Professional Documents
Culture Documents
Synthesis and Reporting (Step 7) : 1. How Is Pathophysiologi and Pathogenesis of Filariasis?
Uploaded by
Rhara Aulia0 ratings0% found this document useful (0 votes)
13 views4 pagesxxxwfetg
Original Title
Synthesis and Reporting
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentxxxwfetg
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
13 views4 pagesSynthesis and Reporting (Step 7) : 1. How Is Pathophysiologi and Pathogenesis of Filariasis?
Uploaded by
Rhara Auliaxxxwfetg
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 4
Synthesis and Reporting (Step 7)
1. How is pathophysiologi and pathogenesis of filariasis?
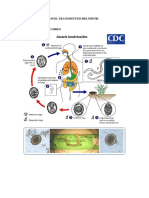
The filarial life cycle, like that of all nematodes, consists of 5 developmental
(larval) stages in a vertebral host and an arthropod intermediate host and
vector. Adult female worms produce thousands of first-stage larvae, or
microfilariae, which are ingested by a feeding insect vector. Some
microfilariae have a unique daily circadian periodicity in the peripheral
circulation. The arthropod vectors (mosquitoes and flies) also have a circadian
rhythm in which they obtain blood meals. The highest concentration of
microfilariae usually occurs when the local vector is feeding most actively.
Microfilariae undergo two developmental changes in the insect. Third-stage
larvae then are inoculated back into the vertebral host during the act of feeding
for the final two stages of development. These larvae travel through the dermis
and enter regional lymphatic vessels. During the next 9 months, the larvae
develop into mature worms (20-100 mm in length). An average parasite can
survive for about 5 years.
The pre-patent period is defined as the interval between a vector bite and the
appearance of microfilariae in blood, with an estimated duration of about 12
months.
The following factors affect the pathogenesis of filariasis:
The quantity of accumulating adult worm antigen in the lymphatics
The duration and level of exposure to infective insect bites
The number of secondary bacterial and fungal infections
The degree of host immune response
Filarial infection generates significant inflammatory immune responses that
participate in the development of symptomatic lymphatic obstruction.
Increased levels of immunoglobulin E (IgE) and immunoglobulin G4 (IgG4)
secondary to antigenic (from dead worms) stimulation of Th2-type immune
response have been demonstrated.
Studies indicate that there is a familial tendency to lymphatic obstruction. This
provides support for the hypothesis that host genes influence lymphedema
susceptibility. Studies also suggest that microfilaremia may be increased in
individuals with low levels of mannose-binding lectin, suggesting a genetic
predisposition. Furthermore, a propensity to develop chronic disease has been
demonstrated in patients with polymorphisms of endothelin-1 and tumor
necrosis factor receptor II.
2. What are the types of filariasis?
Type of filariasis Clinical symptoms Diagnosis Treatment
Filariasis Bancrofti Acute manifestation Identification of DEC 6mg/kgBB/hari
Aetiology: Limfadenitis often micofilariaes in for 12 days, divided
Wuchereria bancrofti accompanied by local blood smear by into 3 doses, take
(tropic) pain, local tenderness, microscopic after meal
fever, headache, examination
Habitat: lymphatic vomiting, nausea Eosinophilia
systems,blood Chronic manifestation Leukositosis
Hydrocele, chiluria,
Epidemiology : lymphedema,
filariasis bancrofti (it elephantiasis
can be found both in
urban and rural but
mstly can be found in
rural, risk group are
young adults
Filariasis Malayi and The amount of Identification of DEC 5mg/kgbb/hari
Timori microfilarias are lower microfilariaes in for 10 days
Aetiology: Brugia than filariasis bancrofti blood smear by
malayi (south east Typical symptoms: microscopic
asia) and Brugia superficial examination
Timori (lesser sunda) lymphadenopathy Eosinophilia up
Do not affect urinary to 70%
Habitat: lymphatic tract and breasts The difference
systems,blood between timori
and malayi are in
Epidemiology: the shape of the
filariasis malayi (can microfilariaes
be found in rural only In timori
due to its vector 1. The shape of the
especially in sumatra length of the head
untul maluku) is 3 times bigger
filariasis timori (can than the width
be found in rural only 2. The tails have 2
too but only in east additional core
indonesia (NTT) and 3. The length of the
timor timor microfilariae is
longer
3. Prevention programs for filariasis
Global Programme to Eliminate Lymphatic Filariasis by WHO
In 1997, following advances in diagnosis and treatment of the disease, WHO
classified lymphatic filariasis, along with five other infectious diseases, as
eradicable or potentially eradicable. The same year, the World Health
Assembly adopted Resolution WHA 50.29, which called on Member States to
initiate steps to eliminate lymphatic filariasis as a public health problem. In
response to this call, WHO launched the Global Programme to Eliminate
Lymphatic Filariasis (GPELF) in 2000.
The elimination strategy has two components:
1. to stop the spread of infection (interrupting transmission)
2. to alleviate the suffering of affected populations (controlling morbidity).
In order to interrupt transmission, districts in which lymphatic filariasis is
endemic must be mapped and community-wide mass treatment programmes
implemented to treat the entire at-risk population. Most of these programmes
are based on once-yearly administration of single doses of two drugs given
together. The following recommended drug regimens need to be administered
once a year for at least 5 years, with a coverage of at least 65% of the total at-
risk population:
1. Stop the spread of infection – MDA
In order to interrupt transmission, districts in which lymphatic filariasis is
endemic must be mapped and a strategy of preventive chemotherapy called
mass drug administration (MDA) implemented to treat the entire at-risk
population. The following drug regimens are recommended for use in
annual MDA for at least 5 years with a coverage of at least 65% of the total
at-risk population:
6 mg/kg of body weight diethylcarbamazine citrate (DEC) + 400 mg
albendazole
150 µg/kg of body weight ivermectin + 400 mg albendazole (in areas
that are also endemic for onchocerciasis)
400 mg albendazole preferably twice per year (in areas that are also
endemic for Loa loa).
An alternative and equally effective community-wide regimen in endemic
regions is the use of common table salt or cooking salt fortified with DEC.
DEC fortified salt has been used in only a few settings.
2. Alleviate suffering – MMDP
Successful MDA will prevent new infections and no new cases of clinical
disease. To achieve the second aim of GPELF, a core strategy of morbidity
management and disability prevention (MMDP) is needed. Suffering
caused by the disease can be alleviated through a minimum recommended
package of care to manage lymphedema and hydrocele. These services
should be available within primary health care systems in all areas of
known patients.
You might also like
- Epidemiology of Lymphatic FilariasisDocument26 pagesEpidemiology of Lymphatic FilariasisvaishnaviNo ratings yet
- Epidemiology and Control of Filariasis: Reshma Ann MathewDocument59 pagesEpidemiology and Control of Filariasis: Reshma Ann MathewHanna HonorisNo ratings yet
- Frequently Asked Quaestions (FAQs)Document22 pagesFrequently Asked Quaestions (FAQs)Drop Ur.questionNo ratings yet
- Filariasis: Dr. Saida SharminDocument35 pagesFilariasis: Dr. Saida SharminBishwajit BhattacharjeeNo ratings yet
- Filariasis in NepalDocument41 pagesFilariasis in NepalBinaya100% (1)
- Filariasis Control ProgramDocument3 pagesFilariasis Control ProgramCate LagradaNo ratings yet
- FILARIASIS PPT FINALDocument39 pagesFILARIASIS PPT FINALBinita Shakya100% (1)
- Filariasis A. Readings: CD ReportDocument6 pagesFilariasis A. Readings: CD ReportAisa ShaneNo ratings yet
- Text Latian Meeting 12Document2 pagesText Latian Meeting 12Empi RamliNo ratings yet
- Guidelines Filariasis Elimination IndiaDocument108 pagesGuidelines Filariasis Elimination IndiaegalivanNo ratings yet
- FilariaDocument16 pagesFilariaJessa MayNo ratings yet
- EPIDIMIOLOGYDocument4 pagesEPIDIMIOLOGYMaxamed Ali AadenNo ratings yet
- Lymphatic FilariasisDocument5 pagesLymphatic FilariasisBALAJI POTBHARENo ratings yet
- LF Anil KRDocument122 pagesLF Anil KRanil sahNo ratings yet
- Filariasis: Signs and SymptomsDocument30 pagesFilariasis: Signs and SymptomsAnonymous VJjzqbAlNo ratings yet
- Blood and Tissue NematodesDocument37 pagesBlood and Tissue NematodesjelenaNo ratings yet
- Lymphatic Filariasis Disease Causes, Symptoms, TreatmentDocument9 pagesLymphatic Filariasis Disease Causes, Symptoms, TreatmentRizzy UgayNo ratings yet
- Filariasis: A Global Parasitic DiseaseDocument5 pagesFilariasis: A Global Parasitic DiseaseHpu JogindernagerNo ratings yet
- Lymphatic Filariasis GuideDocument23 pagesLymphatic Filariasis GuidePrincess Gutierrez RositaNo ratings yet
- Filariasis LYMPHATIC FILARIASISDocument8 pagesFilariasis LYMPHATIC FILARIASISapih_2629No ratings yet
- Filarial NematodeDocument18 pagesFilarial NematodeHawre NajmaddinNo ratings yet
- Malaria Pathology, Transmission & TreatmentDocument12 pagesMalaria Pathology, Transmission & TreatmentMdhe asif alamNo ratings yet
- Filariasis, Schistosomiasis, Leprosy Control Program and Malaria Control ProgramDocument26 pagesFilariasis, Schistosomiasis, Leprosy Control Program and Malaria Control ProgramPC NNo ratings yet
- Filariasis: By: Barrantes, Patrick and Garcia, Charina JaneDocument17 pagesFilariasis: By: Barrantes, Patrick and Garcia, Charina Janerhimineecat71No ratings yet
- FILARIASIS: A Chronic Parasitic InfectionDocument8 pagesFILARIASIS: A Chronic Parasitic InfectionTheeya QuigaoNo ratings yet
- Lymphatic filariasis causes painful swellingDocument3 pagesLymphatic filariasis causes painful swellingArdya GarryNo ratings yet
- Vector Borne DiseasesDocument7 pagesVector Borne DiseasesAbuzar KhanNo ratings yet
- Brugia malayi causes elephantiasis in Southeast AsiaDocument4 pagesBrugia malayi causes elephantiasis in Southeast AsiaJericha IsidroNo ratings yet
- Name:Renelyn C. Candidider Prof - Doc. Josephine Suyamin Subject:Health Econ Filariasis DiseaseDocument4 pagesName:Renelyn C. Candidider Prof - Doc. Josephine Suyamin Subject:Health Econ Filariasis DiseaseAbelardo CuizonNo ratings yet
- FilariasisDocument4 pagesFilariasisKASHYAPNo ratings yet
- Malaria: by Marinel M. CayloDocument33 pagesMalaria: by Marinel M. Caylolorella_abejuelaNo ratings yet
- MaleriaDocument41 pagesMaleriadeepak_143No ratings yet
- B IngDocument8 pagesB IngsanggrainiNo ratings yet
- Lymphatic FilariasisDocument4 pagesLymphatic FilariasisGuna SoundariNo ratings yet
- Elimination of Lymphatic Filariasis: A Neglected Disease of IndiaDocument6 pagesElimination of Lymphatic Filariasis: A Neglected Disease of IndiamuskaanNo ratings yet
- FILARIASIS INFECTIONS & RELATED DISEASESDocument21 pagesFILARIASIS INFECTIONS & RELATED DISEASESInthanNo ratings yet
- ElephantiasisDocument23 pagesElephantiasisNitin0% (1)
- Filariasis - Causes, Types, Symptoms, and ManagementDocument6 pagesFilariasis - Causes, Types, Symptoms, and ManagementChumaNo ratings yet
- Vectors Are Living Organisms That Can Transmit Infectious DiseasesDocument23 pagesVectors Are Living Organisms That Can Transmit Infectious DiseasesLord SivaNo ratings yet
- Harrison's Internal Medicine Chapter 211. Filarial and Related InfectionsDocument9 pagesHarrison's Internal Medicine Chapter 211. Filarial and Related InfectionsGaniNo ratings yet
- Filariasis Control ProgramDocument17 pagesFilariasis Control ProgramLharra Cagulada-PostranoNo ratings yet
- Original ArticleDocument11 pagesOriginal ArticleLisamoni 786No ratings yet
- Lymphatic Filariasis Causes, Symptoms, TreatmentDocument4 pagesLymphatic Filariasis Causes, Symptoms, TreatmentCamela Joy Domingo CentenoNo ratings yet
- Filariasis ParasitesDocument3 pagesFilariasis ParasitesJurel GaoatNo ratings yet
- Understanding Lymphatic FilariasisDocument5 pagesUnderstanding Lymphatic FilariasisawilakNo ratings yet
- Leprosy Disease OverviewDocument44 pagesLeprosy Disease OverviewKamlesh SharmaNo ratings yet
- Filariasis: Fever Skin Lesions ElephantiasisDocument3 pagesFilariasis: Fever Skin Lesions ElephantiasisFoysal SirazeeNo ratings yet
- Brugia Malayi in Cervical Lymph Node Aspirate: A Rare Case ReportDocument2 pagesBrugia Malayi in Cervical Lymph Node Aspirate: A Rare Case ReportzzakieNo ratings yet
- Project Report On MaleriaDocument50 pagesProject Report On Maleriaasutosh0% (1)
- Filariasis: Dr.T.V.Rao MDDocument62 pagesFilariasis: Dr.T.V.Rao MDDipankar NathNo ratings yet
- Mycology VirologyDocument23 pagesMycology VirologymikeNo ratings yet
- Community Health Nursing: Christian Parabuac BS Nursing 3 Ms. FabianDocument14 pagesCommunity Health Nursing: Christian Parabuac BS Nursing 3 Ms. FabianMr. Christian ParabuacNo ratings yet
- Blood and Tissue Nematodes Classification and DiseasesDocument89 pagesBlood and Tissue Nematodes Classification and DiseasesRediat GossayeNo ratings yet
- LeprosyDocument3 pagesLeprosyNadim DinaniNo ratings yet
- Lymphatic Filariasis Elimination ProgramDocument14 pagesLymphatic Filariasis Elimination ProgramIvan Matthew SuperioNo ratings yet
- Leprae and Mycobacterium Lepromatosis. Named After Physician Gerhard Armauer HansenDocument5 pagesLeprae and Mycobacterium Lepromatosis. Named After Physician Gerhard Armauer HansenTim-Tim FerrerNo ratings yet
- HELMINTOLOGIDocument20 pagesHELMINTOLOGIAnggis KusumaningtyasNo ratings yet
- Lymphatic FillairiasisDocument7 pagesLymphatic FillairiasisRitche DamasinNo ratings yet
- FilariasisDocument4 pagesFilariasisapi-371817475% (4)
- Health Effects On Climate Change-Induced Wildfires and HeatwavesDocument6 pagesHealth Effects On Climate Change-Induced Wildfires and HeatwavesRhara AuliaNo ratings yet
- Global Warming and Its Health ImpactDocument14 pagesGlobal Warming and Its Health ImpactAde KurniaNo ratings yet
- RISK ASSESSMENT AND CONTROL OF AGRICULTURAL SECTORDocument8 pagesRISK ASSESSMENT AND CONTROL OF AGRICULTURAL SECTORAde margusNo ratings yet
- Parasit Uget-Uget (SN, JN, HB, JM, CC)Document31 pagesParasit Uget-Uget (SN, JN, HB, JM, CC)Rhara AuliaNo ratings yet
- PHCL Midterms - Lesson 1 (Calculation of Doses General Consideration)Document4 pagesPHCL Midterms - Lesson 1 (Calculation of Doses General Consideration)Lazaro, Javen Andrie A.No ratings yet
- Sandaletal UpdateonITPDocument18 pagesSandaletal UpdateonITPMarcellia AngelinaNo ratings yet
- Keratitis: Causes, Symptoms and TreatmentDocument28 pagesKeratitis: Causes, Symptoms and TreatmentjantyNo ratings yet
- Clinicopathologic Conference: Tejada, Michael Leonelle A. Block 10Document14 pagesClinicopathologic Conference: Tejada, Michael Leonelle A. Block 10Michael TejadaNo ratings yet
- Chapter 5 Prep U.odtDocument6 pagesChapter 5 Prep U.odtLivan MartellNo ratings yet
- Tugas Teks Cause and Effect SmokingDocument9 pagesTugas Teks Cause and Effect SmokingMonica Tia Rissandy100% (1)
- Community Health Nursing ProcessDocument8 pagesCommunity Health Nursing ProcessAncy Varkey0% (1)
- JurdingDocument19 pagesJurdingifaans16No ratings yet
- Placenta PreviaDocument7 pagesPlacenta PreviaMarhina Asarabi MukimNo ratings yet
- 1 SMDocument11 pages1 SMyuniNo ratings yet
- Wa0007.Document47 pagesWa0007.KARLA JOHANNA TARIRA BARROSONo ratings yet
- What's New in Sports Medicine (Primary Care) - UpToDateDocument8 pagesWhat's New in Sports Medicine (Primary Care) - UpToDateManuel OpazoNo ratings yet
- Applying Precision Medicine to Improve Pain Management in SCDDocument2 pagesApplying Precision Medicine to Improve Pain Management in SCDSeemron BiswalNo ratings yet
- Otitis Media: Prepared By: - Priyanka ThapaDocument38 pagesOtitis Media: Prepared By: - Priyanka ThapaKalo kajiNo ratings yet
- Nejmoa 1805374Document10 pagesNejmoa 1805374Lia Diana RaileanuNo ratings yet
- Botulism EpiDocument26 pagesBotulism EpiMaria PavlovaNo ratings yet
- IV Course Curriculum Online Modules and Skills TrainingDocument1 pageIV Course Curriculum Online Modules and Skills TrainingBhuvaneshwariNo ratings yet
- Work Comp C4Document5 pagesWork Comp C4ijustwanawriteNo ratings yet
- Nayor Et Al-2017-European Journal of Heart FailureDocument9 pagesNayor Et Al-2017-European Journal of Heart FailurelacatisNo ratings yet
- Benefits of Zinc:: 1 - Zinc and Regulating Immune FunctionDocument3 pagesBenefits of Zinc:: 1 - Zinc and Regulating Immune FunctionHuda BaharoonNo ratings yet
- ElvisDocument85 pagesElvisPaul SimoneNo ratings yet
- NP IiiDocument9 pagesNP IiiVia LatrasNo ratings yet
- Jurnal Hipertiroid KehamilanDocument5 pagesJurnal Hipertiroid KehamilanAbdurrohman IzzuddinNo ratings yet
- ALGORITHMDocument9 pagesALGORITHMNathalie DeebNo ratings yet
- Couden - Community Health Promotion PaperDocument11 pagesCouden - Community Health Promotion Paperapi-449016836No ratings yet
- Long COVIDDocument8 pagesLong COVIDSMIBA MedicinaNo ratings yet
- Jurnal KeperawatanDocument4 pagesJurnal KeperawatanEko Nur PujiyantoNo ratings yet
- Principles of Spine Trauma and Spinal Deformities PDFDocument34 pagesPrinciples of Spine Trauma and Spinal Deformities PDFVirlan Vasile CatalinNo ratings yet
- Patient Engagement and Safety - PSNetDocument5 pagesPatient Engagement and Safety - PSNetanithaNo ratings yet
- The Aging Population The Increasing Effects On Health CareDocument4 pagesThe Aging Population The Increasing Effects On Health CaredsshhkNo ratings yet