquième Leçon [Twenty-fifth Lesson]" . Leçons sur Oxygen (PDF), Albany, New York: Albany Medical College, archived from the
les effets des substances toxiques et médicamenteuses [Lessons on the effects of toxic original (PDF) on August 21, 2011
and medicinal substances] (in French). Paris: J.B. Baillière. pp. 369–80. 40. ^ Damasio, Antonio R. (1999). The Feeling of What Happens: Body and Emotion in the
18. ^ Dale, H. H. (1 November 1914). "THE ACTION OF CERTAIN ESTERS AND ETHERS Making of Consciousness . San Diego: Harcourt Brace. p. 357. ISBN 978-0-15-601075-
OF CHOLINE, AND THEIR RELATION TO MUSCARINE" . Journal of Pharmacology 7.
and Experimental Therapeutics. The American Society for Pharmacology and 41. ^ For therapeutic dose of tubocurarine by shorter limit as given in: Rang, H. P. (2003).
Experimental Therapeutics. 6: 147-190. Pharmacology. Edinburgh: Churchill Livingstone. p. 151. ISBN 978-0-443-07145-4.
19. ^ Dale, Henry (12 May 1934). "CHEMICAL TRANSMISSION OF THE EFFECTS OF OCLC 51622037 .
NERVE IMPULSES" . British Medical Journal (3827): 835–841. 42. ^ For 20-fold paralytic dose of toxiferine ("calabash curare"), according to: The Alkaloids:
doi:10.1136/bmj.1.3827.835 . PMC 2445804 . PMID 20778253 . v. 1: A Review of Chemical Literature (Specialist Periodical Reports) . Cambridge,
20. ^ King, H. (1935). "Curare alkaloids: Part 1, Tubocurarine" . Journal of the Chemical Engand: Royal Society of Chemistry. 1971. p. 330. ISBN 978-0-85186-257-6.
Society. The Royal Society of Chemistry. 57: 1381–1389. 43. ^ "Cardiopulmonary Resuscitation (CPR)" , Gale Encyclopedia of Medicine, The Gale
21. ^ King, Harold (1935). "Curare" . Nature. The Physical Society. 135: 469–470. Group, Inc., 2008 – via The Free Dictionary by Farlex
22. ^ a b Eger, Edmond I II; Saidman, Lawrence J.; Westhorpe, Rod N., eds. (2014). The 44. ^ Morgan, Thomas III; Kalman, Bernadette (2007). Neuroimmunology in Clinical
Wondrous Story of Anesthesia . Springer. p. 438. ISBN 978-1-4614-8440-0. Practice . Wiley-Blackwell. p. 153. ISBN 978-1-4051-5840-4.
Further reading [ edit ]
Foldes, F. F. (1993), "Anästhesie vor und nach Curare" [Anesthesia before and after curare], Anaesthesiol Reanim (in German), 18 (5), pp. 128–131, PMID 8280340 ,
retrieved June 20, 2005
"Harold Griffith, Fonds P090" . Archival Collections Catalogue. Osler Library of the History of Medicine, McGill University Library, McGill University. – contains papers and
records pertaining to Griffith's introduction of curare into anesthesiology
James, Mel, "Harold Griffith" , Canada Heirloom Series, Volume 6, retrieved June 20, 2005
Raghavendra, Thandla (July 2002). "Neuromuscular blocking drugs: discovery and development" . Journal of the Royal Society of Medicine. 95 (7): 363–367.
PMC 1279945 . PMID 12091515 .
Smith, Roger P., "Cholernergic Transmission" , Dartmouth College, Trustees of Dartmouth College, archived from the original on December 29, 2007, retrieved March 13,
2007
Strecker, G.J.; Jackson, M.B. (October 1989). "Curare binding and the curare-induced subconductance state of the acetylcholine receptor channel" . Biophysical Journal. 56
(4): 795–806. doi:10.1016/S0006-3495(89)82726-2 . PMC 1280535 . PMID 2479422 . Retrieved 11 July 2019.
Waterton, Charles. Bullen, A. H. (ed.). Wanderings In South America – via Project Gutenberg.
V·T·E Skeletal muscle relaxants (M03) [show]
V·T·E Nicotinic acetylcholine receptor modulators [show]
Authority control BNF: cb11957231d (data) · GND: 4300192-0 · LCCN: sh85034841 · SUDOC: 027557308
Categories: Muscle relaxants Neuromuscular blockers Neurotoxins Nicotinic antagonists Plant toxins
This page was last edited on 26 April 2020, at 07:23 (UTC).
Text is available under the Creative Commons Attribution-ShareAlike License; additional terms may apply. By using this site, you agree to the Terms of Use and Privacy Policy. Wikipedia® is a registered trademark of the
Wikimedia Foundation, Inc., a non-profit organization.
Privacy policy About Wikipedia Disclaimers Contact Wikipedia Developers Statistics Cookie statement Mobile view
You might also like
- Luffa Cylindrica: Medicinal Benefits and UsesNo ratings yetLuffa Cylindrica: Medicinal Benefits and Uses6 pages
- Surigao Del Sur State University: Review of Related LiteratureNo ratings yetSurigao Del Sur State University: Review of Related Literature9 pages
- Antibacterial Activities of Extracts of Leaf, Fruit, Seed and Bark of Phoenix DactyliferaNo ratings yetAntibacterial Activities of Extracts of Leaf, Fruit, Seed and Bark of Phoenix Dactylifera5 pages
- Ziziphus Nummularia: Medicinal Uses & BenefitsNo ratings yetZiziphus Nummularia: Medicinal Uses & Benefits10 pages
- Indigenous Traditional Knowledge About Medicinal Plants of Rajasthan State To Cure Respiratory and Liver DiseasesNo ratings yetIndigenous Traditional Knowledge About Medicinal Plants of Rajasthan State To Cure Respiratory and Liver Diseases6 pages
- Ethnobotanical Survey of Vellore Medicinal PlantsNo ratings yetEthnobotanical Survey of Vellore Medicinal Plants8 pages
- FINAL THESIS TECOMA Species-Varshita Batch 1No ratings yetFINAL THESIS TECOMA Species-Varshita Batch 145 pages
- Larvicidal Activity of Some Saponin Containing Plants Against The Dengue Vector Aedes Aegypti.100% (1)Larvicidal Activity of Some Saponin Containing Plants Against The Dengue Vector Aedes Aegypti.11 pages
- Wandera - A Preliminary Phytochemical Investigation of Croton Macrostachyus Stem BarkNo ratings yetWandera - A Preliminary Phytochemical Investigation of Croton Macrostachyus Stem Bark55 pages
- Prosea 12.1 Medicinal and Poisonous Plants 1No ratings yetProsea 12.1 Medicinal and Poisonous Plants 1708 pages
- Crude Drugs of Bangladesh: Uses & BenefitsNo ratings yetCrude Drugs of Bangladesh: Uses & Benefits33 pages
- Visiting Taro From A Botanical and Phytochemical PerspectiveNo ratings yetVisiting Taro From A Botanical and Phytochemical Perspective6 pages
- Antimicrobial Properties of Herbal PlantsNo ratings yetAntimicrobial Properties of Herbal Plants39 pages
- Introduction To Natural Products Chemistry: Rensheng Xu Yang Ye Weimin ZhaoNo ratings yetIntroduction To Natural Products Chemistry: Rensheng Xu Yang Ye Weimin Zhao4 pages
- Antibiotic Activity in BC Medicinal PlantsNo ratings yetAntibiotic Activity in BC Medicinal Plants11 pages
- Botany Uses Chemistry and Pharmacology of Ficus MiNo ratings yetBotany Uses Chemistry and Pharmacology of Ficus Mi9 pages
- Phytochemical Screening of Methanolic Extracts of Different Parts of Rudraksh Plant (Elaeocarpus Ganitrus)No ratings yetPhytochemical Screening of Methanolic Extracts of Different Parts of Rudraksh Plant (Elaeocarpus Ganitrus)2 pages
- Shades and Variations: Indigofera TinctoriaNo ratings yetShades and Variations: Indigofera Tinctoria1 page
- Green: Etymology and Linguistic DefinitionsNo ratings yetGreen: Etymology and Linguistic Definitions1 page
- In Mathematics: List of Basic CalculationsNo ratings yetIn Mathematics: List of Basic Calculations1 page
- Shades and Variations: This Article Is About The Color. For Other Uses, SeeNo ratings yetShades and Variations: This Article Is About The Color. For Other Uses, See1 page
- Nursing Care Plan for Impaired BreathingNo ratings yetNursing Care Plan for Impaired Breathing2 pages
- Hyderabad Based Pharma Companies UpdatedNo ratings yetHyderabad Based Pharma Companies Updated16 pages
- CARESCAPE R860 Ventilator Clinical Accessories Guide - JB79010XX100% (1)CARESCAPE R860 Ventilator Clinical Accessories Guide - JB79010XX16 pages
- Dupax del Norte I COVID-19 Contingency PlanNo ratings yetDupax del Norte I COVID-19 Contingency Plan11 pages
- Vaginal Discharge-Causes, Diagnosis, and Treatment: ABC of Sexually Transmitted InfectionsNo ratings yetVaginal Discharge-Causes, Diagnosis, and Treatment: ABC of Sexually Transmitted Infections3 pages
- List of Expenses Generally Excluded ("Non-Medical") in Hospital Indemnity PolicyNo ratings yetList of Expenses Generally Excluded ("Non-Medical") in Hospital Indemnity Policy6 pages
- Betahistine Dihydrochloride in The Treatment of Peripheral Vestibular VertigoNo ratings yetBetahistine Dihydrochloride in The Treatment of Peripheral Vestibular Vertigo6 pages
- Chulalongkorn University 2022 Scholarship AwardeesNo ratings yetChulalongkorn University 2022 Scholarship Awardees5 pages
- ESOT 2015 Transplantation Congress GuideNo ratings yetESOT 2015 Transplantation Congress Guide33 pages
- Condylar Fracture Overview and ManagementNo ratings yetCondylar Fracture Overview and Management36 pages
- Program and Proceedings - 8th International Neuroscience and Biological Psychiatry Regional ISBS Conference "STRESS AND BEHAVIOR: YOKOHAMA-2016", July 23-25, 2016, Yokohama, JapanNo ratings yetProgram and Proceedings - 8th International Neuroscience and Biological Psychiatry Regional ISBS Conference "STRESS AND BEHAVIOR: YOKOHAMA-2016", July 23-25, 2016, Yokohama, Japan31 pages